Recent Advances in the Development of Mincle-Targeting Vaccine Adjuvants
Abstract
1. The Role of Adjuvants in Vaccine Development
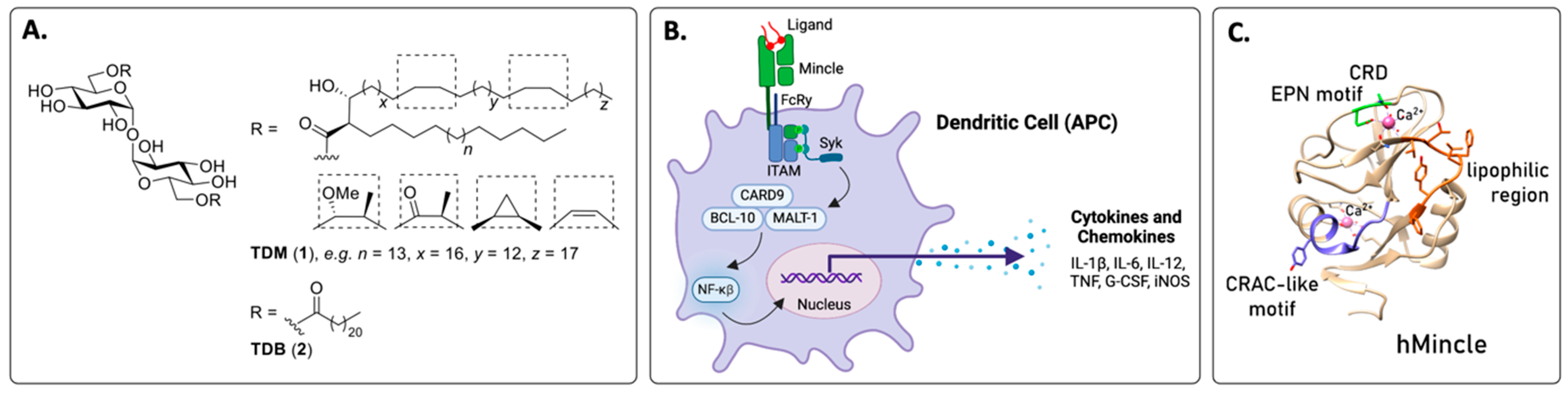
2. The Macrophage-Inducible C-Type Lectin (Mincle)
2.1. The Identification of Mincle and Signalling Pathways
2.2. Ligand Binding Sites in Mincle
2.3. The Multimerisation of Mincle for an Immune Response
2.4. Mincle Activation Leads to a TH1 and TH17 Phenotype
3. Mincle Agonists
3.1. In Vitro Assays Used to Identify Mincle Agonists
3.2. Classes of Mincle Agonists with Adjuvant Activity
3.2.1. Linear and Branched Trehalose Glycolipids (TGLs)
3.2.2. Hydrolytically Stable Linear Trehalose Glycolipids
3.2.3. Lipidated Brartemicin Derivatives
3.2.4. Fatty Acid Derivatives of Glucose, Mannose, and Arabinose
3.2.5. Glycerolipids
3.2.6. Phenololic Glycolipid-III
3.2.7. Archaeal Glycerolipids
3.3. New Mincle Agonists with Unknown Adjuvanticity (Identified After 2017)
4. Mincle-Targeting Adjuvants Used in Preventative or Therapeutic Vaccines
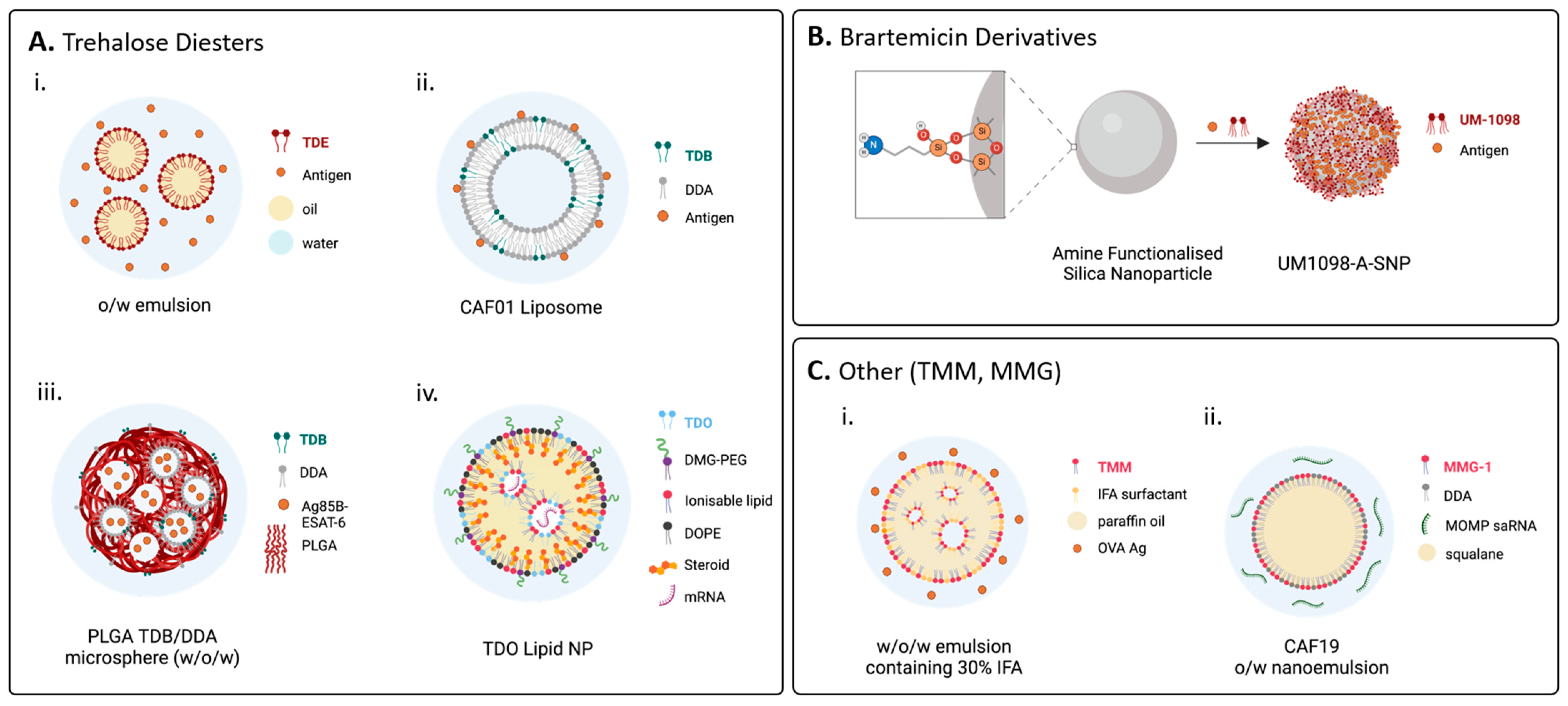
4.1. Formulation Is Critical for In Vivo Applications
4.2. Single Mincle Ligand Agonists as Adjuvants for Preventative Vaccines
4.2.1. Linear Trehalose Diesters
4.2.2. Branched Trehalose Diesters
4.2.3. Trehalose Monoesters
4.2.4. Hydrolytically Stable Linear Trehalose Glycolipids
4.2.5. Lipidated Brartemicin Derivatives
4.2.6. Glycerolipids
4.2.7. Fatty Acid Derivatives of Glucose, Mannose, and Arabinose
4.2.8. Phenololic Glycolipid-III
4.2.9. Archaeal Glycolipids
4.3. Single Mincle Ligand Agonists as Adjuvants for Anti-Cancer Vaccines
Trehalose Diesters (TDM, TDB and TDCM)
5. Codelivery of Mincle Ligands and Other PAMPs or Immunomodulators
5.1. Codelivery of PAMPs or Other Immunomodulators for Applications in Prophylactic Vaccines
5.1.1. Complex Mixture of PAMPs
5.1.2. Mincle Ligands and TLR2 Ligands
5.1.3. Mincle Ligands and TLR3 Ligands
5.1.4. Mincle Ligands and TLR4 Ligands
5.1.5. Mincle Ligands and TLR5 Ligands
5.1.6. Mincle Ligands and TLR7/8 Ligands
5.1.7. Mincle Ligands and TLR9 Ligands
5.1.8. Mincle Ligands and Peptidoglycan Fragments
5.1.9. Mincle Ligands and STING Agonists
5.1.10. Mincle Ligands and Retinoic Acid
5.2. Codelivery of PAMPs for Application in Therapeutic (Anti-Cancer) Vaccines
5.2.1. Complex Mixtures of PAMPs
5.2.2. Mincle Ligands and TLR3 Antigens
5.2.3. Mincle Ligands and TLR4 Ligands
5.2.4. Mincle Ligands and Anti-PD-1 Ligands
5.3. Chimeric Adjuvants: Prophylactic Vaccines
6. Mincle-Targeting Adjuvant–Antigen Conjugates
6.1. Adjuvant–Antigen Conjugates for Prophylactic Vaccines
6.2. Adjuvant–Antigen Conjugates for Therapeutic (Anti-Cancer) Vaccines
7. Therapeutic Application
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Hagan, D.T.; Lodaya, R.N.; Lofano, G. The continued advance of vaccine adjuvants—‘We can work it out’. Semin. Immunol. 2020, 50, 101426. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ho, M.; Hu, Y.; Shi, Y. Vaccine adjuvants: Current status research, and development, licensing, and future opportunities. J. Mater. Chem. B 2024, 12, 4118–4137. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Mahajan, P.; Singh, N.K.; Gupta, A.; Aggarwal, R.; Rappuoli, R.; Johri, A.K. New-age vaccine adjuvants, their development and future perspective. Front. Immunol. 2023, 14, 1043109. [Google Scholar] [CrossRef]
- Ong, G.H.; Lian, B.S.X.; Kawasakai, T.; Kawai, T. Exploration of Pattern Recognition Receptor Agonists as Candidate Adjuvants. Front. Cell. Infect. Microbiol. 2021, 11, 745016. [Google Scholar] [CrossRef]
- Sarkar, I.; Garg, R.; van Drunen Little-van den Hurk, S. Selection of adjuvants for vaccines targeting specific pathogens. Expert Rev. Vaccines 2019, 18, 505–521. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2021, 22, 236–250. [Google Scholar] [CrossRef]
- Tom, J.K.; Albin, T.J.; Manna, S.; Moser, B.A.; Steinhardt, R.C.; Esser-Kahn, A.P. Applications of Immunomodulatory Immune Synergies to Adjuvant Discovery and Vaccine Development. Trends Biotechnol. 2019, 37, 373–388. [Google Scholar] [CrossRef]
- Li, W.-H.; Li, Y.-M. Chemical Strategies to Boost Cancer Vaccines. Chem. Rev. 2020, 120, 11420–11478. [Google Scholar] [CrossRef]
- Lu, X.; Nagata, M.; Yamasaki, S. Mincle: 20 years of a versatile sensor of insults. Int. Immunol. 2018, 30, 233–239. [Google Scholar] [CrossRef]
- Lin, Y.; Slight, S.R.; Khader, S.A. Th17 cytokines and vaccine induced immunity. Semin. Immunopathol. 2020, 32, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Tanaka, T.; Kaisho, T.; Sanjo, H.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Akira, S. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J. Immunol. 1999, 163, 5039–5048. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, E.; Ishikawa, T.; Morita, Y.S.; Toyonaga, K.; Yamada, H.; Takeuchi, O.; Kinoshita, T.; Akira, S.; Yoshikai, Y.; Yamasaki, S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 2009, 206, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Werninghaus, K.; Babiak, A.; Gross, O.; Hölscher, C.; Dietrich, H.; Agger, E.M.; Mages, J.; Mocsai, A.; Schoenen, H.; Finger, K.; et al. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRγ-Syk-Card9-dependent innate immune activation. J. Exp. Med. 2009, 206, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Schoenen, H.; Bodendorfer, B.; Hitchens, K.; Manzanero, S.; Werninghaus, K.; Nimmerjahn, F.; Agger, E.M.; Stenger, S.; Andersen, P.; Ruland, J.; et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J. Immunol. 2010, 184, 2756–2760. [Google Scholar] [CrossRef]
- Balch, S.G.; McKnight, A.J.; Seldin, M.F.; Gordon, S. Cloning of a novel C-type lectin expressed by murine macrophages. J. Biol. Chem. 1998, 273, 18656–18664. [Google Scholar] [CrossRef]
- Braganza, C.D.; Teunissen, T.; Timmer, M.S.M.; Stocker, B.L. Identification and Biological Activity of Synthetic Macrophage Inducible C-Type Lectin Ligands. Front. Immunol. 2018, 8, 1940. [Google Scholar] [CrossRef]
- Williams, S.J. Sensing Lipids with Mincle: Structure and Function. Front. Immunol. 2017, 8, 1662. [Google Scholar] [CrossRef]
- Cramer, J. Medicinal chemistry of the myeloid C-type lectin receptors Mincle, Langerin, and DC-SIGN. RSC Med. Chem. 2021, 12, 1985–2000. [Google Scholar] [CrossRef]
- Braganza, C.D.; Kodar, K.; Teunissen, T.; Andreassend, S.K.; Khan, A.; Timmer, M.S.M.; Stocker, B.L. Lipophilic glycose monoesters and glycosides are potent human Mincle agonists. Org. Biomol. Chem. 2022, 20, 3096–3104. [Google Scholar] [CrossRef]
