Longitudinal Immunological Analysis of Portuguese Healthcare Workers Across the COVID-19 Pandemic Reveals Differences in the Humoral Immune Response to Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Processing and Analysis
2.3. Statistical Analysis
3. Results
3.1. Participants
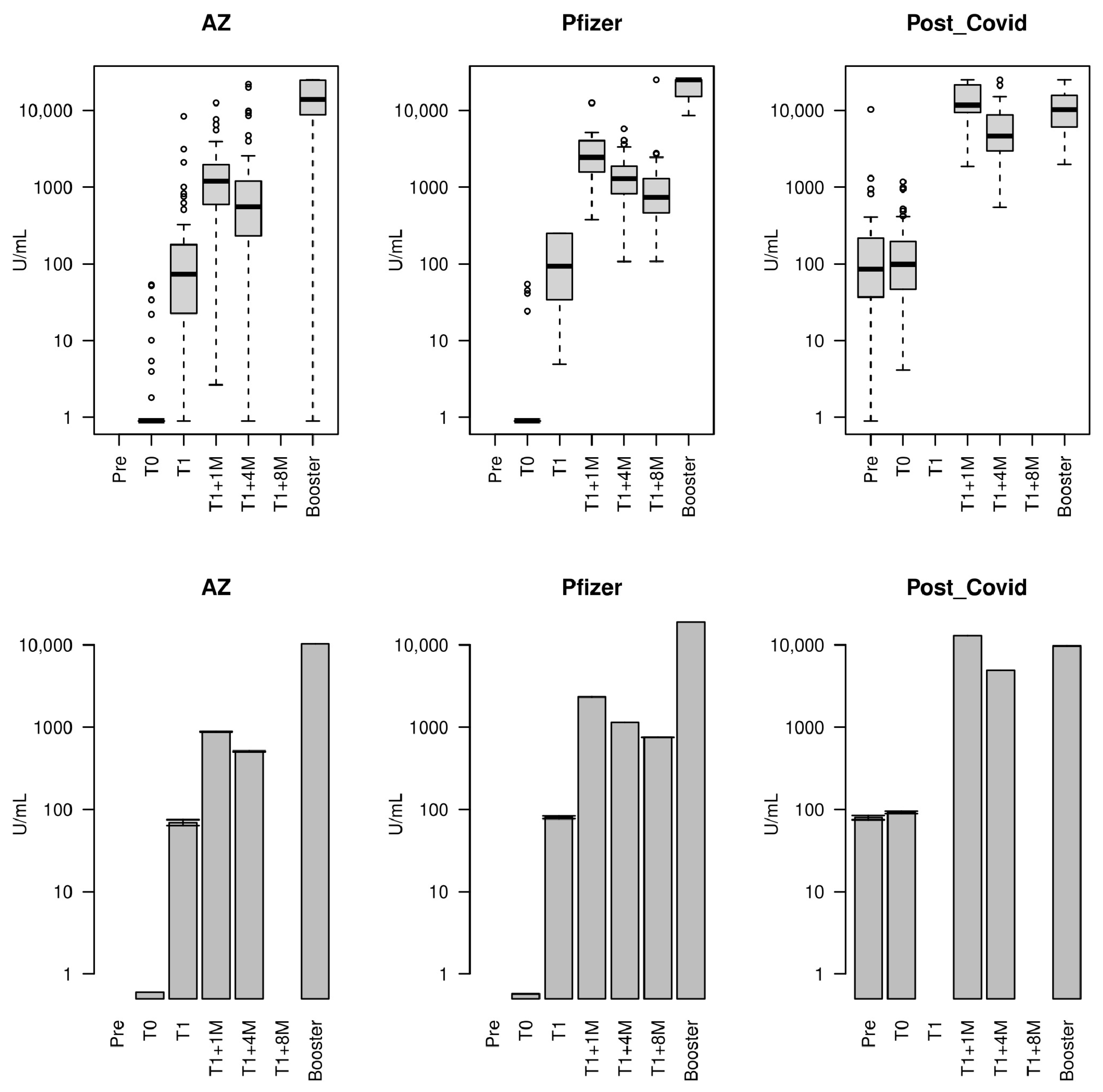
3.2. Concentration of Antibodies Against the SARS-CoV-2 Spike RBD over Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 11 March 2020).
