Longitudinal Comparison of Three T-Cell Assays and Three Antibody Assays Against SARS-CoV-2 Following Homologous mRNA-1273/mRNA-1273/mRNA-1273 and Heterologous ChAdOx1/ChAdOx1/BNT162b2 Vaccination: A Prospective Cohort in Naïve Healthcare Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. SARS-CoV-2-Specific Interferon-γ Enzyme-Linked Immunospot Assay
2.3. SARS-CoV-2-Specific Interferon-γ Release Assay
2.4. SARS-CoV-2 Antibody Assays
2.5. Statistical Analysis
3. Results
3.1. Study Population
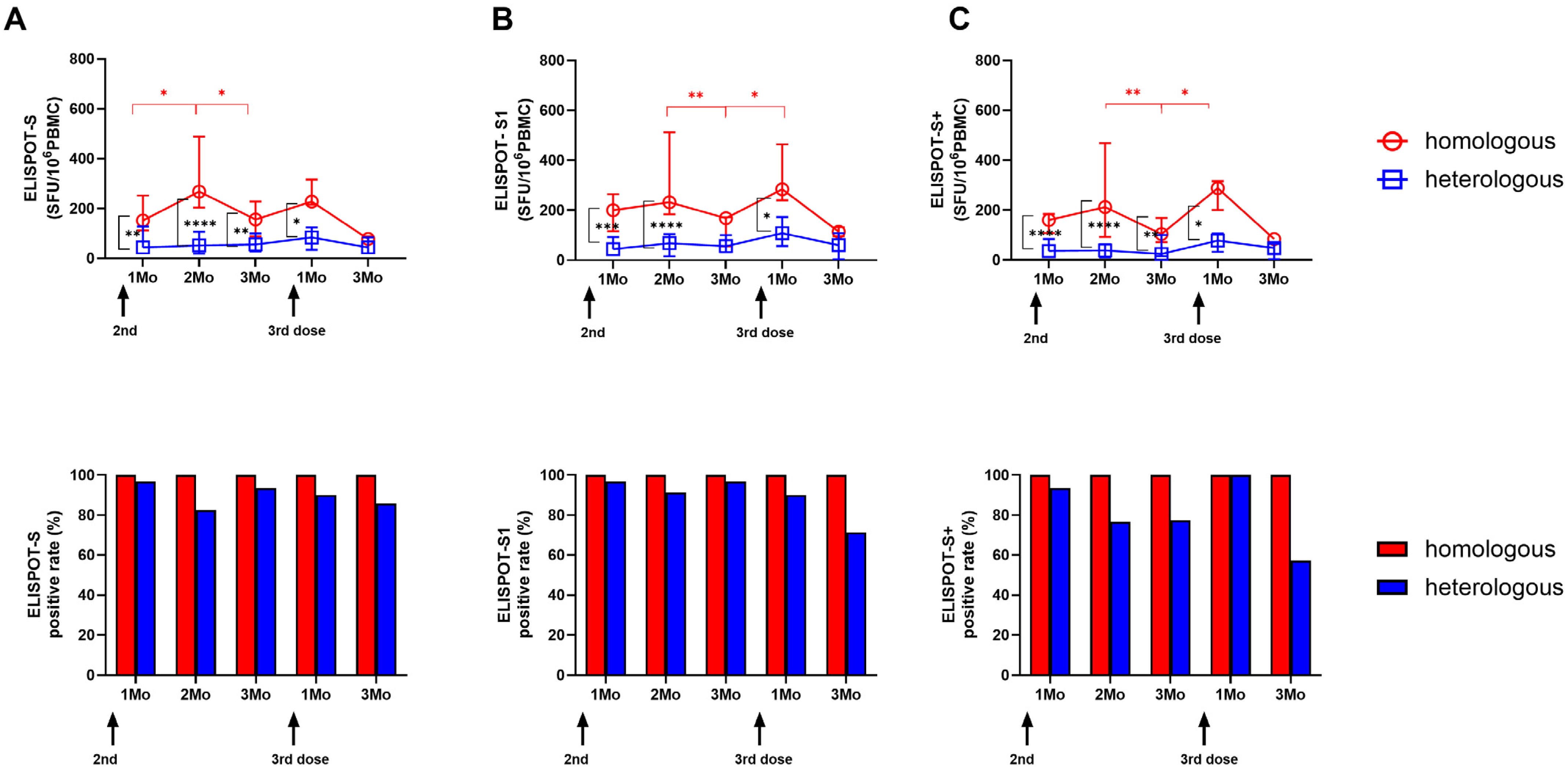
3.2. Longitudinal SARS-CoV-2-Specific ELISPOT Responses in the Two Vaccine Groups
3.3. Longitudinal SARS-CoV-2-Specific Interferon-Gamma Release Assays in the Two Vaccine Groups
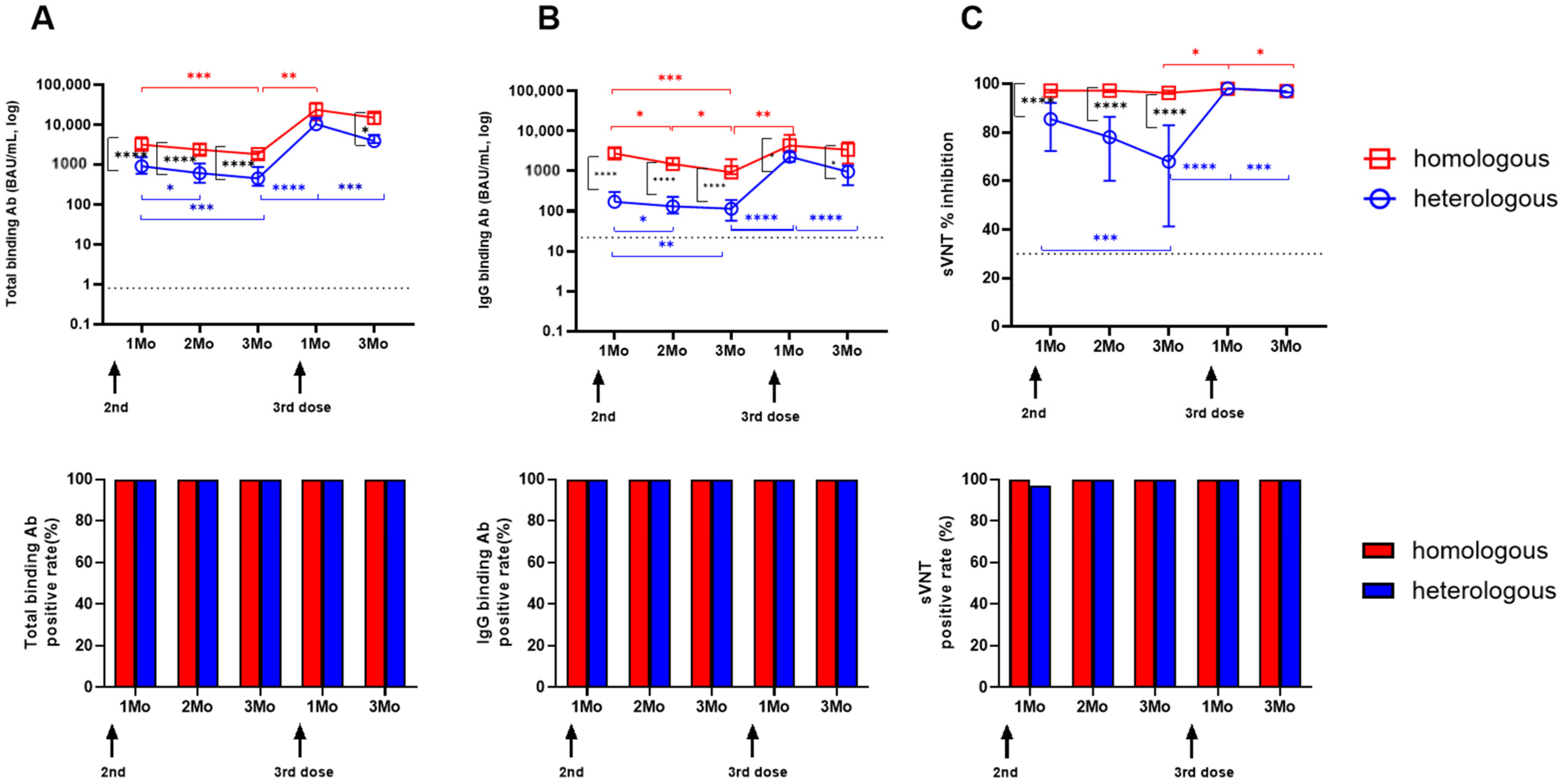
3.4. Longitudinal SARS-CoV-2-Specific Antibody Levels
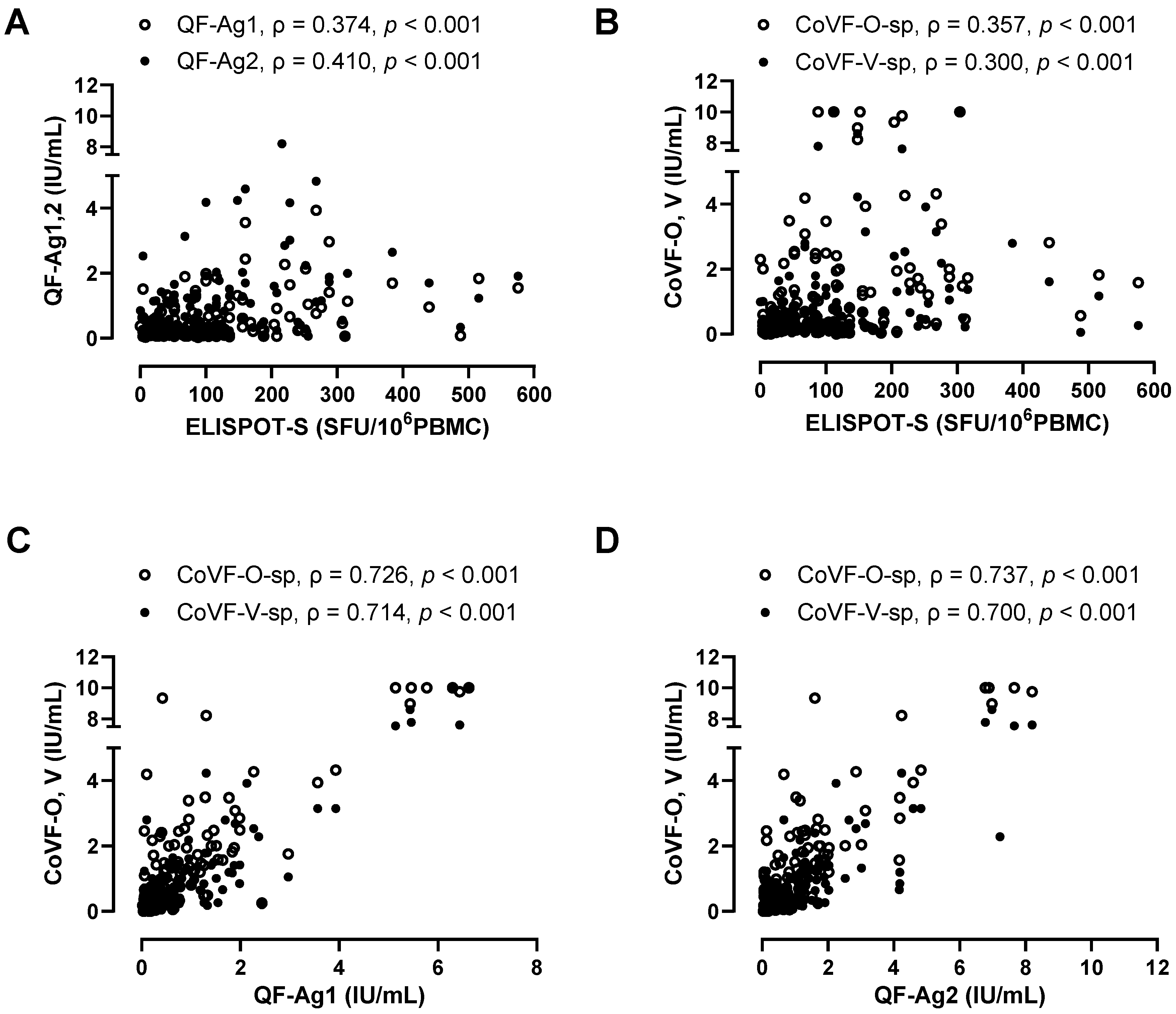
3.5. Correlation and Agreement Between Three SARS-CoV-2-Specific Cellular Assays (ELISPOT, QF-IGRA, CoVF-IGRA)
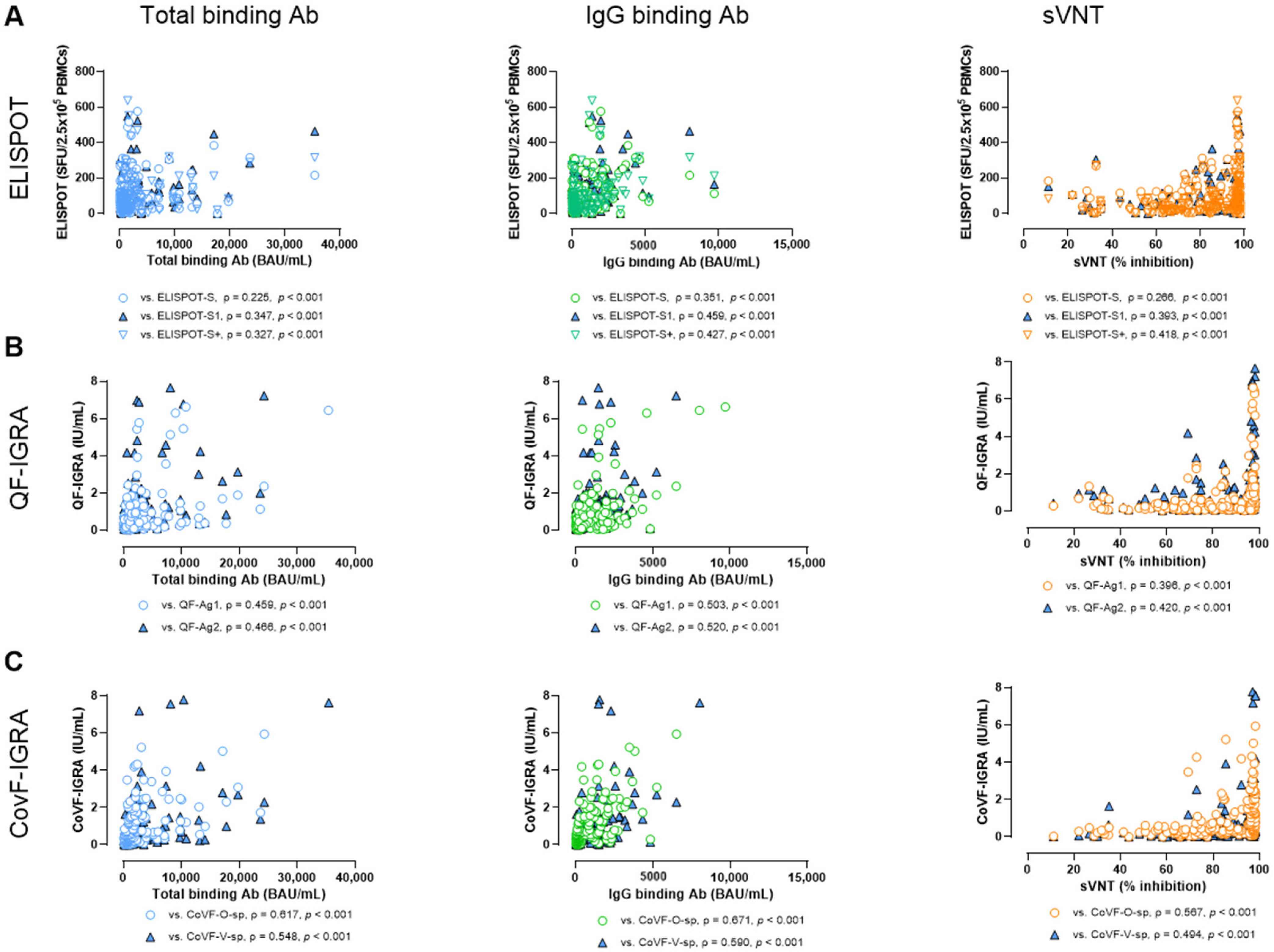
3.6. Correlation and Agreement Between SARS-CoV-2-Specific Antibody Assays and Cellular Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Kang, H.; Jung, J.; Ko, G.Y.; Lee, J.; Oh, E.-J. Evaluation of Long-Term Adaptive Immune Responses Specific to SARS-CoV-2: Effect of Various Vaccination and Omicron Exposure. Vaccines 2024, 12, 301. [Google Scholar] [CrossRef]
- Ssentongo, P.; Ssentongo, A.E.; Voleti, N.; Groff, D.; Sun, A.; Ba, D.M.; Nunez, J.; Parent, L.J.; Chinchilli, V.M.; Paules, C.I. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2022, 22, 439. [Google Scholar] [CrossRef]
- Park, A.K.; Kim, I.H.; Lee, C.Y.; Kim, J.A.; Lee, H.; Kim, H.M.; Lee, N.J.; Woo, S.; Lee, J.; Rhee, J.; et al. Rapid Emergence of the Omicron Variant of Severe Acute Respiratory Syndrome Coronavirus 2 in Korea. Ann. Lab. Med. 2023, 43, 211–213. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Yin, S.; Tao, Y.; Zhu, L.; Tong, X.; Mao, M.; Li, M.; Wan, Y.; Ni, J.; et al. The Third dose of CoronVac vaccination induces broad and potent adaptive immune responses that recognize SARS-CoV-2 Delta and Omicron variants. Emerg. Microbes Infect. 2022, 11, 1524–1536. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. COVID-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Scurr, M.J.; Lippiatt, G.; Capitani, L.; Bentley, K.; Lauder, S.N.; Smart, K.; Somerville, M.S.; Rees, T.; Stanton, R.J.; Gallimore, A.; et al. Magnitude of venous or capillary blood-derived SARS-CoV-2-specific T cell response determines COVID-19 immunity. Nat. Commun. 