- Van Huy, L.; Tanaka, C.; Imai, T.; Yamasaki, S.; Miyamoto, T. Synthesis of 12-O-Mono- and Diglycosyl-oxystearates, a New Class of Agonists for the C-type Lectin Receptor Mincle. ACS Med. Chem. Lett. 2018, 10, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Miller, S.M.; Buhl, C.; Child, R.; Whitacre, M.; Schoener, R.; Ettenger, G.; Burkhart, D.; Ryter, K.; Evans, J.T. Species-Specific Structural Requirements of Alpha-Branched Trehalose Diester Mincle Agonists. Front. Immunol. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Dangerfield, E.M.; Lynch, A.T.; Kodar, K.; Stocker, B.L.; Timmer, M.S.M. Amide-linked brartemicin glycolipids exhibit Mincle-mediated agonist activity in vitro. Carbohydr. Res. 2022, 511, 108461. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Morita, D.; Fujiwara, N.; Mori, D.; Nakamura, T.; Harashima, H.; Yamasaki, S.; Sugita, M. Glycerol Monomycolate Is a Novel Ligand for the Human, but Not Mouse Macrophage Inducible C-type Lectin, Mincle. J. Biol. Chem. 2014, 289, 15405–15412. [Google Scholar] [CrossRef]
- Yamasaki, S.; Ishikawa, E.; Sakuma, M.; Hara, H.; Ogata, K.; Saito, T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 2008, 9, 1179. [Google Scholar] [CrossRef]
- Patin, E.C.; Orr, S.J.; Schaible, U.E. Macrophage Inducible C-Type Lectin as a Multifunctional Player in Immunity. Front. Immunol. 2017, 8, 861. [Google Scholar] [CrossRef]
- Shenderov, K.; Barber, D.L.; Mayer-Barber, K.D.; Gurcha, S.S.; Jankovic, D.; Feng, C.G.; Oland, S.; Hieny, S.; Caspar, P.; Yamasaki, S.; et al. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. J. Immunol. 2013, 190, 5722–5730. [Google Scholar] [CrossRef]
- Khan, A.A.; Stocker, B.L.; Timmer, M.S. Trehalose glycolipids-synthesis and biological activities. Carbohydr. Res. 2012, 15, 25–36. [Google Scholar] [CrossRef]
- Furukawa, A.; Kamishikiryo, J.; Mori, D.; Toyonaga, K.; Okabe, Y.; Toji, A.; Kanda, R.; Miyake, Y.; Ose, T.; Yamasaki, S.; et al. Structural analysis for glycolipid recognition by the C-type lectins Mincle and MCL. Proc. Natl. Acad. Sci. USA 2013, 110, 17438–17443. [Google Scholar] [CrossRef]
- Feinberg, H.; Jégouzo, S.A.; Rowntree, T.J.; Guan, Y.; Brash, M.A.; Taylor, M.E.; Weis, W.I.; Drickamer, K. Mechanism for recognition of an unusual mycobacterial glycolipid by the macrophage receptor mincle. J. Biol. Chem. 2013, 288, 28457–28465. [Google Scholar] [CrossRef]
- Jégouzo, S.A.; Harding, E.C.; Acton, O.; Rex, M.J.; Fadden, A.J.; Taylor, M.E.; Drickamer, K. Defining the conformation of human Mincle that interacts with mycobacterial trehalose dimycolate. Glycobiology 2014, 24, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, H.; Rambaruth, N.D.S.; Jégouzo, S.A.; Jacobsen, K.M.; Djurhuus, R.; Poulsen, T.B.; Weis, W.I.; Taylor, M.E.; Drickamer, K. Binding sites for acylated trehalose analogs of glycolipid ligands on an extended carbohydrate recognition domain of the macrophage receptor Mincle. J. Biol. Chem. 2016, 291, 21222–21233. [Google Scholar] [CrossRef] [PubMed]
- Decout, A.; Silva-Gomes, S.; Drocourt, D.; Barbe, S.; André, I.; Cueto, F.J.; Lioux, T.; Sancho, D.; Pérouzel, E.; Vercellone, A.; et al. Rational design of adjuvants targeting the C-type lectin Mincle. Proc. Natl. Acad. Sci. USA 2017, 114, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Söldner, C.A.; Horn, A.H.C.; Sticht, H. Interaction of Glycolipids with the Macrophage Surface Receptor Mincle—A Systematic Molecular Dynamics Study. Sci. Rep. 2018, 8, 5374. [Google Scholar] [CrossRef] [PubMed]
- Riel, A.M.S.; Rungelrath, V.; Elwaie, T.A.; Rasheed, O.K.; Hicks, L.; Ettenger, G.; You, D.-C.; Smith, M.; Buhl, C.; Abdelwahab, W.; et al. Systematic Evaluation of Regiochemistry and Lipidation of Aryl Trehalose Mincle Agonists. Int. J. Mol. Sci. 2024, 25, 10031. [Google Scholar] [CrossRef]
- Kiyotake, R.; Oh-hora, M.; Ishikawa, E.; Miyamoto, T.; Ishibashi, T.; Yamasaki, S. Human Mincle binds to cholesterol crystals and triggers innate immune responses. J. Biol. Chem. 2015, 290, 25322–25332. [Google Scholar] [CrossRef]
- Kostarnoy, A.V.; Gancheva, P.G.; Lepenies, B.; Tukhvatulin, A.I.; Dzharullaeva, A.S.; Polyakov, N.B.; Grumov, D.A.; Egorova, D.A.; Kulibin, A.Y.; Bobrov, M.A.; et al. Receptor Mincle promotes skin allergies and is capable of recognizing cholesterol sulfate. Proc. Natl. Acad. Sci. USA 2017, 114, E2758–E2765. [Google Scholar] [CrossRef]
- Grebe, A.; Latz, E. Cholesterol crystals and inflammation. Curr. Rheumatol. Rep. 2013, 15, 313. [Google Scholar] [CrossRef]
- Lobato-Pascual, A.; Saether, P.C.; Fossum, S.; Dissen, E.; Daws, M.R. Mincle, the receptor for mycobacterial cord factor, forms a functional receptor complex with MCL and FceRI-g. Eur. J. Immunol. 2013, 43, 3167–3317. [Google Scholar] [CrossRef]
- Yamasaki, S. Signaling while eating: MCL is coupled with Mincle. Eur. J. Immunol. 2013, 43, 3156–3158. [Google Scholar] [CrossRef]
- Liu, Y.; Drickamer, K.; Taylor, M.E. Preformed Mincle dimers stabilized by an interchain disulfide bond in the neck region. Glycobiology 2024, cwae083, in press. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, Y. Innate immune receptor clustering and its role in immune regulation. J. Cell Sci. 2021, 134, jcs249318. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, H.S.; Reyes, C.N.; Becker, C.A.; Katsumoto, T.R.; Ma, J.; Wolf, A.J.; Bose, N.; Chan, A.S.H.; Magee, A.S.; Danielson, M.E.; et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature 2011, 472, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Vercellone, A.; Chimen, M.; Gaibelet, G.; Mazères, S.; Nigou, J.; Dufrêne, Y.F. Nanoscale clustering of mycobacterial ligands and DC-SIGN host receptors are key determinants for pathogen recognition. Sci. Adv. 2023, 9, eadf9498. [Google Scholar] [CrossRef]
- Manthrirathna, M.A.T.P.; Dangerfield, E.M.; Ishizuka, S.; Woods, A.; Luong, B.S.; Yamasaki, S.; Timmer, M.S.M.; Stocker, B.L. Water-soluble trehalose glycolipids show superior Mincle binding and signaling but impaired phagocytosis and IL-1β production. Front. Mol. Biosci. 2022, 9, 1015210. [Google Scholar] [CrossRef]
- Stocker, B.L.; Kodar, K.; Wahi, K.; Foster, A.J.; Harper, J.L.; Mori, D.; Yamasaki, S.; Timmer, M.S.M. The effects of trehalose glycolipid presentation on cytokine production by GM-CSF macrophages. Glycoconj. J. 2019, 36, 69–78. [Google Scholar] [CrossRef]
- Ishizuka, S.; van Dijk, J.H.M.; Kawakita, T.; Miyamoto, Y.; Maeda, Y.; Goto, M.; Le Calvez, G.; Groot, L.M.; Witte, M.D.; Minnaard, A.J.; et al. PGL-III, a rare intermediate of mycobacterium leprae phenolic glycolipid biosynthesis, is a potent mincle ligand. ACS Cent. Sci. 2023, 9, 1388–1399. [Google Scholar] [CrossRef]
- Abdelwahab, W.M.; Riffey, A.; Buhl, C.; Johnson, C.; Ryter, K.; Evans, J.T.; Burkhart, D.J. Co-adsorption of synthetic Mincle agonists and antigen to silica nanoparticles for enhanced vaccine activity: A formulation approach to co-delivery. Int. J. Pharm. 2021, 25, 120119. [Google Scholar] [CrossRef]
- Jones, G.D.; Riel, A.M.S.; Riffey, A.; Buhl, C. Combinatorial Administration of Synthetic Trehalose TLR4 Agonist INI-2002 and Novel Mincle Agonist UM-1098 Delivered via A-SNPs Result in Synergistic IL-1b Production in Human Primary Cells and Enhances TH1 and TH17 Responses In Vivo. Master’s Thesis, University of Montana, Missoula, MT, USA, 2022; p. 11994. Available online: https://scholarworks.umt.edu/etd/11994/ (accessed on 15 November 2024).