- Carvalho, T.; Krammer, F.; Iwasaki, A. The First 12 Months of COVID-19: A Timeline of Immunological Insights. Nat. Rev. Immunology 2021, 21, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19; Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- EPortugal. Available online: https://eportugal.gov.pt/en/noticias/plano-de-vacinacao-para-a-covid-19 (accessed on 7 December 2020).
- WHO Regional Office for Europe. Strategic Considerations in Preparing for Deployment of COVID-19 Vaccine and Vaccination in the WHO European Region. Who/Euro:2020-1148-40894-55356. Available online: https://apps.who.int/iris/bitstream/handle/10665/335940/WHO-EURO2020-1148-40894-55356-eng.pdf?sequence=1&isAllowed=y (accessed on 9 October 2020).
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.-D.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N. Effect of Delta Variant on Viral Burden and Vaccine Effectiveness against New SARS-CoV-2 Infections in the UK. Nat. Med. 2021, 27, 2127–2135. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a Third Dose of the BNT162b2 MRNA COVID-19 Vaccine for Preventing Severe Outcomes in Israel: An Observational Study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Lopez Bernal, J. Effectiveness of COVID-19 Booster Vaccines against COVID-19-Related Symptoms, Hospitalization and Death in England. Nat. Med. 2022, 28, 831–837. [Google Scholar] [CrossRef]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous Covid-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Edridge, A.W.D.; Kaczorowska, J.; Hoste, A.C.R.; Bakker, M.; Klein, M.; Loens, K.; Jebbink, M.F.; Matser, A.; Kinsella, C.M.; Rueda, P.; et al. Seasonal Coronavirus Protective Immunity Is Short-Lasting. Nat. Med. 2020, 26, 1691–1693. [Google Scholar] [CrossRef]
- Roche Diagnostics. Elecsys® Anti-Sars-Cov-2 S Cobas. Available online: https://www.fda.gov/media/144037/download (accessed on 18 January 2021).
- Lunt, R.; Quinot, C.; Kirsebom, F.; Andrews, N.; Skarnes, C.; Letley, L.; Haskins, D.; Angel, C.; Firminger, S.; Ratcliffe, K.; et al. The impact of vaccination and SARS-CoV-2 variants on the virological response to SARS-CoV-2 infections during the Alpha, Delta, and Omicron waves in England. J. Infect. 2024, 88, 21–29. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Henry, B.M.; Pighi, L.; De Nitto, S.; Gianfilippi, G.L.; Lippi, G. Waning Antibodies in SARS-CoV-2 Naïve Vaccinees: Results of a Three-Month Interim Analysis of Ongoing Immunogenicity and Efficacy Surveillance of the MRNA-1273 Vaccine in Healthcare Workers. J. Infect. 2021, 83, e4–e5. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, D.; Antonazzo, I.C.; Caci, G.; Vitale, A.; Della Ragione, G.; Romano, M.L.; Borrelli, M.; Schiavone, B.; Polosa, R.; Ferrara, P. Dynamics of Antibody Response to BNT162b2 MRNA COVID-19 Vaccine After 6 Months. J. Travel Med. 2021, 28, taab173. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Bayart, J.L.; Mullier, F.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Roy, T.; Wieers, G.; Laurent, C.; Dogné, J.M.; et al. Antibody Titres Decline 3-Month Post-Vaccination with BNT162b2. Emerg. Microbes Infect. 2021, 10, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.; Najjar-Debbiny, R.; Hanna, A.; Jabbour, A.; Abu Ahmad, Y.; Saffuri, A.; Abu-Sinni, M.; Shkeiri, R.; Elemy, A.; Hakim, F. COVID-19 Vaccine–Long Term Immune Decline and Breakthrough Infections. Vaccine 2021, 39, 6984–6989. [Google Scholar] [CrossRef]