2022, 13, 5422. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.; Grigoropoulos, I.; Alexopoulou, P.; Karofylakis, E.; Galani, E.; Papadopoulou, K.K.; Tsiavou, A.; Ntourou, A.; Mavrou, E.; Qevani, I.; et al. Sustained cell-mediated but not humoral responses in rituximab-treated rheumatic patients after vaccination against SARS-CoV-2. Rheumatology 2023, 63, 534–541. [Google Scholar] [CrossRef]
- Lochmanova, A.; Martinek, J.; Tomaskova, H.; Zelena, H.; Dieckmann, K.; Grage-Griebenow, E.; Jezo, E.; Janosek, J. Comparison of Two Commercially Available Interferon-gamma Release Assays for T-Cell-Mediated Immunity and Evaluation of Humoral Immunity against SARS-CoV-2 in Healthcare Workers. Diagnostics 2023, 13, 637. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.Y.; Lee, J.; Bae, H.; Ryu, J.H.; Park, H.S.; Kang, H.; Jung, J.; Choi, A.R.; Lee, R.; Lee, D.G.; et al. Longitudinal Analysis of SARS-CoV-2-Specific Cellular and Humoral Immune Responses and Breakthrough Infection following BNT162b2/BNT162b2/BNT162b2 and ChAdOx1/ChAdOx1/BNT162b2 Vaccination: A Prospective Cohort in Naive Healthcare Workers. Vaccines 2023, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.; Kang, H.; Jung, J.; Lee, J.; Ko, G.Y.; Park, H.S.; Choi, J.; Park, K.; Oh, E.J. Comparison of humoral and cellular immune responses between ChAd-BNT heterologous vaccination and BNT-BNT homologous vaccination following the third BNT dose: A prospective cohort study. Front. Immunol. 2023, 14, 1120556. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Binayke, A.; Zaheer, A.; Vishwakarma, S.; Singh, S.; Sharma, P.; Chandwaskar, R.; Gosain, M.; Raghavan, S.; Murugesan, D.R.; Kshetrapal, P.; et al. A quest for universal anti-SARS-CoV-2 T cell assay: Systematic review, meta-analysis, and experimental validation. NPJ Vaccines 2024, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Vardhana, S.; Baldo, L.; Morice, W.G., 2nd; Wherry, E.J. Understanding T cell responses to COVID-19 is essential for informing public health strategies. Sci. Immunol. 2022, 7, eabo1303. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Phillips, E.; Adele, S.; Longet, S.; Malone, T.; Mason, C.; Stafford, L.; Jamsen, A.; Gardiner, S.; Deeks, A.; et al. Evaluation of QuantiFERON SARS-CoV-2 interferon-gamma release assay following SARS-CoV-2 infection and vaccination. Clin. Exp. Immunol. 2023, 212, 249–261. [Google Scholar] [CrossRef]