- Desel, C.; Werninghaus, K.; Ritter, M.; Jozefowski, K.; Wenzel, J.; Russkamp, N.; Schleicher, U.; Christensen, D.; Wirtz, S.; Kirschning, C.; et al. The Mincle-activating adjuvant TDB induces MyD88-dependent Th1 and Th17 responses through IL-1R signaling. PLoS ONE 2013, 8, e53531. [Google Scholar] [CrossRef]
- Foster, A.J.; Nagata, M.; Lu, X.; Lynch, A.T.; Omahdi, Z.; Ishikawa, E.; Yamasaki, S.; Timmer, M.S.M.; Stocker, B.L. Lipidated Brartemicin Analogues Are Potent Th1-Stimulating Vaccine Adjuvants. J. Med. Chem. 2018, 61, 1045–1060. [Google Scholar] [CrossRef]
- Dhume, K.; Finn, C.M.; Devarajan, P.; Singh, A.; Tejero, J.D.; Prokop, E.; Strutt, T.M.; Sell, S.; Swain, S.L.; McKinstry, K.K. Bona Fide Th17 Cells without Th1 Functional Plasticity Protect against Influenza. J. Immunol. 2022, 208, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Counoupas, C.; Ferrell, K.C.; Ashhurst, A.; Bhattacharyya, N.D.; Nagalingam, G.; Stewart, E.L.; Feng, C.G.; Petrovsky, N.; Britton, W.J.; Triccas, J.A. Mucosal delivery of a multistage subunit vaccine promotes development of lung-resident memory T cells and affords interleukin-17-dependent protection against pulmonary tuberculosis. NPJ Vaccines 2020, 5, 105. [Google Scholar] [CrossRef] [PubMed]
- Dijkman, K.; Sombroek, C.C.; Vervenne, R.A.W.; Hofman, S.O.; Boot, C.; Remarque, E.J.; Kocken, C.H.M.; Ottenhoff, T.H.M.; Kondova, I.; Khayum, M.A.; et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat. Med. 2019, 25, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.L.; Counoupas, C.; Quan, D.H.; Wang, T.; Petrovsky, N.; Britton, W.J.; Triccas, J.A. Lung IL-17A-Producing CD4+ T Cells Correlate with Protection after Intrapulmonary Vaccination with Differentially Adjuvanted Tuberculosis Vaccines. Vaccines 2024, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Wilk, M.M.; Misiak, A.; McManus, R.M.; Allen, A.C.; Lynch, M.A.; Mills, K.H.G. Lung CD4 Tissue-Resident Memory T Cells Mediate Adaptive Immunity Induced by Previous Infection of Mice with Bordetella pertussis. J. Immunol. 2017, 199, 233–243. [Google Scholar] [CrossRef]
- Kapil, P.; Merkel, T.J. Pertussis vaccines and protective immunity. Curr. Opin. Immunol. 2019, 59, 72–78. [Google Scholar] [CrossRef]
- Huangfu, L.; Li, R.; Huang, Y.; Wang, S. The IL-17 family in diseases: From bench to bedside. Signal Transduct. Target. Ther. 2023, 8, 402. [Google Scholar] [CrossRef]
- Yamasaki, S.; Matsumoto, M.; Takeuchi, O.; Matsuzawa, T.; Ishikawa, E.; Sakuma, M.; Tateno, H.; Uno, J.; Hirabayashi, J.; Mikami, Y.; et al. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc. Natl. Acad. Sci. USA 2009, 106, 1897–1902. [Google Scholar] [CrossRef]
- Wørzner, K.; Hvannastein, J.; Schmidt, S.T.; Foged, C.; Rosenkrands, I.; Pedersen, G.K.; Christensen, D. Adsorption of protein antigen to the cationic liposome adjuvant CAF01 is required for induction of Th1 and Th17 responses but not for antibody induction. Eur. J. Pharm. Biopharm. 2021, 165, 293–305. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; van der Most, R.; Lodaya, R.N.; Coccia, M.; Lofano, G. “World in motion”—Emulsion adjuvants rising to meet the pandemic challenges. NPJ Vaccines 2021, 6, 158. [Google Scholar] [CrossRef]
- Woodworth, J.S.; Christensen, D.; Cassidy, J.P.; Agger, E.M.; Mortensen, R.; Andersen, P. Mucosal boosting of H56:CAF01 immunization promotes lung-localized T cells and an accelerated pulmonary response to Mycobacterium tuberculosis infection without enhancing vaccine protection. Mucosal Immunol. 2019, 12, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Van Dis, E.; Sogi, K.M.; Rae, C.S.; Sivick, K.E.; Surh, N.H.; Leong, M.L.; Kanne, D.B.; Metchette, K.; Leong, J.J.; Bruml, J.R.; et al. STING-Activating Adjuvants Elicit a Th17 Immune Response and Protect against Mycobacterium tuberculosis Infection. Cell Rep. 2018, 23, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Orr, M.T.; Beebe, E.A.; Hudson, T.E.; Argilla, D.; Huang, P.W.; Reese, V.A.; Fox, C.B.; Reed, S.G.; Coler, R.N. Mucosal delivery switches the response to an adjuvanted tuberculosis vaccine from systemic TH1 to tissue-resident TH17 responses without impacting the protective efficacy. Vaccine 2015, 33, 6570–6578. [Google Scholar] [CrossRef] [PubMed]
- Carroll, E.C.; Jin, L.; Mori, A.; Muñoz-Wolf, N.; Oleszycka, E.; Moran, H.B.T.; Mansouri, S.; McEntee, C.P.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Oleszycka, E.; Sharp, F.A.; Coleman, M.; Ozasa, Y.; Singh, M.; O’Hagan, D.T.; Tajber, L.; Corrigan, O.I.; McNeela, E.A.; et al. The vaccine adjuvant alum inhibits IL-12 by promoting PI3 kinase signaling while chitosan does not inhibit IL-12 and enhances Th1 and Th17 responses. Eur. J. Immunol. 2012, 42, 2709–2719. [Google Scholar] [CrossRef]
- Woodworth, J.S.; Contreras, V.; Christensen, D.; Naninck, T.; Kahlaoui, N.; Gallouët, A.S.; Langlois, S.; Burban, E.; Joly, C.; Gros, W.; et al. MINCLE and TLR9 agonists synergize to induce Th1/Th17 vaccine memory and mucosal recall in mice and non-human primates. Nat. Commun. 2024, 15, 8959. [Google Scholar] [CrossRef]
- Enriquez, A.B.; Izzo, A.; Miller, S.M.; Stewart, E.L.; Mahon, R.N.; Frank, D.J.; Evans, J.T.; Rengarajan, J.; Triccas, J.A. Advancing Adjuvants for Mycobacterium tuberculosis Therapeutics. Front. Immunol. 2021, 12, 740117. [Google Scholar] [CrossRef]
- Burchill, L.; Williams, S.J. From the banal to the bizarre: Unravelling immune recognition and response to microbial lipids. Chem. Commun. 2022, 58, 925–940. [Google Scholar] [CrossRef]
- Rambaruth, N.; Jégouzo, S.; Marlor, H.; Taylor, M.; Drickamer, K. Mouse Mincle: Characterization as a Model for Human Mincle and Evolutionary Implications. Molecules 2015, 20, 6670–6682. [Google Scholar] [CrossRef]
- Vijayan, D.; Radford, K.J.; Beckhouse, A.G.; Ashman, R.B.; Wells, C.A. Mincle polarizes human monocyte and neutrophil responses to Candida albicans. Immunol. Cell Biol. 2012, 90, 889–895. [Google Scholar] [CrossRef]
- Ozeki, Y.; Tsutsui, H.; Kawada, N.; Suzuki, H.; Kataoka, M.; Kodama, T.; Yano, I.; Kaneda, K.; Kobayashi, K. Macrophage Scavenger Receptor Down-Regulates Mycobacterial Cord Factor-Induced Proinflammatory Cytokine Production by Alveolar and Hepatic Macrophages. Microb. Pathog. 2006, 40, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Kodar, K.; McConnell, M.J.; Harper, J.L.; Timmer, M.S.M.; Stocker, B.L. The Coadministration of Trehalose Dibehenate and Monosodium Urate Crystals Promotes an Antitumor Phenotype in Human-Derived Myeloid Cells. Immunol. Cell Biol. 2020, 98, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L.; Armitige, L.; Jagannath, C.; Actor, J.K. TB research at UT-Houston-a review of cord factor: New approaches to drugs, vaccines and the pathogenesis of tuberculosis. Tuberculosis 2009, 89, S18–S25. [Google Scholar] [CrossRef] [PubMed]
- Stocker, B.L.; Khan, A.A.; Chee, S.H.; Kamena, F.; Timmer, M.S.M. On one leg: Trehalose mono-esters activate macrophages in a Mincle-dependant Manner. ChemBiochem 2014, 15, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.; Kallerup, R.S.; Korsholm, K.S.; Franzyk, H.; Lepenies, B.; Christensen, D.; Foged, C.; Lang, R. Trehalose diester glycolipids are superior to the monoesters in binding to Mincle, activation of macrophages in vitro and adjuvant activity in vivo. Innate Immun. 2016, 22, 405–418. [Google Scholar] [CrossRef]
- Kodar, K.; Dangerfield, E.M.; Foster, A.J.; Forsythe, D.; Ishizuka, S.; McConnell, M.J.; Yamasaki, S.; Timmer, M.S.M.; Stocker, B.L. Aryl-functionalised α,α′-Trehalose 6,6′-Glycolipid Induces Mincle-independent Pyroptotic Cell Death. Inflammation 2023, 46, 1365–1380. [Google Scholar] [CrossRef]
- Stocker, B.L.; Dangerfield, E.M.; Gupta, S.K.; Parlane, N.A.; Foster, A.J.; Wedlock, D.N.; Timmer, M.S.M. Lipidated brartemicin adjuvant p-C18Brar is a promising α,α′-trehalose 6,6′-dilipid for use in ovine pneumonia vaccines. Pure Appl. Chem. 2023, 95, 979–990. [Google Scholar] [CrossRef]
- Martin-Bertelsen, B.; Korsholm, K.S.; Rose, F.; Nordly, P.; Franzyk, H.; Andersen, P.; Agger, E.M.; Christensen, D.; Yaghmur, A.; Foged, C. The supramolecular structure is decisive for the immunostimulatory properties of synthetic analogues of a mycobacterial lipid in vitro. RSC Adv. 2013, 3, 20673–20683. [Google Scholar] [CrossRef]
- Martin-Bertelsen, B.; Korsholm, K.S.; Roces, C.B.; Nielsen, M.H.; Christensen, D.; Franzyk, H.; Yaghmur, A.; Foged, C. Nano-Self-Assemblies Based on Synthetic Analogues of Mycobacterial Monomycoloyl Glycerol and DDA: Supramolecular Structure and Adjuvant Efficacy. Mol. Pharm. 2016, 13, 2771–2781. [Google Scholar] [CrossRef]
- Rasheed, O.K.; Buhl, C.; Evans, J.T.; Ryter, K.T. Design of Trehalose-Based Amide/Sulfonamide C-type Lectin Receptor Signaling Compounds. ChemMedChem 2021, 16, 1246–1251. [Google Scholar] [CrossRef]
- Parant, M.; Audibert, F.; Parant, F.; Chedid, L.; Soler, E.; Polonsky, J.; Lederer, E. Nonspecific immunostimulant activities of synthetic trehalose-6,6′-diesters (lower homologs of cord factor). Infect. Immun. 1978, 20, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Yarkoni, E.; Rapp, H.J.; Polonsky, J.; Lederer, E. Immunotherapy with an intralesionally administered synthetic cord factor analogue. Int. J. Cancer 1978, 22, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Davidsen, J.; Rosenkrands, I.; Christensen, D.; Vangala, A.; Kirby, D.; Perrie, Y.; Agger, E.M.; Andersen, P. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)—A novel adjuvant inducing both strong CMI and antibody responses. Biochim. Biophys. Acta 2005, 1718, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Chee, S.H.; McLaughlin, R.J.; Harper, J.L.; Kamena, F.; Timmer, M.S.M.; Stocker, B.L. Long-chain lipids are required for the innate immune recognition of trehalose diesters by macrophages. ChemBioChem 2011, 12, 2572–2576. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Kodar, K.; Timmer, M.S.M.; Stocker, B.L. Lipid length and iso-branching of trehalose diesters influences Mincle agonist activity. Tetrahedron 2018, 74, 1269–1277. [Google Scholar] [CrossRef]
- Lynch, A.T.; Motozono, C.; Foster, A.J.; Kodar, K.; Dangerfied, E.M.; Yamasaki, S.; Wedlock, D.N.; Timmer, M.S.M.; Stocker, B.L. Trehalose diamide glycolipids augment antigen-specific antibody responses in a Mincle-dependent manner. Bioorg. Chem. 2021, 110, 104747. [Google Scholar] [CrossRef]
- Kallerup, R.S.; Madsen, C.M.; Schiøth, M.L.; Franzyk, H.; Rose, F.; Christensen, D.; Korsholm, K.S.; Foged, C. Influence of trehalose 6,6′-diester (TDX) chain length on the physicochemical and immunopotentiating properties of DDA/TDX liposomes. Eur. J Pharm. Biopharm. 2015, 90, 80–89. [Google Scholar] [CrossRef]
- Bae, S.-H.; Yoo, S.; Lee, J.; Park, H.-J.; Kwon, S.P.; Jin, H.; Park, S.-I.; Lee, Y.-S.; Bang, Y.-J.; Roh, G.; et al. A lipid nanoparticle platform incorporating trehalose glycolipid for exceptional mRNA vaccine safety. Bioact. Mater. 2024, 14, 486–498. [Google Scholar] [CrossRef]
- Barry, C.E., III; Lee, R.E.; Mdluli, K.; Sampson, A.E.; Schroeder, B.G.; Slayden, R.A.; Yuan, Y. Mycolic acids: Structure, biosynthesis and physiological functions. Prog. Lipid Res. 1998, 37, 143–179. [Google Scholar] [CrossRef]