- Grupel, D.; Gazit, S.; Schreiber, L.; Nadler, V.; Wolf, T.; Lazar, R.; Supino-Rosin, L.; Perez, G.; Peretz, A.; Ben Tov, A.; et al. Kinetics of SARS-CoV-2 Anti-S IgG after BNT162b2 Vaccination. Vaccine 2021, 39, 5337–5340. [Google Scholar] [CrossRef]
- Wei, J.; Pouwels, K.B.; Stoesser, N.; Matthews, P.C.; Diamond, I.; Studley, R.; Rourke, E.; Cook, D.; Bell, J.I.; Newton, J.N. Antibody Responses and Correlates of Protection in the General Population after Two Doses of the ChAdOx1 or BNT162b2 Vaccines. Nat. Med. 2022, 28, 1072–1082. [Google Scholar] [CrossRef]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J. Spike-Antibody Waning after Second Dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Robertson, L.J.; Price, R.; Moore, J.S.; Curry, G.; Farnan, J.; Black, A.; Blighe, K.; Nesbit, M.A.; McLaughlin, J.A.D.; Moore, T. IgG Antibody Production and Persistence to 6 Months Following SARS-CoV-2 Vaccination: A Northern Ireland Observational Study. Vaccine 2022, 40, 2535–2539. [Google Scholar] [CrossRef]
- Mishra, S.K.; Pradhan, S.K.; Pati, S.; Sahu, S.; Nanda, R.K. Waning of Anti-Spike Antibodies in AZD1222 (ChAdOx1) Vaccinated Healthcare Providers: A Prospective Longitudinal Study. Cureus 2021, 13, e19879. [Google Scholar] [CrossRef]
- Choudhary, H.R.; Parai, D.; Chandra Dash, G.; Kshatri, J.S.; Mishra, N.; Choudhary, P.K.; Pattnaik, D.; Panigrahi, K.; Behera, S.; Ranjan Sahoo, N. Persistence of Antibodies against Spike Glycoprotein of SARS-CoV-2 in Healthcare Workers Post Double Dose of BBV-152 and AZD1222 Vaccines. Front. Med. 2021, 8, 778129. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and Immunogenicity of ChAdOx1 NCoV-19 Vaccine Administered in a Prime-Boost Regimen in Young and Old Adults (COV002): A Single-Blind, Randomised, Controlled, Phase 2/3 Trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck Jr, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and Immunogenicity of Heterologous versus Homologous Prime-Boost Schedules with an Adenoviral Vectored and MRNA COVID-19 Vaccine (Com-COV): A Single-Blind, Randomised, Non-Inferiority Trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Klemis, V.; Schub, D.; Schneitler, S.; Reichert, M.C.; Wilkens, H.; Sester, U.; Sester, M.; Mihm, J. Cellular Immunity Predominates over Humoral Immunity after Homologous and Heterologous MRNA and Vector-Based COVID-19 Vaccine Regimens in Solid Organ Transplant Recipients. Am. J. Transplant. 2021, 21, 3990–4002. [Google Scholar] [CrossRef]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schäffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-Scale Study of Antibody Titer Decay Following BNT162b2 MRNA Vaccine or SARS-CoV-2 Infection. Vaccines 2022, 10, 64. [Google Scholar] [CrossRef]
- L’Huillier, A.G.; Meyer, B.; Andrey, D.O.; Arm-Vernez, I.; Baggio, S.; Didierlaurent, A.; Eberhardt, C.S.; Eckerle, I.; Grasset-Salomon, C.; Huttner, A.; et al. Antibody Persistence in the First 6 Months Following SARS-CoV-2 Infection among Hospital Workers: A Prospective Longitudinal Study. Clin. Microbiol. Infect. 2021, 27, 784.e1–784.e8. [Google Scholar] [CrossRef]