- Phillips, E.; Adele, S.; Malone, T.; Deeks, A.; Stafford, L.; Dobson, S.L.; Amini, A.; Skelly, D.; Eyre, D.; Jeffery, K.; et al. Comparison of two T-cell assays to evaluate T-cell responses to SARS-CoV-2 following vaccination in naive and convalescent healthcare workers. Clin. Exp. Immunol. 2022, 209, 90–98. [Google Scholar] [CrossRef]
- Kapten, K.; Orczyk, K.; Smolewska, E. Application of Interferon-γ Release Assay in the Assessment of T-Cell Immunity to SARS-CoV-2 Antigens in the Cohort of Pediatric Patients with Juvenile Idiopathic Arthritis. Children 2024, 11, 736. [Google Scholar] [CrossRef]
- Seo, Y.J.; Oh, I.; Nam, M.; Shin, S.; Roh, E.Y.; Song, E.Y. Comparison of Four T-cell Assays and Two Binding Antibody Assays in SARS-CoV-2 Vaccinees With or Without Omicron Breakthrough Infection. Ann. Lab. Med. 2023, 43, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Ryu, J.H.; Jang, J.H.; Bae, H.; Yoo, S.H.; Choi, A.R.; Jo, S.J.; Lim, J.; Lee, J.; Ryu, H.; et al. Comparison of SARS-CoV-2 Antibody Responses and Seroconversion in COVID-19 Patients Using Twelve Commercial Immunoassays. Ann. Lab. Med. 2021, 41, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, J.H.; Ko, G.Y.; Ryu, J.H.; Jang, J.H.; Bae, H.; Yoo, S.H.; Choi, A.R.; Jung, J.; Lee, J.; et al. Quantitative SARS-CoV-2 Spike Antibody Response in COVID-19 Patients Using Three Fully Automated Immunoassays and a Surrogate Virus Neutralization Test. Diagnostics 2021, 11, 1496. [Google Scholar] [CrossRef] [PubMed]
- Vogel, E.; Kocher, K.; Priller, A.; Cheng, C.C.; Steininger, P.; Liao, B.H.; Korber, N.; Willmann, A.; Irrgang, P.; Held, J.; et al. Dynamics of humoral and cellular immune responses after homologous and heterologous SARS-CoV-2 vaccination with ChAdOx1 nCoV-19 and BNT162b2. EBioMedicine 2022, 85, 104294. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.L.; Oh, J. COVID-19 vaccination program in South Korea: A long journey toward a new normal. Health Policy Technol. 2022, 11, 100601. [Google Scholar] [CrossRef]
- Jaganathan, S.; Stieber, F.; Rao, S.N.; Nikolayevskyy, V.; Manissero, D.; Allen, N.; Boyle, J.; Howard, J. Preliminary Evaluation of QuantiFERON SARS-CoV-2 and QIAreach Anti-SARS-CoV-2 Total Test in Recently Vaccinated Individuals. Infect. Dis. Ther. 2021, 10, 2765–2776. [Google Scholar] [CrossRef]
- Gandolfo, C.; Anichini, G.; Mugnaini, M.; Bocchia, M.; Terrosi, C.; Sicuranza, A.; Gori Savellini, G.; Gozzetti, A.; Franchi, F.; Cusi, M.G. Overview of Anti-SARS-CoV-2 Immune Response Six Months after BNT162b2 mRNA Vaccine. Vaccines 2022, 10, 171. [Google Scholar] [CrossRef]