- Tima, H.G.; Al Dulayymi, J.R.; Denis, O.; Lehebel, P.; Baols, K.S.; Mohammed, M.O.; L’Homme, L.; Sahb, M.M.; Potemberg, G.; Legrand, S.; et al. Inflammatory Properties and Adjuvant Potential of Synthetic Glycolipids Homologous to Mycolate Esters of the Cell Wall of Mycobacterium tuberculosis. J. Innate Immun. 2017, 9, 162–180. [Google Scholar] [CrossRef]
- van der Peet, P.L.; Gunawan, C.; Torigoe, S.; Yamasaki, S.; Williams, S.J. Corynomycolic acid-containing glycolipids signal through the pattern recognition receptor Mincle. Chem. Commun. 2015, 51, 5100–5103. [Google Scholar] [CrossRef] [PubMed]
- Bird, J.H.; Khan, A.A.; Nishimura, N.; Yamasaki, S.; Timmer, M.S.M.; Stocker, B.L. Synthesis of Branched Trehalose Glycolipids and their Mincle agonist activity. J. Org. Chem. 2018, 83, 7593–7605. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Yamamoto, H.; Kawakami, T. Maintenance of homeostasis by TLR4 ligands. Front. Immunol. 2024, 15, 1286270. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Lian, Q.; Li, W.; Chen, L.; Zhang, R.; Yang, D.; Gao, L.; Qi, X.; Liu, Z.; Liao, G. Fully synthetic Mincle-dependent self-adjuvanting cancer vaccines elicit robust humoral and T cell-dependent immune responses and protect mice from tumor development. Chem. Sci. 2021, 12, 15998–16013. [Google Scholar] [CrossRef] [PubMed]
- Manthrirathna, M.A.T.P.; Kodar, K.; Ishizuka, S.; Dangerfield, E.M.; Xiuyuan, L.; Yamasaki, S.; Stocker, B.L.; Timmer, M.S.M. 6-C-Linked trehalose glycolipids signal through Mincle and exhibit potent adjuvant activity. Bioorg. Chem. 2023, 133, 106345. [Google Scholar] [CrossRef]
- Jacobsen, K.M.; Keiding, U.; Clement, L.L.; Schaffert, E.S.; Rambaruth, N.D.S.; Johannsen, M.; Drickamer, K.; Poulsen, T.B. The natural product brartemicin is a high affinity ligand for the carbohydrate-recognition domain of the macrophage receptor Mincle. MedChemComm 2015, 6, 647–652. [Google Scholar] [CrossRef]
- Foster, A.J.; Kodar, K.; Timmer, M.S.M.; Stocker, B.L. Ortho-substituted lipidated Brartemicin derivative shows promising Mincle-mediated adjuvant activity. Org. Biomol. Chem. 2020, 18, 1095–1103. [Google Scholar] [CrossRef]
- Ryter, K.T.; Ettenger, G.; Rasheed, O.K.; Buhl, C.; Child, R.; Miller, S.M.; Holley, D.; Smith, A.J.; Evans, J.T. Aryl Trehalose Derivatives as Vaccine Adjuvants for Mycobacterium tuberculosis. J. Med. Chem. 2020, 63, 309–320. [Google Scholar] [CrossRef]
- Rasheed, O.K.; Ettenger, G.; Buhl, C.; Child, R.; Miller, S.M.; Evans, J.T.; Ryter, K.T. 6,6′-Aryl trehalose analogs as potential Mincle ligands. Bioorg. Med. Chem. 2020, 28, 115564. [Google Scholar] [CrossRef]
- Abdelwahab, W.M.; Le-Vinh, B.; Riffey, A.; Hicks, L.; Buhl, C.; Ettenger, G.; Jackson, K.J.; Weiss, A.M.; Miller, S.; Ryter, K.; et al. Promotion of Th17 Polarized Immunity via Co-Delivery of Mincle Agonist and Tuberculosis Antigen Using Silica Nanoparticles. ACS Appl. Bio Mater. 2024, 7, 3877–3889. [Google Scholar] [CrossRef]
- Brennan, P.J.; Lehane, D.P.; Thomas, D.W. Acylglucoses of the corynebacteria and mycobacteria. Eur. J. Biochem. 1970, 13, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Moody, D.B.; Reinhold, B.B.; Guy, M.R.; Beckman, E.M.; Frederique, D.E.; Furlong, S.T.; Ye, S.; Reinhold, V.N.; Sieling, P.A.; Modlin, R.L.; et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science 1997, 278, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Sahb, M.M.; Al Dulayymi, J.R.; Baird, M.S. Glucose monomycolates based on single synthetic mycolic acids. Chem. Phys. Lipids 2015, 190, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.O.; Baird, M.S.; Al Dulayymi, J.R.; Jones, A.; Gwenin, C.D. Arabino mycolates from synthetic mycolic acids. Tetrahedron 2016, 72, 2849–2857. [Google Scholar] [CrossRef]
- Andersen, C.S.; Agger, E.M.; Rosenkrands, I.; Gomes, J.M.; Bhowruth, V.; Gibson, K.J.C.; Petersen, R.V.; Minnikin, D.E.; Besra, G.S.; Andersen, P. A Simple Mycobacterial Monomycolated Glycerol Lipid Has Potent Immunostimulatory Activity. J. Immunol. 2009, 182, 424–432. [Google Scholar] [CrossRef]
- Bhowruth, V.; Minnikin, D.E.; Agger, E.M.; Andersen, P.; Bramwell, V.W.; Perrie, Y.; Besra, G.S. Adjuvant properties of a simplified C32 monomycolyl glycerol analogue. Bioorg. Med. Chem. Lett. 2009, 19, 2029–2032. [Google Scholar] [CrossRef]
- Ali, O.T.; Sahb, M.M.; Al Dulayymi, J.R.; Baird, M.S. Glycerol mycolates from synthetic mycolic acids. Carbohydr. Res. 2017, 448, 67–73. [Google Scholar] [CrossRef]
- Pedersen, G.K.; Andersen, P.; Christensen, D. Immunocorrelates of CAF family adjuvants. Semin. Immunol. 2018, 39, 4–13. [Google Scholar] [CrossRef]
- Oka, S.; Watanabe, M.; Ito, E.; Takeyama, A.; Matsuoka, T.; Arichi, N.; Ohno, H.; Yamasaki, S.; Inuki, S. Archaeal Glycerolipids Are Recognized by C-type Lectin Receptor Mincle. J. Am. Chem. Soc. 2023, 145, 18538–18548. [Google Scholar] [CrossRef]
- Nagata, M.; Izumi, Y.; Ishikawa, E.; Kiyotake, R.; Doi, R.; Iwai, S.; Omahdi, Z.; Yamaji, T.; Miyamoto, T.; Bamba, T.; et al. Intracellular metabolite β-glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity. Proc. Natl. Acad. Sci. USA 2017, 114, E3285–E3294. [Google Scholar] [CrossRef]
- van der Peet, P.L.; Gunawn, C.; Wantanabe, M.; Yamaski, S. Synthetic b-1,2-mannosyl Synthetic β-1,2-mannosyloxymannitol glycolipid from the Fungus Malassezia pachydermatis signals through human Mincle. J. Org. Chem. 2019, 84, 6788–6797. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Nagata, M.; Yamasaki, S.; Williams, S.J. Total synthesis of a cyclopropane-fatty acid α-glucosyl diglyceride from Lactobacillus plantarum and identification of its ability to signal through Mincle. Chem. Commun. 2016, 52, 10902–10905. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Itoh, F.; Yoshida, S.; Saijo, S.; Matsuzawa, T.; Gonoi, T.; Saito, T.; Okawa, Y.; Shibata, N.; Miyamoto, T.; et al. Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia. Cell Host Microbe 2013, 13, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Hollwedel, F.; Maus, U.A.; Stocker, B.L.; Timmer, M.S.M. Synthesis of α-Glucosyl Diacylglycerides as potential adjuvants for Streptococcus pneumoniae vaccines. Carbohydr. Res. 2020, 489, 107951. [Google Scholar] [CrossRef] [PubMed]
- Behler-Janbeck, F.; Takano, T.; Maus, R.; Stolper, J.; Jonigk, D.; Tort Tarrés, M.; Fuehner, T.; Prasse, A.; Welte, T.; Timmer, M.S.M.; et al. C-type lectin Mincle recognizes glucosyl-diacylglycerol of Streptococcus pneumoniae and plays a protective role in pneumococcal pneumonia. PLoS Pathog. 2016, 12, e1006038. [Google Scholar] [CrossRef]
- Reinink, P.; Buter, J.; Mishra, V.K.; Ishikawa, E.; Cheng, T.Y.; Willemsen, P.T.J.; Porwollik, S.; Brennan, P.J.; Heinz, E.; Mayfield, J.A.; et al. Discovery of Salmonella trehalose phospholipids reveals functional convergence with mycobacteria. J. Exp. Med. 2019, 216, 757–771. [Google Scholar] [CrossRef]
- Mishra, V.K.; Buter, J.; Blevins, M.S.; Witte, M.D.; van Rhijn, I.; Moody, D.B.; Brodbelt, J.S.; Minnaard, A.J. Total synthesis of an immunogenic trehalose phospholipid from Salmonella Typhi and elucidation of its sn-regiochemistry by mass spectrometry. Org. Lett. 2019, 21, 5126–5131. [Google Scholar] [CrossRef]
- Holzheimer, M.; Reijneveld, J.F.; Ramnarine, A.K.; Misiakos, G.; Young, D.C.; Ishikawa, E.; Cheng, T.Y.; Yamasaki, S.; Moody, D.B.; Van Rhijn, I.; et al. Asymmetric Total Synthesis of Mycobacterial Diacyl Trehaloses Demonstrates a Role for Lipid Structure in Immunogenicity. ACS Chem. Biol. 2020, 15, 1835–1841. [Google Scholar] [CrossRef]
- Reijneveld, J.F.; Holzheimer, M.; Young, D.C.; Lopez, K.; Suliman, S.; Jimenez, J.; Calderon, R.; Lecca, L.; Murray, M.B.; Ishikawa, E.; et al. Synthetic mycobacterial diacyl trehaloses reveal differential recognition by human T cell receptors and the C-type lectin Mincle. Sci. Rep. 2021, 11, 2010. [Google Scholar] [CrossRef]
- Rasheed, O.K.; Buhl, C.; Evan, J.T.; Holley, D.; Ryter, K.T. Synthesis and biological evaluation of trehalose-based bi-aryl derivatives as C-type lectins. Tetrahedron 2023, 132, 133241. [Google Scholar] [CrossRef]
- Ikazaki, T.; Ishikawa, E.; Tamashima, H.; Akiyama, H.; Kimuro, Y.; Yoritate, M.; Matoba, H.; Imamura, A.; Ishida, H.; Yamasaki, S.; et al. Ligand-Controlled Stereoselective Synthesis and Biological Activity of 2-Exomethylene Pseudo-glycoconjugates: Discovery of Mincle-Selective Ligands. Angew. Chem. Int. Ed. Engl. 2023, 62, e202302569. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.J.; Dangerfield, E.M.; Timmer, M.S.M.; Stocker, B.L.; Wilkinson, B.L. Probing Isosteric Replacement for Immunoadjuvant Design: Bis-Aryl Triazole Trehalolipids are Mincle Agonists. ACS Med. Chem. Lett. 2024, 15, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Toyonaga, K.; Ishikawa, E.; Haji, S.; Okahashi, N.; Takahashi, M.; Izumi, Y.; Imamura, A.; Takato, K.; Ishida, H.; et al. Helicobacter pylori metabolites exacerbate gastritis through C-type lectin receptors. J. Exp. Med. 2021, 218, e20200815. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.G.M.; Ito, E.; Yamasaki, S.; Williams, S.J. Cholesteryl 6-O-acyl-α-glucosides from diverse Helicobacter spp. signal through the C-type lectin receptor Mincle. Org. Biomol. Chem. 2020, 18, 7907–7915. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.G.M.; Hosono, Y.; Nagata, M.; Yamasaki, S.; Williams, S.J. Design of potent Mincle signalling agonists based on an alkyl β-glucoside template. Chem. Commun. 2020, 56, 4292–4295. [Google Scholar] [CrossRef] [PubMed]
- Timmer, M.S.M.; Teunissen, T.J.; Kodar, K.; Foster, A.J.; Yamasaki, S.; Stocker, B.L. Cholesteryl glucosides signal through the carbohydrate recognition domain of the macrophage inducible C-type lectin (mincle). Org. Biomol. Chem. 2021, 19, 2198–2202. [Google Scholar] [CrossRef]
- Rajendran, L.; Knölker, H.-J.; Simons, K. Subcellular targeting strategies for drug design and delivery. Nat. Rev. Drug Discov. 2010, 9, 29–42. [Google Scholar] [CrossRef]
- Gupta, S.K.; Parlane, N.; Bridgeman, B.; Lynch, A.T.; Dangerfield, E.M.; Timmer, M.S.M.; Stocker, B.L.; Wedlock, D.N. The trehalose glycolipid C18Brar promotes antibody and T-cell immune responses to Mannheimia haemolytica and Mycoplasma ovipneumoniae whole cell antigens in sheep. PLoS ONE 2023, 18, e0278853. [Google Scholar] [CrossRef]
- Agger, E.M.; Rosenkrands, I.; Hansen, J.; Brahimi, K.; Vandahl, B.S.; Aagaard, C.; Werninghaus, K.; Kirschning, C.; Lang, R.; Christensen, D.; et al. Cationic Liposomes Formulated with Synthetic Mycobacterial Cordfactor (CAF01): A Versatile Adjuvant for Vaccines with Different Immunological Requirements. PLoS ONE 2008, 3, e3116. [Google Scholar] [CrossRef]
- Kirby, D.J.; Rosenkrands, I.; Agger, E.M.; Andersen, P.; Coombes, A.G.; Perrie, Y. Liposomes act as stronger sub-unit vaccine adjuvants when compared to microspheres. J. Drug Target. 2008, 16, 543–554. [Google Scholar] [CrossRef]