- Cho, A.; Muecksch, F.; Schaefer-Babajew, D.; Wang, Z.; Finkin, S.; Gaebler, C.; Ramos, V.; Cipolla, M.; Mendoza, P.; Agudelo, M.; et al. Anti-SARS-CoV-2 Receptor-Binding Domain Antibody Evolution after MRNA Vaccination. Nature 2021, 600, 517–522. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. Distinguishing Features of Current COVID-19 Vaccines: Knowns and Unknowns of Antigen Presentation and Modes of Action. npj Vaccines 2021, 6, 104. [Google Scholar] [CrossRef]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II Study of COVID-19 RNA Vaccine BNT162b1 in Adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Graham, S.P.; McLean, R.K.; Spencer, A.J.; Belij-Rammerstorfer, S.; Wright, D.; Ulaszewska, M.; Edwards, J.C.; Hayes, J.W.P.; Martini, V.; Thakur, N.; et al. Evaluation of the Immunogenicity of Prime-Boost Vaccination with the Replication-Deficient Viral Vectored COVID-19 Vaccine Candidate ChAdOx1 NCoV-19. npj Vaccines 2020, 5, 2–7. [Google Scholar] [CrossRef]
- Ryan, F.J.; Norton, T.S.; McCafferty, C.; Blake, S.J.; Stevens, N.E.; James, J.; Eden, G.L.; Tee, Y.C.; Benson, S.C.; Masavuli, M.G.; et al. Systems Immunology Study Comparing Innate and Adaptive Immune Responses in Adults to COVID-19 MRNA and Adenovirus Vectored Vaccines. Cell Rep. Med. 2023, 4, 100971. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and Immunogenicity of Seven COVID-19 Vaccines as a Third Dose (Booster) Following Two Doses of ChAdOx1 NCov-19 or BNT162b2 in the UK (COV-BOOST): A Blinded, Multicentre, Randomised, Controlled, Phase 2 Trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Frenck, R.W., Jr.; Walsh, E.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Bailey, R.; Swanson, K.A.; Xu, X.; et al. SARS-CoV-2 Neutralization with BNT162b2 Vaccine Dose 3. N. Engl. J. Med. 2021, 385, 1627–1629. [Google Scholar] [CrossRef] [PubMed]
- Wolszczak-Biedrzycka, B.; Bieńkowska, A.; Cieślikiewicz, B.; Smolińska-Fijołek, E.; Biedrzycki, G.; Dorf, J. The Effect of the Third Dose of the BNT162b2 Vaccine on Anti-SARS-CoV-2 Spike Antibody Levels in Healthcare Workers with and without COVID-19 Infection. Ann. Med. 2023, 55, 722–732. [Google Scholar] [CrossRef]
- Kester, K.E.; Mckinney, D.A.; Tornieporth, N.; Ockenhouse, C.F.; Heppner, D.G.; Hall, T.; Krzych, U.; Delchambre, M.; Voss, G.; Dowler, M.G.; et al. Efficacy of Recombinant Circumsporozoite Protein Vaccine Regimens against Experimental Plasmodium Falciparum Malaria. J. Infect. Dis. 2001, 183, 640–647. [Google Scholar] [CrossRef]
- White, M.T.; Verity, R.; Griffi, J.T.; Asante, K.P.; Owusu-agyei, S.; Greenwood, B.; Drakeley, C.; Gesase, S.; Kremsner, P.; Hoff, I.; et al. Immunogenicity of the RTS, S/AS01 Malaria Vaccine and Implications for Duration of Vaccine Effi Cacy: Secondary Analysis of Data from a Phase 3 Randomised Controlled Trial. Lancet Infect. Dis. 2015, 15, 1450–1458. [Google Scholar] [CrossRef]
- McNamara, H.A.; Idris, A.H.; Sutton, H.J.; Vistein, R.; Flynn, B.J.; Cai, Y.; Wiehe, K.; Lyke, K.E.; Chatterjee, D.; KC, N.; et al. Antibody Feedback Limits the Expansion of B Cell Responses to Malaria Vaccination but Drives Diversification of the Humoral Response. Cell Host Microbe 2020, 28, 572–585.e7. [Google Scholar] [CrossRef]
- Zarnitsyna, V.I.; Ellebedy, A.H.; Davis, C.; Jacob, J.; Ahmed, R.; Antia, R. Masking of Antigenic Epitopes by Antibodies Shapes the Humoral Immune Response to Influenza. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef]
- Zarnitsyna, V.I.; Lavine, J.; Ellebedy, A.; Ahmed, R.; Antia, R. Multi-Epitope Models Explain How Pre-Existing Antibodies Affect the Generation of Broadly Protective Responses to Influenza. PLoS Pathog. 2016, 12, e1005692. [Google Scholar] [CrossRef]