- Yang, J.; Ko, J.H.; Baek, J.Y.; Hong, J.; Ha, S.; Lee, B.; Huh, K.; Cho, S.Y.; Kang, C.I.; Chung, D.R.; et al. Effects of Short-Term Corticosteroid Use on Reactogenicity and Immunogenicity of the First Dose of ChAdOx1 nCoV-19 Vaccine. Front. Immunol. 2021, 12, 744206. [Google Scholar]
- Jeong, S.; Lee, N.; Lee, S.K.; Cho, E.J.; Hyun, J.; Park, M.J.; Song, W.; Kim, H.S. Humoral and Cellular Responses to BNT162b2 as a Booster Following Two Doses of ChAdOx1 nCov-19 Determined Using Three SARS-CoV-2 Antibody Assays and an Interferon-Gamma Release Assay: A Prospective Longitudinal Study in Healthcare Workers. Front. Immunol. 2022, 13, 859019. [Google Scholar] [CrossRef]
- Nandakumar, V.; Profaizer, T.; Lozier, B.K.; Elgort, M.G.; Larragoite, E.T.; Williams, E.; Solis-Leal, A.; Lopez, J.B.; Berges, B.K.; Planelles, V.; et al. Evaluation of a Surrogate Enzyme-Linked Immunosorbent Assay-Based Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) cPass Neutralization Antibody Detection Assay and Correlation With Immunoglobulin G Commercial Serology Assays. Arch. Pathol. Lab. Med. 2021, 145, 1212–1220. [Google Scholar] [CrossRef]
- Park, Y.; Hong, K.H.; Lee, S.K.; Hyun, J.; Oh, E.J.; Lee, J.; Lee, H.; Song, S.H.; Kee, S.J.; Kwon, G.C.; et al. Performance Comparison of Five SARS-CoV-2 Antibody Assays for Seroprevalence Studies. Ann. Lab. Med. 2022, 42, 71–78. [Google Scholar] [CrossRef]
- Janosek, J.; Komarek, A. Post-infection immunity provides excellent protection from COVID-19 ICU hospitalization during Delta and Omicron waves. Infect. Dis. 2023, 55, 74–77. [Google Scholar] [CrossRef]
- Mak, W.A.; Visser, W.; Koeleman, J.G.M.; Ong, D.S.Y. SARS-CoV-2-specific T cell responses: A comparative analysis between QuantiFERON SARS-CoV-2, T-SPOT.COVID, and an in-house Omicron ELISpot. J. Virol. Methods 2024, 327, 114949. [Google Scholar] [CrossRef] [PubMed]
- Borobia, A.M.; Carcas, A.J.; Perez-Olmeda, M.; Castano, L.; Bertran, M.J.; Garcia-Perez, J.; Campins, M.; Portoles, A.; Gonzalez-Perez, M.; Garcia Morales, M.T.; et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef]
- Pedersen, R.M.; Tornby, D.S.; Bistrup, C.; Johansen, I.S.; Andersen, T.E.; Justesen, U.S. Negative SARS-CoV-2 antibodies, T-cell response and virus neutralization following full vaccination in a renal transplant recipient: A call for vigilance. Clin. Microbiol. Infect. 2021, 27, 1371–1373. [Google Scholar] [CrossRef]