- Schmidt, S.T.; Neustrup, M.A.; Harloff-Helleberg, S.; Korsholm, K.S.; Rades, T.; Andersen, P.; Christensen, D.; Foged, C. Systematic Investigation of the Role of Surfactant Composition and Choice of oil: Design of a Nanoemulsion-Based Adjuvant Inducing Concomitant Humoral and CD4+ T-Cell Responses. Pharm. Res. 2017, 34, 1716–1727. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.; Juel, H.B.; Bang, P.; Cheeseman, H.M.; Dohn, R.B.; Cole, T.; Kristiansen, M.P.; Korsholm, K.S.; Lewis, D.; Olsen, A.W.; et al. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: A first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2019, 19, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Dejon-Agobe, J.C.; Ateba-Ngoa, U.; Lalremruata, A.; Homoet, A.; Engelhorn, J.; Nouatin, O.P.; Edoa, J.R.; Fernandes, J.F.; Esen, M.; Mouwenda, Y.D.; et al. Controlled Human Malaria Infection of Healthy Adults With Lifelong Malaria Exposure to Assess Safety, Immunogenicity, and Efficacy of the Asexual Blood Stage Malaria Vaccine Candidate GMZ2. Clin. Infect. Dis. 2019, 69, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, I.; Brandt, L.; Vinner, L.; Kromann, I.; Andreasen, L.V.; Andersen, P.; Gerstoft, J.; Kronborg, G.; Fomsgaard, A. Adjuvanted HLA-supertype restricted subdominant peptides induce new T-cell immunity during untreated HIV-1-infection. Clin. Immunol. 2013, 146, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Kloverpris, H.; Karlsson, I.; Bonde, J.; Thorn, M.; Vinner, L.; Pedersen, A.E.; Hentze, J.L.; Andresen, B.S.; Svane, I.M.; Gerstoft, J.; et al. Induction of novel CD8+ T-cell responses during chronic untreated HIV-1 infection by immunization with subdominant cytotoxic T-lymphocyte epitopes. AIDS 2009, 23, 1329–1340. [Google Scholar] [CrossRef]
- Ottenhoff, T.H.M.; Doherty, T.M.; van Dissel, J.T.; Bang, P.; Lingnau, K.; Kromann, I.; Andersen, P. First in humans: A new molecularly defined vaccine shows excellent safety and strong induction of long-lived Mycobacterium tuberculosis -specific Th1-cell like responses. Hum. Vaccines 2010, 6, 1007–1015. [Google Scholar] [CrossRef]
- Pollock, K.M.; Borges, Á.H.; Cheeseman, H.M.; Rosenkrands, I.; Schmidt, K.L.; Søndergaard, R.E.; Day, S.; Evans, A.; McFarlane, L.R.; Joypooranachandran, J.; et al. An investigation of trachoma vaccine regimens by the chlamydia vaccine CTH522 administered with cationic liposomes in healthy adults (CHLM-02): A phase 1, double-blind trial. Lancet Infect. Dis. 2024, 24, 829–844. [Google Scholar] [CrossRef]
- Román, V.R.; Jensen, K.J.; Jensen, S.S.; Leo-Hansen, C.; Jespersen, S.; da Silva Té, D.; Rodrigues, C.M.; Janitzek, C.M.; Vinner, L.; Katzenstein, T.L.; et al. Therapeutic vaccination using cationic liposome-adjuvanted HIV type 1 peptides representing HLA-supertype-restricted subdominant T cell epitopes: Safety, immunogenicity, and feasibility in Guinea-Bissau. AIDS Res. Hum. Retroviruses 2013, 29, 1504–1512. [Google Scholar] [CrossRef]
- Feather, L.A.J.; Nadella, V.; Kastner, E.; Perrie, Y.; Hilton, A.C.; Devitt, A. Development of a rapid in vitro pre-screen for distinguishing effective liposome-adjuvant delivery systems. Sci. Rep. 2022, 12, 12448. [Google Scholar] [CrossRef]
- Lemaire, G.; Tenu, J.-P.; Petit, J.-F.; Lederer, E. Natural and synthetic trehalose diesters as immunomodulators. Med. Res. Rev. 1986, 6, 243–274. [Google Scholar] [CrossRef]
- Shiga, M.; Miyazaki, J.; Tanuma, K.; Nagumo, Y.; Yoshino, T.; Kandori, S.; Negoro, H.; Kojima, T.; Tanaka, R.; Okiyama, N.; et al. The liposome of trehalose dimycolate extracted from M. bovis BCG induces antitumor immunity via the activation of dendritic cells and CD8+ T cells. Cancer Immunol. Immunother. 2021, 70, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.B.; Geary, S.M.; Salem, A.K. Biodegradable particles as vaccine antigen delivery systems for stimulating cellular immune responses. Hum. Vaccines Immunother. 2013, 9, 2584–2590. [Google Scholar] [CrossRef] [PubMed]
- Rose, F.; Wern, J.E.; Ingvarsson, P.T.; van de Weert, M.; Andersen, P.; Follmann, F.; Foged, C. Engineering of a novel adjuvant based on lipid-polymer hybrid nanoparticles: A quality-by-design approach. J. Control. Release 2015, 210, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Garcia-Contreras, L.; Muttil, P.; Padilla, D.; Xu, D.; Liu, J.; Braunstein, M.; McMurray, D.N.; Hickey, A.J. Pulmonary Immunization Using Antigen 85-B Polymeric Microparticles to Boost Tuberculosis Immunity. AAPS J. 2010, 12, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Ashhurst, A.S.; Parumasivam, T.; Chan, J.G.Y.; Lin, L.C.W.; Flórido, M.; West, N.P.; Chan, H.-K.; Britton, W.J. PLGA particulate subunit tuberculosis vaccines promote humoral and Th17 responses but do not enhance control of Mycobacterium tuberculosis infection. PLoS ONE 2018, 13, e0194620. [Google Scholar] [CrossRef] [PubMed]
- Rungelrath, V.; Ahmed, M.; Hicks, L.; Miller, S.M.; Ryter, K.T.; Montgomery, K.; Ettenger, G.; Riffey, A.; Abdelwahab, W.M.; Khader, S.A.; et al. Vaccination with Mincle agonist UM-1098 and mycobacterial antigens induces protective Th1 and Th17 responses. NPJ Vaccines 2024, 9, 100. [Google Scholar] [CrossRef]
- Nordly, P.; Korsholm, K.S.; Pedersen, E.A.; Khilji, T.S.; Franzyk, H.; Jorgensen, L.; Nielsen, H.M.; Agger, E.M.; Foged, C. Incorporation of a synthetic mycobacterial monomycoloyl glycerol analogue stabilizes dimethyldioctadecylammonium liposomes and potentiates their adjuvant effect in vivo. Eur. J. Pharm. Biopharm. 2011, 77, 89–98. [Google Scholar] [CrossRef]
- Knudsen, N.P.H.; Olsen, A.; Buonsanti, C.; Follmann, F.; Zhang, Y.; Coler, R.N.; Fox, C.B.; Meinke, A.; D’Oro, U.; Casini, D.; et al. Different human vaccine adjuvants promote distinct antigen-independent immunological signatures tailored to different pathogens. Sci. Rep. 2016, 6, 19570. [Google Scholar] [CrossRef]
- Blakney, A.K.; McKay, P.F.; Christensen, D.; Yus, B.I.; Aldon, Y.; Follmann, F.; Shattock, R.J. Effects of cationic adjuvant formulation particle type, fluidity and immunomodulators on delivery and immunogenicity of saRNA. J. Control. Release 2019, 304, 65–74. [Google Scholar] [CrossRef]
- Desel, C.; Murray, P.J.; Lehmann, C.H.K.; Heger, L.; Christensen, D.; Andersen, P.; Mack, M.; Dudziak, D.; Lang, R. Monocytes Elicit a Neutrophil-Independent Th1/Th17 Response Upon Immunization With a Mincle-Dependent Glycolipid Adjuvant. Front. Immunol. 2022, 13, 880474. [Google Scholar] [CrossRef]
- Schmidt, S.T.; Khadke, S.; Korsholm, K.S.; Perrie, Y.; Rades, T.; Andersen, P.; Foged, C.; Christensen, D. The administration route is decisive for the ability of the vaccine adjuvant CAF09 to induce antigen-specific CD8 + T-cell responses: The immunological consequences of the biodistribution profile. J. Control. Release 2016, 239, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.; Lindenstrøm, T.; van de Wijdeven, G.; Andersen, P.; Agger, E.M. Syringe Free Vaccination with CAF01 Adjuvated Ag85B-ESAT-6 in Bioneedles Provides Strong and Prolonged Protection Against Tuberculosis. PLoS ONE 2010, 5, e15043. [Google Scholar] [CrossRef] [PubMed]
- Van Dissel, J.T.; Joosten, S.A.; Hoff, S.T.; Soonawala, D.; Prins, C.; Hokey, D.A.; O’Dee, D.M.; Graves, A.; Thierry-Carstensen, B.; Andreasen, L.V.; et al. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine 2014, 32, 7098–7107. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Oliveira, L.V.N.; Hester, M.M.; Carlson, D.; Christensen, D.; Specht, C.A.; Levitz, S.M. Protection against experimental cryptococcosis elicited by Cationic Adjuvant Formulation 01-adjuvanted subunit vaccines. PLoS Pathog. 2024, 20, e1012220. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.M.; Mosquini, M.; Covre, L.P.; Stagmiller, N.P.; Rodrigues, R.R.; Christensen, D.; de Matos Guedes, H.L.; Rossi-Bergmann, B.; Gomes, D.C. Intranasal vaccination with killed Leishmania amazonensis promastigotes antigen (LaAg) associated with CAF01 adjuvant induces partial protection in BALB/c mice challenged with Leishmania (infantum) chagasi. Parasitology 2015, 142, 1640–1646. [Google Scholar] [CrossRef]
- Wørzner, K.; Sheward, D.J.; Schmidt, S.T.; Hanke, L.; Zimmermann, J.; McInerney, G.; Karlsson Hedestam, G.B.; Murrell, B.; Christensen, D.; Pedersen, G.K. Adjuvanted SARS-CoV-2 spike protein elicits neutralizing antibodies and CD4 T cell responses after a single immunization in mice. eBioMedicine 2021, 63, 103197. [Google Scholar] [CrossRef]
- Araújo, M.V.; Santos Júnior, S.R.D.; Nosanchuk, J.D.; Taborda, C.P. Therapeutic Vaccination with Cationic Liposomes Formulated with Dioctadecyldimethylammonium and Trehalose Dibehenate (CAF01) and Peptide P10 Is Protective in Mice Infected with Paracoccidioides brasiliensis. J. Fungi 2020, 6, 347. [Google Scholar] [CrossRef]
- Tomás-Cortázar, J.; Quinn, C.; Corcoran, N.; Blanco, A.; Christensen, D.; McClean, S. BpOmpW antigen administered with CAF01 adjuvant stimulates comparable T cell responses to Sigma adjuvant system. Vaccine X 2024, 17, 100438. [Google Scholar] [CrossRef]
- Sisteré-Oró, M.; Pedersen, G.K.; Córdoba, L.; López-Serrano, S.; Christensen, D.; Darji, A. Influenza NG-34 T cell conserved epitope adjuvanted with CAF01 as a possible influenza vaccine candidate. Vet. Res. 2020, 51, 57. [Google Scholar] [CrossRef]
- López-Serrano, S.; Cordoba, L.; Pérez-Maillo, M.; Pleguezuelos, P.; Remarque, E.J.; Ebensen, T.; Guzmán, C.A.; Christensen, D.; Segalés, J.; Darji, A. Immune Responses to Pandemic H1N1 Influenza Virus Infection in Pigs Vaccinated with a Conserved Hemagglutinin HA1 Peptide Adjuvanted with CAF®01 or CDA/αGalCerMPEG. Vaccines 2021, 9, 751. [Google Scholar] [CrossRef]
- López-Serrano, S.; Mahmmod, Y.S.; Christensen, D.; Ebensen, T.; Guzmán, C.A.; Rodríguez, F.; Segalés, J.; Aragón, V. Immune responses following neonatal vaccination with conserved F4 fragment of VtaA proteins from virulent Glaesserella parasuis adjuvanted with CAF®01 or CDA. Vaccine X 2023, 14, 100330. [Google Scholar] [CrossRef] [PubMed]
- Okoth, W.A.; Ho, M.-F.; Zaman, M.; Cooper, E.; Som, P.; Burgess, M.; Walton, M.; Nevagi, R.J.; Beattie, L.; Murphy, D.; et al. A CAF01-adjuvanted whole asexual blood-stage liposomal malaria vaccine induces a CD4+ T-cell-dependent strain-transcending protective immunity in rodent models. mBio 2023, 14, e0254723. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.; Andreasen, L.V.; Andersen, P.; Agger, E.M. Inducing Dose Sparing with Inactivated Polio Virus Formulated in Adjuvant CAF01. PLoS ONE 2014, 9, e100879. [Google Scholar] [CrossRef] [PubMed]
- Martel, C.J.-M.; Agger, E.M.; Poulsen, J.J.; Hammer Jensen, T.; Andresen, L.; Christensen, D.; Nielsen, L.P.; Blixenkrone-Møller, M.; Andersen, P.; Aasted, B. CAF01 Potentiates Immune Responses and Efficacy of an Inactivated Influenza Vaccine in Ferrets. PLoS ONE 2011, 6, e22891. [Google Scholar] [CrossRef]
- Bhide, Y.; Dong, W.; Gribonika, I.; Voshart, D.; Meijerhof, T.; de Vries-Idema, J.; Norley, S.; Guilfoyle, K.; Skeldon, S.; Engelhardt, O.G.; et al. Cross-Protective Potential and Protection-Relevant Immune Mechanisms of Whole Inactivated Influenza Virus Vaccines Are Determined by Adjuvants and Route of Immunization. Front. Immunol. 2019, 10, 646. [Google Scholar] [CrossRef]