- Ellebedy, A.H.; Krammer, F.; Li, G.-M.; Miller, M.S.; Chiu, C.; Wrammert, J.; Chang, C.Y.; Davis, C.W.; McCausland, M.; Elbein, R. Induction of Broadly Cross-Reactive Antibody Responses to the Influenza HA Stem Region Following H5N1 Vaccination in Humans. Proc. Natl. Acad. Sci. USA 2014, 111, 13133–13138. [Google Scholar] [CrossRef]
- Röltgen, K.; Nielsen, S.C.A.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune Imprinting, Breadth of Variant Recognition, and Germinal Center Response in Human SARS-CoV-2 Infection and Vaccination. Cell 2022, 185, 1025–1040.e14. [Google Scholar] [CrossRef]
- da Rocha Queiroz Lima, M.; Nogueira, R.M.R.; Nogueira, R.M.R.; Nunes, P.C.G.; de Sousa, C.S.; da Silva, M.H.; Santos, F.B.D. A Simple Heat Dissociation Method Increases Significantly the ELISA Detection Sensitivity of the Nonstructural-1 Glycoprotein in Patients Infected with DENV Type-4. J. Virol. Methods 2014, 204, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Bollinger, J.R.; Richard, L.; Kline, H.L.; Francis, M.W.; Moss, J.G.; Bartlett, T.C.Q. Acid Dissociation Increases the Sensitivity of P24 Antigen Detection for the Evaluation of Antiviral Therapy and Disease Progression in Asymptomatic Human Immunodeficiency Virus-Infected Persons. J. Infect. Dis. 1992, 165, 913–916. [Google Scholar]
- Tas, J.M.J.; Koo, J.H.; Lin, Y.C.; Xie, Z.; Steichen, J.M.; Jackson, A.M.; Hauser, B.M.; Wang, X.; Cottrell, C.A.; Torres, J.L.; et al. Antibodies from Primary Humoral Responses Modulate the Recruitment of Naive B Cells during Secondary Responses. Immunity 2022, 55, 1856–1871.e6. [Google Scholar] [CrossRef]
- Schaefer-Babajew, D.; Wang, Z.; Muecksch, F.; Cho, A.; Loewe, M.; Cipolla, M.; Raspe, R.; Johnson, B.; Canis, M.; DaSilva, J.; et al. Antibody Feedback Regulates Immune Memory after SARS-CoV-2 MRNA Vaccination. Nature 2023, 613, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Dangi, T.; Sanchez, S.; Lew, M.H.; Awakoaiye, B.; Visvabharathy, L.; Richner, J.M.; Koralnik, I.J.; Penaloza-MacMaster, P. Pre-Existing Immunity Modulates Responses to MRNA Boosters. Cell Rep. 2023, 42, 112167. [Google Scholar] [CrossRef]
- Parry, H.; Bruton, R.; Stephens, C.; Bentley, C.; Brown, K.; Amirthalingam, G.; Hallis, B.; Otter, A.; Zuo, J.; Moss, P. Extended Interval BNT162b2 Vaccination Enhances Peak Antibody Generation. NPJ Vaccines 2022, 7, 14. [Google Scholar] [CrossRef]
- INSPQ. Efficacité de Deux Doses de Vaccin Contre La COVID-9 Chez Les Adultes Québécois Vivant Dans La Communauté. Available online: https://www.inspq.qc.ca/covid-19/vaccination/efficacite-2-doses (accessed on 2 December 2021).
- Delaunay, C.L.; Melo, A.; Maurel, M.; Mazagatos, C.; Goerlitz, L.; O’Donnell, J.; Oroszi, B.; Sève, N.; Rodrigues, A.P.; Martínez-Baz, I.; et al. Effectiveness of COVID-19 vaccines administered in the 2023 autumnal campaigns in Europe: Results from the VEBIS primary care test-negative design study, September 2023–January 2024. Vaccine 2024, 42, 3931–3937. [Google Scholar] [CrossRef]
- Begovac, J. First real-life data on COVID-19 vaccine effectiveness against hospitalisation and severe disease from the eastern part of the WHO European Region. Lancet Reg. Health-Eur. 2024, 47, 101130. [Google Scholar] [CrossRef]
- WHO. COVID-19 Epidemiological Update-6 November 2024, 173rd ed.; WHO: Geneva, Switzerland, 2024. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update-edition-173 (accessed on 25 November 2024).