- Van Praet, J.T.; Vandecasteele, S.; De Roo, A.; De Vriese, A.S.; Reynders, M. Humoral and Cellular Immunogenicity of the BNT162b2 Messenger RNA Coronavirus Disease 2019 Vaccine in Nursing Home Residents. Clin. Infect. Dis. 2021, 73, 2145–2147. [Google Scholar] [CrossRef]
- Widyasari, K.; Jang, J.; Lee, S.; Kang, T.; Kim, S. Evaluation of the T cell and B cell response following the administration of COVID-19 vaccines in Korea. J. Microbiol. Immunol. Infect. 2022, 55, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Widyasari, K.; Kim, S. Comparative analysis between STANDARD-E Covi-FERON ELISA with pre-existing IFN-gamma release assays and determination of the optimum cutoff value for assessment of T-Cell response to SARS-CoV-2. J. Clin. Lab. Anal. 2023, 37, e24882. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.D.; van der Heiden, M.; Geers, D.; Imhof, C.; van Baarle, D. RECOVAC-IR Collaborators. Difference in sensitivity between SARS-CoV-2-specific T cell assays in patients with underlying conditions. J. Clin. Investig. 2021, 131, e155499. [Google Scholar] [CrossRef]
- Barbeau, D.J.; Martin, J.M.; Carney, E.; Dougherty, E.; Doyle, J.D.; Dermody, T.S.; Hoberman, A.; Williams, J.V.; Michaels, M.G.; Alcorn, J.F.; et al. Comparative analysis of human immune responses following SARS-CoV-2 vaccination with BNT162b2, mRNA-1273, or Ad26.COV2.S. NPJ Vaccines 2022, 7, 77. [Google Scholar] [CrossRef]
- van Gils, M.J.; Lavell, A.; van der Straten, K.; Appelman, B.; Bontjer, I.; Poniman, M.; Burger, J.A.; Oomen, M.; Bouhuijs, J.H.; van Vught, L.A.; et al. Antibody responses against SARS-CoV-2 variants induced by four different SARS-CoV-2 vaccines in health care workers in the Netherlands: A prospective cohort study. PLoS Med. 2022, 19, e1003991. [Google Scholar] [CrossRef]
- Favresse, J.; Tre-Hardy, M.; Gillot, C.; Cupaiolo, R.; Wilmet, A.; Beukinga, I.; Blairon, L.; Bayart, J.L.; Closset, M.; Wauthier, L.; et al. Vaccine-induced humoral response of BNT162b2 and mRNA-1273 against BA.1, BA.5, and XBB.1.5. (sub)variants 6 months after a homologous booster: Is immunogenicity equivalent? Heliyon 2024, 10, e36116. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.; Yun, S.G.; Kim, S.W.; Kim, C.G.; Cha, J.H.; Lee, C.; Kang, S.; Park, S.G.; Kim, S.B.; Lee, K.B.; et al. Humoral and Cellular Immune Responses to Vector, Mix-and-Match, or mRNA Vaccines against SARS-CoV-2 and the Relationship between the Two Immune Responses. Microbiol. Spectr. 2022, 10, e0249521. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Lamara Mahammed, L.; Bensaid, K.; Ait-Seddik, S.; Larinouna, A.; Brahimi, G.; Belkaid, R.; Hamzaoui, O.; Rouaki, S.M.; Idder, C.; Allam, I.; et al. Improved Performance of the QuantiFERON-SARS-CoV-2 Assay with the Extended Set. Viruses 2023, 15, 1179. [Google Scholar] [CrossRef]