- Larrouy-Maumus, G.; Layre, E.; Clark, S.; Prandi, J.; Rayner, E.; Lepore, M.; de Libero, G.; Williams, A.; Puzo, G.; Gilleron, M. Protective efficacy of a lipid antigen vaccine in a guinea pig model of tuberculosis. Vaccine 2017, 35, 1395–1402. [Google Scholar] [CrossRef]
- Ciabattini, A.; Pettini, E.; Fiorino, F.; Lucchesi, S.; Pastore, G.; Brunetti, J.; Santoro, F.; Andersen, P.; Bracci, L.; Pozzi, G.; et al. Heterologous Prime-Boost Combinations Highlight the Crucial Role of Adjuvant in Priming the Immune System. Front. Immunol. 2018, 9, 380. [Google Scholar] [CrossRef]
- Ingvarsson, P.T.; Rasmussen, I.S.; Viaene, M.; Irlik, P.J.; Nielsen, H.M.; Foged, C. The surface charge of liposomal adjuvants is decisive for their interactions with the Calu-3 and A549 airway epithelial cell culture models. Eur. J. Pharm. Biopharm. 2014, 87, 480–488. [Google Scholar] [CrossRef]
- Müllertz, O.A.O.; Andersen, P.; Christensen, D.; Foged, C.; Thakur, A. Pulmonary Administration of the Liposome-Based Adjuvant CAF01: Effect of Surface Charge on Mucosal Adjuvant Function. Mol. Pharm. 2023, 20, 953–970. [Google Scholar] [CrossRef]
- Qu, W.; Li, N.; Yu, R.; Zuo, W.; Fu, T.; Fei, W.; Hou, Y.; Liu, Y.; Yang, J. Cationic DDA/TDB liposome as a mucosal vaccine adjuvant for uptake by dendritic cells in vitro induces potent humoural immunity. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 852–860. [Google Scholar] [CrossRef]
- Christensen, D.; Mortensen, R.; Rosenkrands, I.; Dietrich, J.; Andersen, P. Vaccine-induced Th17 cells are established as resident memory cells in the lung and promote local IgA responses. Mucosal Immunol. 2017, 10, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Pinto, F.E.; Hansen, H.S.; Andersen, P.; Christensen, D.; Janfelt, C.; Foged, C. Intrapulmonary (i.pulmon.) Pull Immunization With the Tuberculosis Subunit Vaccine Candidate H56/CAF01 After Intramuscular (i.m.) Priming Elicits a Distinct Innate Myeloid Response and Activation of Antigen-Presenting Cells Than i.m. or i.pulmon. Prime Immunization Alone. Front. Immunol. 2020, 11, 803. [Google Scholar] [CrossRef]
- Olsen, A.W.; Rosenkrands, I.; Jacobsen, C.S.; Cheeseman, H.M.; Kristiansen, M.P.; Dietrich, J.; Shattock, R.J.; Follmann, F. Immune signature of Chlamydia vaccine CTH522/CAF®01 translates from mouse-to-human and induces durable protection in mice. Nat. Commun. 2024, 15, 1665. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrands, I.; Olsen, A.W.; Knudsen, S.; Dehari, N.; Juel, H.B.; Cheeseman, H.M.; Andersen, P.; Shattock, R.J.; Follmann, F. Human antibody signatures towards the Chlamydia trachomatis major outer membrane protein after natural infection and vaccination. EBioMedicine 2024, 104, 105140. [Google Scholar] [CrossRef] [PubMed]
- Garcia-del Rio, L.; Diaz-Rodriguez, P.; Pedersen, G.K.; Christensen, D.; Landin, M. Sublingual Boosting with a Novel Mucoadhesive Thermogelling Hydrogel Following Parenteral CAF01 Priming as a Strategy Against Chlamydia trachomatis. Adv. Healthc. Mater. 2022, 11, e2102508. [Google Scholar] [CrossRef]
- Carlsen, P.H.R.; Kjeldsen, R.B.; Pedersen, G.K.; Christensen, D.; Nielsen, L.H.; Boisen, A. Oral vaccination using microdevices to deliver α-GalCer adjuvanted vaccine afford a mucosal immune response. J. Control. Release 2023, 353, 134–146. [Google Scholar] [CrossRef]
- Lorenzen, E.; Contreras, V.; Olsen, A.W.; Andersen, P.; Desjardins, D.; Rosenkrands, I.; Juel, H.B.; Delache, B.; Langlois, S.; Delaugerre, C.; et al. Multi-component prime-boost Chlamydia trachomatis vaccination regimes induce antibody and T cell responses and accelerate clearance of infection in a non-human primate model. Front. Immunol. 2022, 13, 1057375. [Google Scholar] [CrossRef]
- Verma, A.; Hawes, C.E.; Elizaldi, S.R.; Smith, J.C.; Rajasundaram, D.; Pedersen, G.K.; Shen, X.; Williams, L.D.; Tomaras, G.D.; Kozlowski, P.A.; et al. Tailoring Tfh profiles enhances antibody persistence to a clade C HIV-1 vaccine in rhesus macaques. ELife 2024, 12, RP89395. [Google Scholar] [CrossRef]
- Christensen, D.; Foged, C.; Rosenkrands, I.; Nielsen, H.M.; Andersen, P.; Agger, E.M. Trehalose preserves DDA/TDB liposomes and their adjuvant effect during freeze-drying. Biochim. Biophys. Acta (BBA)—Biomembr. 2007, 1768, 2120–2129. [Google Scholar] [CrossRef]
- Thakur, A.; Ingvarsson, P.T.; Schmidt, S.T.; Rose, F.; Andersen, P.; Christensen, D.; Foged, C. Immunological and physical evaluation of the multistage tuberculosis subunit vaccine candidate H56/CAF01 formulated as a spray-dried powder. Vaccine 2018, 36, 3331–3339. [Google Scholar] [CrossRef]
- Thakur, A.; Xu, Y.; Cano-Garcia, G.; Feng, S.; Rose, F.; Gerde, P.; Andersen, P.; Christensen, D.; Foged, C. Optimizing the design and dosing of dry powder inhaler formulations of the cationic liposome adjuvant CAF®01 for pulmonary immunization. Front. Drug Deliv. 2022, 2, 973599. [Google Scholar] [CrossRef]
- Roces, C.B.; Khadke, S.; Christensen, D.; Perrie, Y. Scale-Independent Microfluidic Production of Cationic Liposomal Adjuvants and Development of Enhanced Lymphatic Targeting Strategies. Mol. Pharm. 2019, 16, 4372–4386. [Google Scholar] [CrossRef] [PubMed]
- Hovav, A.-H.; Fishman, Y.; Bercovier, H. Gamma interferon and monophosphoryl lipid A-trehalose dicorynomycolate are efficient adjuvants for Mycobacterium tuberculosis multivalent acellular vaccine. Infect. Immun. 2005, 73, 250–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, F.; Feng, X.; Huang, J.; Zhang, M.; Liu, W.; Wang, X.; Zhu, R.; Wang, X.; Wang, P.; Yu, B.; et al. Periodic Mesoporous Organosilica as a Nanoadjuvant for Subunit Vaccines Elicits Potent Antigen-Specific Germinal Center Responses by Activating Naive B Cells. ACS Nano 2023, 17, 15424–15440. [Google Scholar] [CrossRef] [PubMed]
- Lutta, A.; Knopp, M.M.; Tollemeto, M.; Pedersen, G.K.; Schmidt, S.T.; Grohganz, H.; Hagner Nielsen, L. The interplay between trehalose and dextran as spray drying precursors for cationic liposomes. Int. J. Pharm. 2024, 652, 123798. [Google Scholar] [CrossRef]
- Aleynick, M.; Svensson-Arvelund, J.; Flowers, C.R.; Marabelle, A.; Brody, J.D. Pathogen Molecular Pattern Receptor Agonists: Treating Cancer by Mimicking Infection. Clin. Cancer Res. 2019, 25, 6283–6294. [Google Scholar] [CrossRef]
- Li, C.; Xue, V.W.; Wang, Q.-M.; Lian, G.-Y.; Huang, X.-R.; Lee, T.-L.; To, K.-F.; Tang, P.M.-K.; Lan, H.-Y. The Mincle/Syk/NF-KB Signaling Circuit Is Essential for Maintaining the Protumoral Activities of Tumor-Associated Macrophages A C. Cancer Immunol. Res. 2020, 8, 1004–1017. [Google Scholar] [CrossRef]
- Seifert, L.; Werba, G.; Tiwari, S.; Giao Ly, N.N.; Alothman, S.; Alqunaibit, D.; Avanzi, A.; Barilla, R.; Daley, D.; Greco, S.H.; et al. The Necrosome Promotes Pancreatic Oncogenesis via CXCL1 and Mincle-Induced Immune Suppression. Nature 2016, 532, 245–249. [Google Scholar] [CrossRef]
- Haro, M.A.; Dyevoich, A.M.; Phipps, J.P.; Haas, K.M. Tumor Biology and Immunology Activation of B-1 Cells Promotes Tumor Cell Killing in the Peritoneal Cavity. Cancer Res. 2019, 79, 159–170. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, Y.; Li, C.; Li, B.; Hao, H.; Tian, F.; Li, H.; Liu, Z.; Wang, G.; Shen, X.C.; et al. Hydrogel Activation of Mincle Receptors for Tumor Cell Processing: A Novel Approach in Cancer Immunotherapy. Biomaterials 2024, 311, 122703. [Google Scholar] [CrossRef]
- Kodar, K.; Harper, J.L.; McConnell, M.J.; Timmer, M.S.M.; Stocker, B.L. The Mincle ligand trehalose dibehenate differentially modulates M1-like and M2-like macrophage phenotype and function via Syk signaling. Immun. Inflamm. Dis. 2017, 5, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Levast, B.; Awate, S.; Babiuk, L.; Mutwiri, G.; Gerdts, V.; van Drunen Little-van den Hurk, S. Vaccine Potentiation by Combination Adjuvants. Vaccines 2014, 2, 297–322. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Rajakumara, E.; Ahmed, N. Induction of Mincle by Helicobacter pylori and consequent anti-inflammatory signaling denote a bacterial survival strategy. Sci. Rep. 2015, 5, 15049. [Google Scholar] [CrossRef] [PubMed]
- Van Haren, S.D.; Pedersen, G.K.; Kumar, A.; Ruckwardt, T.J.; Moin, S.; Moore, I.N.; Minai, M.; Liu, M.; Pak, J.; Borriello, F.; et al. CAF08 adjuvant enables single dose protection against respiratory syncytial virus infection in murine newborns. Nat. Commun. 2022, 13, 4234. [Google Scholar] [CrossRef]
- Nordly, P.; Agger, E.M.; Andersen, P.; Nielsen, H.M.; Foged, C. Incorporation of the TLR4 Agonist Monophosphoryl Lipid A Into the Bilayer of DDA/TDB Liposomes: Physico-Chemical Characterization and Induction of CD8+ T-Cell Responses In Vivo. Pharm. Res. 2011, 28, 553. [Google Scholar] [CrossRef]
- Nordly, P.; Rose, F.; Christensen, D.; Nielsen, H.M.; Andersen, P.; Agger, E.M.; Foged, C. Immunity by formulation design: Induction of high CD8+ T-cell responses by poly(I:C) incorporated into the CAF01 adjuvant via a double emulsion method. J. Control. Release 2011, 150, 307–317. [Google Scholar] [CrossRef]
- Korsholm, K.S.; Hansen, J.; Karlsen, K.; Filskov, J.; Mikkelsen, M.; Lindenstrøm, T.; Schmidt, S.T.; Andersen, P.; Christensen, D. Induction of CD8+ T-cell responses against subunit antigens by the novel cationic liposomal CAF09 adjuvant. Vaccine 2014, 32, 3927–3935. [Google Scholar] [CrossRef]
- Karlsen, K.; Korsholm, K.S.; Mortensen, R.; Ghiasi, S.M.; Andersen, P.; Foged, C.; Christensen, D. A Stable Nanoparticulate DDA/MMG Formulation Acts Synergistically with CpG ODN 1826 to enhance the CD4 + T-Cell Response. Nanomedicine 2014, 9, 2625–2638. [Google Scholar] [CrossRef]
- Schmidt, S.T.; Pedersen, G.K.; Neustrup, M.A.; Korsholm, K.S.; Rades, T.; Andersen, P.; Foged, C.; Christensen, D. Induction of cytotoxic T-lymphocyte responses upon subcutaneous administration of a subunit vaccine adjuvanted with an emulsion containing the Toll-like receptor 3 ligand poly(I:C). Front. Immunol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Christensen, D.; Bøllehuus Hansen, L.; Leboux, R.; Jiskoot, W.; Christensen, J.P.; Andersen, P.; Dietrich, J. A Liposome-Based Adjuvant Containing Two Delivery Systems with the Ability to Induce Mucosal Immunoglobulin A Following a Parenteral Immunization. ACS Nano 2019, 13, 1116–1126. [Google Scholar] [CrossRef]
- Hansen, J.; Lindenstrøm, T.; Lindberg-Levin, J.; Aagaard, C.; Andersen, P.; Agger, E.M. CAF05: Cationic liposomes that incorporate synthetic cord factor and poly(I:C) induce CTL immunity and reduce tumor burden in mice. Cancer Immunol. Immunother. 2012, 61, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Wu, Y.; Zhang, Y.; Zhou, Z.; Lei, Q.; Ullah, N.; Banga Ndzouboukou, J.L.; Lin, X.; Fan, X. Combinational PRR agonists in liposomal adjuvant enhances immunogenicity and protective efficacy in a tuberculosis subunit vaccine. Front. Immunol. 2020, 1, 575504. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Teng, X.; Wang, X.; Fan, X.; Wu, Y.; Tian, M.; Zhou, Z.; Li, L. A multistage subunit vaccine effectively protects mice against primary progressive tuberculosis, latency and reactivation. EBioMedicine 2017, 22, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Tian, M.; Li, J.; Tan, S.; Yuan, X.; Yu, Q.; Jing, Y.; Zhang, Z.; Yue, T.; Zhou, L.; et al. Immunogenicity and protective efficacy of DMT liposome-adjuvanted tuberculosis subunit CTT3H vaccine. Hum. Vaccines Immunother. 2015, 11, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Liang, J.; Zhang, Y.; Teng, X.; Yuan, X.; Fan, X. Protection against Mycobacterium tuberculosis infection offered by a new multistage subunit vaccine correlates with increased number of IFN-γ+IL-2+ CD4+ and IFN-γ+ CD8+ T Cells. PLoS ONE 2015, 10, e0122560. [Google Scholar] [CrossRef]