- Takei, S.; Ai, T.; Yamamoto, T.; Igawa, G.; Kanno, T.; Tobiume, M.; Hiki, M.; Saito, K.; Khasawneh, A.; Wakita, M.; et al. Performance Evaluation of the Roche Elecsys® Anti-SARS-CoV-2 Immunoassays by Comparison with Neutralizing Antibodies and Clinical Assessment. PLoS ONE 2022, 17, e0274181. [Google Scholar] [CrossRef]
- Taffertshofer, K.; Walter, M.; Mackeben, P.; Kraemer, J.; Potapov, S.; Jochum, S. Design and Performance Characteristics of the Elecsys Anti-SARS-CoV-2 S Assay. Front. Immunol. 2022, 13, 1002576. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | AZ (n = 68) Median (N) | AZ (n = 68) Range (%) | Pfizer (n = 51) Median (N) | Pfizer (n = 51) % (Range) | Post-COVID (n = 72) Median (N) | Post-COVID (n = 72) % (Range) | Difference p-Value **** |
|---|---|---|---|---|---|---|---|
| Age (years) | 44.5 | 20–66 | 41 | 24–65 | 44 | 20–68 | 0.968 |
| Female | 60 | 88.2% | 39 | 76.5% | 61 | 84.7% | 0.218 |
| Serology time diff. (days) | |||||||
| Pre-baseline * to baseline (T0) | ·· | ·· | ·· | ·· | −118 | −118–−118 | ·· |
| T1a (3 weeks) to baseline | ·· | ·· | 23 | 23–23 | ·· | ·· | ·· |
| T1b (3 months) to baseline | 84 | 84–84 | ·· | ·· | ·· | ·· | ·· |
| T1 + 1 month to baseline | 123 | 120–123 | 51 | 51–51 | 36 | 36–36 | <0.001 |
| T1 + 4 months to baseline | 214 | 214–221 | 147 | 147–147 | 118 | 118–118 | <0.001 |
| T1a + 8 months to baseline ** | ·· | ·· | 266 | 266–267 | ·· | ·· | ·· |
| Booster *** to baseline | 286 | 267–328 | 310 | 303–366 | 183 | 176–239 | <0.001 |
| Vaccine | Timepoint | Median | IQR | Geom. Mean (SD) | Fold Change in Geom. Mean [Trend] |
|---|---|---|---|---|---|
| AZ | T0 | 0.400 | 0.400–0.400 | 0.60 (3.28) | Baseline [-] |
| T1b * | 72.57 | 22.08–173.65 | 69.33 (5.59) | 116.23 [>] | |
| T1b + 1 month | 1197 | 592.35–1963.6 | 878.80 (4.16) | 12.68 [>] | |
| T1b + 4 months | 550.8 | 234.7–1200.2 | 507.28 (6.05) | 0.58 [<] | |
| Booster | 13,846 | 9042.5–24,554.5 | 10,251.21 (5.87) | 20.21 [>] | |
| Pfizer | T0 | 0.400 | 0.400–0.400 | 0.57 (3.48) | Baseline [-] |
| T1a * | 93.09 | 36.84–250 | 80.17 (3.05) | 139.95 [>] | |
| T1a + 1 month | 2441.0 | 1564–4030 | 2332.60 (2.04) | 29.10 [>] | |
| T1a + 4 months | 1294.0 | 824.4–1874 | 1145.33 (2.24) | 0.49 [<] | |
| T1a + 8 months | 737.3 | 463.5–1265.2 | 754.91 (2.63) | 0.66 [<] | |
| Booster | 25,000 | 15,220–25,000 | 18,923.95 (1.45) | 25.07 [>] | |
| Post-COVID | 4 months pre-T0 | 84.95 | 36.77–213.85 | 79.45 (4.70) | - |
| T0 | 97.86 | 46.79–192.43 | 92.01 (3.30) | Baseline [-] | |
| T1 ** + 1 month | 11703 | 9406–21,368 | 12,977.29 (1.73) | 141.04 [>] | |
| T1 ** + 4 months | 4610 | 3008.8–8450 | 4920.43 (2.09) | 0.38 [<] | |
| Booster | 10,264 | 6111–15,552 | 9702.92 (1.90) | 1.97 [>] |
| Timepoint Ratio (/T1 + 1M) | Vaccine | Median | IQR | Mean (SD) | Difference p-Value * |
|---|---|---|---|---|---|
| T0 | AZ | 0.0004 | 0.0002–0.0016 | 0.0057 (0.0263) | <10−15 |