- Ishii, T.; Hamada, K.; Jubishi, D.; Hashimoto, H.; Okamoto, K.; Hisasue, N.; Sunohara, M.; Saito, M.; Shinohara, T.; Yamashita, M.; et al. Waning cellular immune responses and predictive factors in maintaining cellular immunity against SARS-CoV-2 six months after BNT162b2 mRNA vaccination. Sci. Rep. 2023, 13, 9607. [Google Scholar] [CrossRef]
- Le Bert, N.; Chia, W.N.; Wan, W.Y.; Teo, A.K.J.; Chong, S.Z.; Tan, N.; Tan, D.S.C.; Chia, A.; Tan, I.B.; Kunasegaran, K.; et al. Widely heterogeneous humoral and cellular immunity after mild SARS-CoV-2 infection in a homogeneous population of healthy young men. Emerg. Microbes Infect. 2021, 10, 2141–2150. [Google Scholar] [CrossRef]
- Bonnet, B.; Chabrolles, H.; Archimbaud, C.; Brebion, A.; Cosme, J.; Dutheil, F.; Lambert, C.; Junda, M.; Mirand, A.; Ollier, A.; et al. Decline of Humoral and Cellular Immune Responses Against SARS-CoV-2 6 Months After Full BNT162b2 Vaccination in Hospital Healthcare Workers. Front. Immunol. 2022, 13, 842912. [Google Scholar] [CrossRef]
- Malipiero, G.; Moratto, A.; Infantino, M.; D’Agaro, P.; Piscianz, E.; Manfredi, M.; Grossi, V.; Benvenuti, E.; Bulgaresi, M.; Benucci, M.; et al. Assessment of humoral and cellular immunity induced by the BNT162b2 SARS-CoV-2 vaccine in healthcare workers, elderly people, and immunosuppressed patients with autoimmune disease. Immunol. Res. 2021, 69, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Brlek, P.; Matisic, V.; Molnar, V.; Vrdoljak, K.; Zadro, R.; Parcina, M. Cellular Immunity-The Key to Long-Term Protection in Individuals Recovered from SARS-CoV-2 and after Vaccination. Vaccines 2022, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, P.; Sanz, J.C.; San Román, J.; Pérez-Abeledo, M.; Carretero, M.; Megías, G.; Viñuela-Prieto, J.M.; Ramos, B.; Canora, J.; Martínez-Peromingo, F.J.; et al. A Pilot Study for the Evaluation of an Interferon Gamma Release Assay (IGRA) To Measure T-Cell Immune Responses after SARS-CoV-2 Infection or Vaccination in a Unique Cloistered Cohort. J. Clin. Microbiol. 2022, 60, e0219921. [Google Scholar] [CrossRef]
- Pitiriga, V.C.; Papamentzelopoulou, M.; Konstantinakou, K.E.; Vasileiou, I.V.; Konstantinidis, A.D.; Spyrou, N.I.; Tsakris, A. Prolonged SARS-CoV-2 T Cell Responses in a Vaccinated COVID-19-Naive Population. Vaccines 2024, 12, 270. [Google Scholar] [CrossRef]