- Tian, M.; Zhou, Z.; Tan, S.; Fan, X.; Li, L.; Ullah, N. Formulation in DDA-MPLA-TDB liposome enhances the immunogenicity and protective efficacy of a dna vaccine against Mycobacterium tuberculosis infection. Front. Immunol. 2018, 9, 310. [Google Scholar] [CrossRef]
- Ullah, N.; Hao, L.; Wu, Y.; Zhang, Y.; Lei, Q.; Banga Ndzouboukou, J.-L.; Lin, X.; Fan, X. differential immunogenicity and protective efficacy elicited by MTO- and DMT-adjuvanted CMFO subunit vaccines against Mycobacterium tuberculosis infection. J. Immunol. Res. 2020, 2020, 2083793. [Google Scholar] [CrossRef]
- Van Haren, S.D.; Dowling, D.J.; Foppen, W.; Christensen, D.; Andersen, P.; Reed, S.G.; Hershberg, R.M.; Baden, L.R.; Levy, O. Age-Specific Adjuvant Synergy: Dual TLR7/8 and Mincle Activation of Human Newborn Dendritic Cells Enables Th1 Polarization. J. Immunol. 2016, 197, 4413–4424. [Google Scholar] [CrossRef]
- Zimmermann, J.; van Haren, S.D.; Diray-Arce, J.; Adriawan, I.R.; Wørzner, K.; Krog, R.T.; Guleed, S.; Hu, T.; Mortensen, R.; Dietrich, J.; et al. Co-adjuvanting DDA/TDB liposomes with a TLR7 agonist allows for IgG2a/c class-switching in the absence of Th1 cells. NPJ Vaccines 2023, 8, 189. [Google Scholar] [CrossRef]
- Wilkinson, A.; Lattmann, E.; Roces, C.B.; Pedersen, G.K.; Christensen, D.; Perrie, Y. Lipid conjugation of TLR7 agonist Resiquimod ensures co-delivery with the liposomal Cationic Adjuvant Formulation 01 (CAF01) but does not enhance immunopotentiation compared to non-conjugated Resiquimod+CAF01. J. Control. Release 2018, 291, 1–10. [Google Scholar] [CrossRef]
- Lee, M.J.; Jo, H.; Shin, S.H.; Kim, S.M.; Kim, B.; Shim, H.S.; Park, J.H. Mincle and STING-Stimulating Adjuvants Elicit Robust Cellular Immunity and Drive Long-Lasting Memory Responses in a Foot-and-Mouth Disease Vaccine. Front. Immunol. 2019, 10, 2509. [Google Scholar] [CrossRef] [PubMed]
- Šťastná, E.; Erbs, G.; Skovgaard, K.; Jakobsen, J.T.; Bailey, M.; Pedersen, G.K.; Jungersen, G. Effects of different immunomodulating liposome-based adjuvants and injection sites on immunogenicity in pigs. Microbes Infect. 2024, 26, 105346. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Andrea, A.; Mikkelsen, H.; Woodworth, J.S.; Andersen, P.; Jungersen, G.; Aagaard, C. Targeting the Mincle and TLR3 receptor using the dual agonist cationic adjuvant formulation 9 (CAF09) induces humoral and polyfunctional memory T cell responses in calves. PLoS ONE 2018, 13, e0201253. [Google Scholar] [CrossRef] [PubMed]
- Billeskov, R.; Wang, Y.; Solaymani-Mohammadi, S.; Frey, B.; Kulkarni, S.; Andersen, P.; Agger, E.M.; Sui, Y.; Berzofsky, J.A. Low Antigen Dose in Adjuvant-Based Vaccination Selectively Induces CD4 T Cells with Enhanced Functional Avidity and Protective Efficacy. J. Immunol. 2017, 198, 3494–3506. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, D.A.; Christensen, D.; Muñoz, C.; Singh, S.; Locke, E.; Andersen, P.; Zavala, F. Robust antibody and CD8+ T-cell responses induced by P. falciparum CSP adsorbed to cationic liposomal adjuvant CAF09 confer sterilizing immunity against experimental rodent malaria infection. NPJ Vaccines 2017, 2, 10. [Google Scholar] [CrossRef]
- Huston, W. Immunological responses in a Chlamydia trachomatis vaccine trial. Lancet Infect. Dis. 2024, 24, 795–796. [Google Scholar] [CrossRef]
- Zimmermann, J.; Schmidt, S.; Trebbien, R.; Cox, R.; Zhou, F.; Follmann, F.; Pedersen, G.; Christensen, D. A Novel Prophylaxis Strategy Using Liposomal Vaccine Adjuvant CAF09b Protects against Influenza Virus Disease. Int. J. Mol. Sci. 2022, 23, 1850. [Google Scholar] [CrossRef]
- Schmidt, S.T.; Olsen, C.L.; Franzyk, H.; Wørzner, K.; Korsholm, K.S.; Rades, T.; Andersen, P.; Foged, C.; Christensen, D. Comparison of two different PEGylation strategies for the liposomal adjuvant CAF09: Towards induction of CTL responses upon subcutaneous vaccine administration. Eur. J. Pharm. Biopharm. 2019, 140, 29–39. [Google Scholar] [CrossRef]
- Mørk, S.K.; Kadivar, M.; Bol, K.F.; Draghi, A.; Westergaard, M.C.W.; Skadborg, S.K.; Overgaard, N.; Sørensen, A.B.; Rasmussen, I.S.; Andreasen, L.V.; et al. Personalized therapy with peptide-based neoantigen vaccine (EVX-01) including a novel adjuvant, CAF®09b, in patients with metastatic melanoma. Oncoimmunology 2022, 11, 2023255. [Google Scholar] [CrossRef]
- Mørk, S.K.; Kongsted, P.; Westergaard, M.C.W.; Albieri, B.; Granhøj, J.S.; Donia, M.; Martinenaite, E.; Holmström, M.O.; Madsen, K.; Kverneland, A.H.; et al. First in man study: Bcl-Xl_42-CAF®09b vaccines in patients with locally advanced prostate cancer. Front. Immunol. 2023, 14, 1122977. [Google Scholar] [CrossRef]
- Thakur, A.; Wadhwa, A.; Lokras, A.; Müllertz, O.A.O.; Christensen, D.; Franzyk, H.; Foged, C. Method of manufacturing CAF®09 liposomes affects immune responses induced by adjuvanted subunit proteins. Eur. J. Pharm. Biopharm. 2023, 189, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Phipps, J.P.; Haas, K.M. An adjuvant that increases protective antibody responses to polysaccharide antigens and enables recall responses. J. Infect. Dis. 2019, 219, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Dyevoich, A.M.; Disher, N.S.; Haro, M.A.; Haas, K.M. A TLR4-TRIF-dependent signaling pathway is required for protective natural tumor-reactive IgM production by B1 cells. Cancer Immunol. Immunother. 2020, 69, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Dubé, J.Y.; McIntosh, F.; Zarruk, J.G.; David, S.; Nigou, J.; Behr, M.A. Synthetic mycobacterial molecular patterns partially complete Freund’s adjuvant. Sci. Rep. 2020, 10, 5874. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Jin, S.; Gilmartin, L.; Toth, I.; Hussein, W.; Stephenson, R. Advances in Infectious Disease Vaccine Adjuvants. Vaccines 2022, 10, 1120. [Google Scholar] [CrossRef]
- Valdemar-Aguilar, C.M.; Manisekaran, R.; Acosta-Torres, L.S.; López-Marín, L.M. Spotlight on mycobacterial lipid exploitation using nanotechnology for diagnosis, vaccines, and treatments. Nanomedicine 2023, 48, 102653. [Google Scholar] [CrossRef]
- Stewart, E.; Triccas, J.A.; Petrovsky, N. Adjuvant Strategies for More Effective Tuberculosis Vaccine Immunity. Microorganisms 2019, 7, 255. [Google Scholar] [CrossRef]
- Das, I.; Padhi, A.; Mukherjee, S.; Dash, D.P.; Kar, S.; Sonawane, A. Biocompatible chitosan nanoparticles as an efficient delivery vehicle for Mycobacterium tuberculosis lipids to induce potent cytokines and antibody response through activation of γδ T cells in mice. Nanotechnology 2017, 28, 165101. [Google Scholar] [CrossRef]
- Pi, J.; Zhang, Z.; Yang, E.; Chen, L.; Zeng, L.; Chen, Y.; Wang, R.; Huang, D.; Fan, S.; Lin, W.; et al. Nanocages engineered from Bacillus Calmette-Guerin facilitate protective Vγ2Vδ2 T cell immunity against Mycobacterium tuberculosis infection. J. Nanobiotechnol. 2022, 20, 36. [Google Scholar] [CrossRef]
- Marino, L. Towards the Development of Synthetic Vaccines Against Tuberculosis. Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 2022. [Google Scholar]
- Spurrier, M.A.; Jennings-Gee, J.E.; Haas, K.M. Type I IFN Receptor Signaling on B Cells Promotes Antibody Responses to Polysaccharide Antigens. J. Immunol. 2023, 210, 148–157. [Google Scholar] [CrossRef]
- Pettenati, C.; Ingersoll, M.A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 2018, 15, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Azuma, I.; Seya, T. Development of Immunoadjuvants for Immunotherapy of Cancer. Int. Immunopharmacol. 2001, 1, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Sato, H.; Hayashi, R.; Asano, T.; Sato, H.; Yamamura, Y. Postoperative adjuvant immunotherapy of gastric cancer with BCG-cell wall skeleton. 3- to 6-year follow up of a randomized clinical trial. Cancer Immunol. Immunother. 1983, 14, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Higashiyama, M.; Takami, K.; Oda, K.; Okami, J.; Maeda, J.; Akazawa, T.; Matsumoto, M.; Seya, T.; Wada, M.; et al. Innate immune therapy with a Bacillus Calmette-Guérin cell wall skeleton after radical surgery for non-small cell lung cancer: A case-control study. Surg. Today 2009, 39, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Tsuboi, A.; Tanemura, A.; Ito, T.; Nakajima, H.; Shirakata, T.; Morimoto, S.; Fujiki, F.; Hosen, N.; Oji, Y.; et al. Immune adjuvant therapy using Bacillus Calmette–Guérin cell wall skeleton (BCG-CWS) in advanced malignancies: A phase 1 study of safety and immunogenicity assessments. Medicine 2019, 98, e16771. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Nakamura, T.; Noma, Y.; Harashima, H. Application of BCG-CWS as a systemic adjuvant by using nanoparticulation technology. Mol. Pharm. 2018, 15, 5762–5771. [Google Scholar] [CrossRef]
- Shibata, T.; Takata, E.; Sakamoto, J.; Shioya, A.; Yamada, S.; Takakura, M.; Sasagawa, T. A Retrospective Study of Immunotherapy Using the Cell Wall Skeleton of Mycobacterium Bovis Bacillus Calmette-Guérin (BCG-CWS) for Cervical Cancer. Medicine 2022, 101, e32481. [Google Scholar] [CrossRef]
- Liu, K.; Peng, J.; Guo, Y.; Li, Y.; Qi, X.; Duan, D.; Li, T.; Li, J.; Niu, Y.; Han, G.; et al. Expanding the Potential of Neoantigen Vaccines: Harnessing Bacille Calmette-Guérin Cell-Wall-Based Nanoscale Adjuvants for Enhanced Cancer Immunotherapy. ACS Nano 2024, 18, 11910–11920. [Google Scholar] [CrossRef]
- Ishida, H.; Ogawa, Y.; Imai, Y.; Kiso, M.; Hasegawa, A.; Sakurai, T.; Azume, I. Chemical combination of 6-deoxy-6-mycoloylamino-α,α-trehalose and N-acetyl-6-O-(aminoacyl)muramoyl dipeptide. Carbohydr. Res. 1989, 194, 199–208. [Google Scholar] [CrossRef]
- Dangerfield, E.M.; Ishizuka, S.; Kodar, K.; Yamasaki, S.; Timmer, M.S.M.; Stocker, B.L. Chimeric NOD2 Mincle agonists as vaccine adjuvants. J. Med. Chem. 2024, 67, 5373–5390. [Google Scholar] [CrossRef]
- Kramer, K.; Shields, N.J.; Poppe, V.; Young, S.L.; Walker, G.F. Intracellular Cleavable CpG Oligodeoxynucleotide-Antigen Conjugate Enhances Anti-tumor Immunity. Mol. Ther. 2017, 25, 62–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blander, J.M.; Medzhitov, R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 2006, 440, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.C.; Maxwell, J.W.C.; Ismanto, H.S.; Ashhurst, A.S.; Artner, L.M.; Rudrawar, S.; Britton, W.J.; Yamasaki, S.; Payne, R.J. Synthetic vaccines targeting Mincle through conjugation of trehalose dibehenate. Chem. Commun. 2022, 58, 6890–6893. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C. Synthesis and Evaluation of Conjugate Vaccines for Tuberculosis. Ph.D. Thesis, The University of Sydney, Sydney, Australia, 2020. Available online: https://hdl.handle.net/2123/22700 (accessed on 15 November 2024).