| T0 | Pfizer | 0.0002 | 0.0001–0.0003 | 0.0005 (0.0010) | |
| T0 | Post-COVID | 0.0078 | 0.0045–0.0166 | 0.0115 (0.0106) | |
| T1 + 4M | AZ | 0.4033 | 0.3185–0.5172 | 0.4170 (0.1623) | 0.0033 |
| T1 + 4M | Pfizer | 0.5402 | 0.3517–0.6536 | 0.5283 (0.1971) | |
| T1 + 4M | Post-COVID | 0.3645 | 0.2903–0.4905 | 0.4045 (0.1770) | |
| Booster | AZ | 12.924 | 7.812–27.356 | 22.165 (27.088) | <10−15 |
| Booster | Pfizer | 7.8852 | 5.942–10.484 | 9.759 (6.612) | |
| Booster | Post-COVID | 0.8558 | 0.5752–1.000 | 0.8787 (0.4360) |
| Fixed Effects | Estimate | LCL | UCL | p-Value |
|---|---|---|---|---|
| Intercept | 0.6716 | 0.1743 | 1.1689 | 0.0088 |
| Age | −0.0083 | −0.0183 | 0.0018 | 0.1103 |
| Male vs. female | 0.0152 | −0.3083 | 0.3386 | 0.9270 |
| T1 + 1M vs. T0 | 6.5991 | 6.3190 | 6.8793 | <2 × 10−16 |
| T1 + 4M vs. T0 | 6.0483 | 5.7734 | 6.3232 | <2 × 10−16 |
| Booster vs. T0 | 9.2521 | 8.9481 | 9.5560 | <2 × 10−16 |
| Pfizer vs. AZ | −0.0336 | −0.4009 | 0.3337 | 0.8578 |
| Post-COVID vs. AZ | 4.1967 | 3.8421 | 4.5512 | <2 × 10−16 |
| T1 + 1M × Pfizer * | 0.8963 | 0.4972 | 1.2955 | 0.00001 |
| T1 + 4M × Pfizer * | 0.7621 | 0.3607 | 1.1635 | 0.00023 |
| Booster × Pfizer * | 0.3556 | −0.0918 | 0.8031 | 0.1200 |
| T1 + 1M × Post-COVID * | −1.6839 | −2.0763 | −1.2916 | <7 × 10−16 |
| T1 + 4M × Post-COVID * | −2.0898 | −2.4749 | −1.7047 | <2 × 10−16 |
| Booster × Post-COVID * | −4.6049 | −5.0305 | −4.1793 | <2 × 10−16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilela, L.; Silva, A.; Cruz, A.; Sousa, M.; Costa, M.; Fonseca, F.; Campino, S.; Clark, T.G.; Miranda, A. Longitudinal Immunological Analysis of Portuguese Healthcare Workers Across the COVID-19 Pandemic Reveals Differences in the Humoral Immune Response to Vaccines. Vaccines 2024, 12, 1358. https://doi.org/10.3390/vaccines12121358
Vilela L, Silva A, Cruz A, Sousa M, Costa M, Fonseca F, Campino S, Clark TG, Miranda A. Longitudinal Immunological Analysis of Portuguese Healthcare Workers Across the COVID-19 Pandemic Reveals Differences in the Humoral Immune Response to Vaccines. Vaccines. 2024; 12(12):1358. https://doi.org/10.3390/vaccines12121358
Chicago/Turabian StyleVilela, Luísa, Anabela Silva, Alberta Cruz, Madalena Sousa, Margarida Costa, Fernando Fonseca, Susana Campino, Taane G. Clark, and Anabela Miranda. 2024. "Longitudinal Immunological Analysis of Portuguese Healthcare Workers Across the COVID-19 Pandemic Reveals Differences in the Humoral Immune Response to Vaccines" Vaccines 12, no. 12: 1358. https://doi.org/10.3390/vaccines12121358
APA StyleVilela, L., Silva, A., Cruz, A., Sousa, M., Costa, M., Fonseca, F., Campino, S., Clark, T. G., & Miranda, A. (2024). Longitudinal Immunological Analysis of Portuguese Healthcare Workers Across the COVID-19 Pandemic Reveals Differences in the Humoral Immune Response to Vaccines. Vaccines, 12(12), 1358. https://doi.org/10.3390/vaccines12121358