- Vitiello, L.; Gatta, L.; Ilari, S.; Bonassi, S.; Cristina, M.; Ciatti, F.; Fini, M.; Proietti, S.; Russo, P.; Tomino, C.; et al. Long Lasting Cellular Immune Response Induced by mRNA Vaccination: Implication for Prevention Strategies. Front. Immunol. 2022, 13, 836495. [Google Scholar] [CrossRef]



| Assay | Platform | Stimulating Agents for T-Cell-Response Assays and Target Epitope for Antibody Assays | Sample | Cut-Off |
|---|---|---|---|---|
| SARS-CoV-2 ELISPOT assay | Interferon-γ ELISPOT | Peptide pools of S, S1, S+ protein of WT | PBMCs | 10 SFU/106 PBMCs |
| QuantiFERON SARS-CoV-2 | Whole blood IGRA | Ag1–CD4+ epitopes derived from the S1 subunit (WT) Ag2–CD4+ and CD8+ epitopes from the S1 and S2 subunits (WT) | Whole blood | 0.15 IU/mL |
| Covi-FERON | Whole blood IGRA | Original spike protein tube—spike protein derived from Wuhan and UK variants (B.1.1.7) Variant spike protein tube—spike protein from South Africa (B.1.351) and Brazil (P.1) strains | Whole blood | 0.25 IU/mL |
| Elecsys Anti-SARS-CoV-2 assay | ECLIA | RBD of S protein | serum | 0.8 U/mL |
| Atellica IM SARS-CoV-2 IgG assay | CLIA | RBD of S1 protein | serum | 1.0 U/mL |
| GenScript SARS-CoV-2 Surrogate Virus Neutralization Test | ELISA | Block the interaction between the RBD with the ACE2 cell surface receptor | serum | 30% inhibition |
| Characteristic | Homologous mRNA-1273 Group (mRNA-1273/mRNA-1273/mRNA-1273) (n = 11) | Heterologous ChAd Group (ChAd/ChAd/BNT) (n = 35) | p-Value |
|---|---|---|---|
| Sex, n (%) | 0.1124 | ||
| Female | 8 (72.7) | 32 (91.4) | |
| Male | 3 (27.3) | 3 (8.6) | |
| Age, years | |||
| mean ± SD | 29.1 +/− 6.5 | 39.2 +/− 7.9 | 0.0004 |
| group, n (%) | |||
| 20–29 | 6 (63.7) | 4 (11.5) | 0.0005 |
| 30–39 | 3 (27.3) | 16 (45.8) | 0.2825 |
| 40–49 | 1 (9.1) | 10 (28.8) | 0.1874 |
| 50–59 | 0 (0) | 5 (14.4) | 0.1871 |
| Vaccination schedule | |||
| First to second dose interval (days) | |||
| Median (range) | 28 (24–36) | 73 (57–91) | |
| Second to third interval (days) | |||
| Median (range) | 161 (154–161) | 167 (147–201) | |
| Breakthrough infection until 7 months after 3rd vaccination, n (%) | |||
| 6 (63.7) | 15 (42.9) | 0.2335 | |
| Assay | ELISPOT | QF-IGRA | CoVF-IGRA | sVNT | IgG Antibody | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | S1 | S+ | Ag1 | Ag2 | O-sp | V-sp | ||||
| ELISPOT | S1 | † 93.7 ‡ (0.874) | ||||||||
| S+ | 89.7 (0.795) | 90.7 (0.815) | ||||||||