- Wang, K.; Zhang, T.; Liu, M.; Wang, D.; Zhu, H.; Wang, Z.; Yu, F.; Liu, Y.; Zhao, W. Synthesis and immunological evaluation of Mincle ligands-based antitumor vaccines. Chin. Chem. Lett. 2023, 34, 108065. [Google Scholar] [CrossRef]
- Yang, D.; Li, X.; Li, J.; Liu, Z.; Li, T.; Liao, P.; Luo, X.; Liu, Z.; Ming, W.; Liao, G. Fully Synthetic TF-Based Self-Adjuvanting Vaccine Simultaneously Triggers iNKT Cells and Mincle and Protects Mice against Tumor Development. J. Med. Chem. 2024, 67, 17640–17656. [Google Scholar] [CrossRef]
- Nouatin, O.; Ibáñez, J.; Fendel, R.; Ngoa, U.A.; Lorenz, F.-R.; Dejon-Agobé, J.-C.; Edoa, J.R.; Flügge, J.; Brückner, S.; Esen, M.; et al. Cellular and antibody response in GMZ2-vaccinated Gabonese volunteers in a controlled human malaria infection trial. Malar. J. 2022, 21, 191. [Google Scholar] [CrossRef]
- Liu, X.; Situ, A.; Kang, Y.; Villabroza, K.R.; Liao, Y.; Chang, C.H.; Donahue, T.; Nel, A.E.; Meng, H. Irinotecan Delivery by Lipid-Coated Mesoporous Silica Nanoparticles Shows Improved Efficacy and Safety over Liposomes for Pancreatic Cancer. ACS Nano 2016, 10, 2702–2715. [Google Scholar] [CrossRef]
- Christensen, D. Development and Evaluation of CAF01. In Immunopotentiators in Modern Vaccines; Elsevier: Amsterdam, The Netherlands, 2017; pp. 333–345. [Google Scholar] [CrossRef]






| Mincle Ligand | Formulation | Disease(s) | Antigen(s) | Reference |
|---|---|---|---|---|
| TDM (1) | Emulsified in IFA or CFA | Mouse model, cancers | OVA, No Ag | [28,91,141] |
| Liposomes (DOPC, Chol) | Bladder and colon cancers, melanoma | No Ag | [142] | |
| TDB (2) | DDA liposomes (CAF01) + variations | Many, i.e., bacterial, viral, fungal, cancers | Proteins, lipids, saRNA, whole parasite/virus | [109,130,133,134,135,136,137,138,139] |
| PLGA micro or nanoparticles | M. Tb, chlamydia | H1, MPT83, MOMP, rAg85B | [131,143,144,145,146] | |
| TDP (3b), TDS (3c), PEG-TDS | DDA liposomes | M. Tb, chlamydia | H56, MOMP | [76,88] |
| TDO (3e) | Lipid NP | Model | Hemagglutinin mRNA | [89] |
| Branched TDE (6a) | DDA/DSPC liposomes | M. Tb | M72 | [22] |
| TMS (4c) | DDA liposomes | Chlamydia | MOMP | [76] |
| TMM (4b) | w/o/w emulsions containing 30% IFA | Model | OVA | [91] |
| Amide-TDB (8c) and inverted ester (9a) | o/w emulsion | Model, ovine pneumonia | OVA, M. haemolytica, and/or M. ovipneumoniae | [87,96] |
|
C18dMeBrar (10a), p- and o-C18Brar (10b and 10c) | o/w emulsion | Model, ovine pneumonia |
OVA, M. haemolytica, and/or M. ovipneumoniae | [51,78,87,123,129] |
| UM-1024 (12a) | DDA/DSPC liposomes | M. Tb | M72 | [99] |
| UM-1024 (12a), UM-1052 (11a), and UM-1098 (11e) | A-SNPs | M. Tb | OVA, M72, ESAT-6/Ag85B | [48,101,147] |
| MMG-1 (17) | DDA liposomes (CAF04) | M. Tb, chlamydia | Ag85B-ESAT-6 and H56, PmpG, and MOMP | [148,149] |
| Squalane o/w nanoemulsion (CAF19) | Model, chlamydia | OVA, MOMP saRNA | [132,149,150] | |
| GlcC14C18 (14c) and ManC14C18 (15c) | DDA liposomes | M. Tb | Ag85A | [33] |
| GMM (13b) and AraMM (16) | w/o/w emulsions containing 30% IFA | Model | OVA | [91] |
| PGL-III (18) | o/w emulsion | Model | OVA | [47] |
| Mincle Ligand | Formulation | Disease | Antigen(s), Route(s) | Highest Phase | Trial #/Reference |
|---|---|---|---|---|---|
| TDB (2) | DDA liposomes (CAF01) | M. Tb | Ag85B-ESAT-6 (H1) recombinant protein i.m. | Phase 1 | NCT00922363 [137] |
| HIV-1 | 18 HIV-1 peptides i.m. | Phase 1 | NCT01141205 [135] | ||
| HIV-1 | 18 HIV-1 peptides i.m. | Phase 1 | NCT01009762 [136] | ||
| Malaria | GMZ-2 recombinant protein i.m. | Phase 1 | PACTR201503001038304 [134] | ||
| Chlamydia | CTH522 recombinant protein, i.m. + intranasal | Phase 1 | NCT02787109 [133] | ||
| Chlamydia | CTH522 recombinant protein, i.m. + topical ocular or intradermal | Phase 1 | NCT03926728 [138] |
| Mincle Ligand | Other PAMP(s) | Formulation | Disease(s) | Antigen(s) | Reference |
|---|---|---|---|---|---|
| TDB (2) | Poly(I:C) (TLR3) | DDA liposomes (CAF05) | Model, cancer (melanoma and TC1 tumors) | OVA, no Ag | [197,202] |
| MPL (TLR4) | DDA liposomes (CAF06) | Model, M. Tb | OVA, A1D4, CTT3H, CMFO, plasmid pCMFO | [196,203,204,205,206,207] | |
| o/w emulsion (MTO) | M. Tb | CMFO | [206,208] | ||
| 3M-052 (TLR7/8) | DDA liposomes (CAF08) | Respiratory syncytial virus (RSV), chlamydia | RSV Pre-F, CTH522, MOMP saRNA | [150,195,209,210] | |
| Resiquimod (TLR7/8) | DDA liposomes | M. Tb | H56 | [211] | |
| c-di-GMP (STING) | o/w emulsion | Foot-and-mouth (FMD) disease | FMD virus particles | [212] | |
| Retinoic acid | DDA liposomes (CAF23) | Chlamydia | MOMP, CTH522 | [201,213] | |
| MMG-1 (17) | Poly(I:C) (TLR3) | DDA liposomes (CAF09) | Model, M avium subsp. paratuberculosis, HIV-1, HPV, malaria, influenza, chlamydia, cancers (prostate, solid) | OVA, MAP, CSP, WIV-H1N1, CTH522, MOMP saRNA, Bcl-XL, patient-derived neoantigens | [138,150,152,198,214,215,216,217,218,219,220,221] |
| o/w nanoemulsion (CAF24) | Model | OVA | [200] | ||
| Poly(I:C) (TLR3) and L5N12 (TLR4) | DDA liposomes | M. Tb, HIV-1 | H56, E7 | [222] | |
| Flagellin (TLR5) | DDA liposomes (CAF11) | M. Tb | H56 | [199] | |
| CpG2006 (TLR9) | DDA liposomes (CAF10) | M. Tb, chlamydia | H56, H107e, CTH522 | [67,199] | |
| TDCM (5) | Salmonella Minnesota MPL (TLR4) | Squalene o/w emulsion (MTS) | S. pneumococcus | Haptenated-Ficoll, S. pneumoniae-derived capsular polysaccharides (PPSs) | [223] |
| MPL from BCG (TLR4) | Squalene o/w emulsion | Peritoneal carcinomatosis | Tumour-associated carbohydrate antigens (TACAs) | [224] | |
| UM-1098 (11e) | INI-2002 (TLR4) | A-SNPs | M.Tb | M72 | [49] |
| GlcC14C18 (14c) | N-glycolyl MDP (NOD2) | Emulsified with IFA in PBS | Model | OVA | [225] |
| Mincle Ligand | Formulation | Disease | Antigen(s), Route(s) | Clinical Trial | Trial #/Reference |
|---|---|---|---|---|---|
| MMG-1 (17) | DDA liposomes with poly(I:C) (TLR3) (CAF09b) | Chlamydia | CTH522 recombinant protein i.m. + topical ocular or intradermal | Phase 1 | NCT03926728 [138] |
| Prostate Cancer | B-cell lymphoma-extra-large (Bcl-XL) peptide, i.m./i.p. | Phase 1 | NCT03412786 [221] | ||
| Advanced Solid Cancers | Up to 15 patient-derived neo-peptide antigens formulated with anti-PD-1 or anti-PD-L1 i.m./i.p. | Phase1/2a | NCT03715985 [220] | ||
| DDA liposomes with CpG2006 (TLR9) (CAF10b) | M.Tb | H107e, 8 different M.Tb antigens i.m. | Phase 1 | NCT06050356 [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weth, A.F.; Dangerfield, E.M.; Timmer, M.S.M.; Stocker, B.L. Recent Advances in the Development of Mincle-Targeting Vaccine Adjuvants. Vaccines 2024, 12, 1320. https://doi.org/10.3390/vaccines12121320
Weth AF, Dangerfield EM, Timmer MSM, Stocker BL. Recent Advances in the Development of Mincle-Targeting Vaccine Adjuvants. Vaccines. 2024; 12(12):1320. https://doi.org/10.3390/vaccines12121320
Chicago/Turabian StyleWeth, Anya F., Emma M. Dangerfield, Mattie S. M. Timmer, and Bridget L. Stocker. 2024. "Recent Advances in the Development of Mincle-Targeting Vaccine Adjuvants" Vaccines 12, no. 12: 1320. https://doi.org/10.3390/vaccines12121320
APA StyleWeth, A. F., Dangerfield, E. M., Timmer, M. S. M., & Stocker, B. L. (2024). Recent Advances in the Development of Mincle-Targeting Vaccine Adjuvants. Vaccines, 12(12), 1320. https://doi.org/10.3390/vaccines12121320