| QF-IGRA | Ag1 | 82.3 (0.649) | 83.3 (0.669) | 79.5 (0.592) | ||||||
| Ag2 | 87.7 (0.755) | 88.6 (0.774) | 84.9 (0.697) | 78.0 (0.560) | ||||||
| CoVF-IGRA | O-sp | 84.9 (0.699) | 85.8 (0.718) | 82.0 (0.641) | 75.3 (0.506) | 80.4 (0.608) | ||||
| V-sp | 75.4 (0.515) | 76.3 (0.534) | 72.6 (0.458) | 66.3 (0.325) | 71.4 (0.428) | 68.7 (0.373) | ||||
| sVNT | 95.3 (0.905) | 96.2 (0.924) | 92.4 (0.848) | 85.2 (0.705) | 90.4 (0.807) | 87.7 (0.753) | 78.6 (0.572) | |||
| IgG antibody | 96.2 (0.924) | 97.2 (0.943) | 93.4 (0.866) | 86.1 (0.723) | 91.3 (0.825) | 88.6 (0.771) | 79.5 (0.590) | 98.5 (0.969) | ||
| Total antibody | 96.5 (0.930) | 97.5 (0.949) | 93.7 (0.873) | 86.4 (0.729) | 91.6 (0.831) | 88.9 (0.777) | 79.8 (0.596) | 98.8 (0.976) | 99.7 (0.994) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Ko, G.Y.; Lee, J.; Bae, H.; Ryu, J.H.; Jung, J.; Kang, H.; Lee, R.; Lee, D.-G.; Oh, E.-J. Longitudinal Comparison of Three T-Cell Assays and Three Antibody Assays Against SARS-CoV-2 Following Homologous mRNA-1273/mRNA-1273/mRNA-1273 and Heterologous ChAdOx1/ChAdOx1/BNT162b2 Vaccination: A Prospective Cohort in Naïve Healthcare Workers. Vaccines 2024, 12, 1350. https://doi.org/10.3390/vaccines12121350
Lee H, Ko GY, Lee J, Bae H, Ryu JH, Jung J, Kang H, Lee R, Lee D-G, Oh E-J. Longitudinal Comparison of Three T-Cell Assays and Three Antibody Assays Against SARS-CoV-2 Following Homologous mRNA-1273/mRNA-1273/mRNA-1273 and Heterologous ChAdOx1/ChAdOx1/BNT162b2 Vaccination: A Prospective Cohort in Naïve Healthcare Workers. Vaccines. 2024; 12(12):1350. https://doi.org/10.3390/vaccines12121350
Chicago/Turabian StyleLee, Hyeyoung, Geon Young Ko, Jihyun Lee, Hyunjoo Bae, Ji Hyeong Ryu, Jin Jung, Hyunhye Kang, Raeseok Lee, Dong-Gun Lee, and Eun-Jee Oh. 2024. "Longitudinal Comparison of Three T-Cell Assays and Three Antibody Assays Against SARS-CoV-2 Following Homologous mRNA-1273/mRNA-1273/mRNA-1273 and Heterologous ChAdOx1/ChAdOx1/BNT162b2 Vaccination: A Prospective Cohort in Naïve Healthcare Workers" Vaccines 12, no. 12: 1350. https://doi.org/10.3390/vaccines12121350
APA StyleLee, H., Ko, G. Y., Lee, J., Bae, H., Ryu, J. H., Jung, J., Kang, H., Lee, R., Lee, D.-G., & Oh, E.-J. (2024). Longitudinal Comparison of Three T-Cell Assays and Three Antibody Assays Against SARS-CoV-2 Following Homologous mRNA-1273/mRNA-1273/mRNA-1273 and Heterologous ChAdOx1/ChAdOx1/BNT162b2 Vaccination: A Prospective Cohort in Naïve Healthcare Workers. Vaccines, 12(12), 1350. https://doi.org/10.3390/vaccines12121350







