Immunosenescence: Aging and Immune System Decline
Abstract
1. Introduction

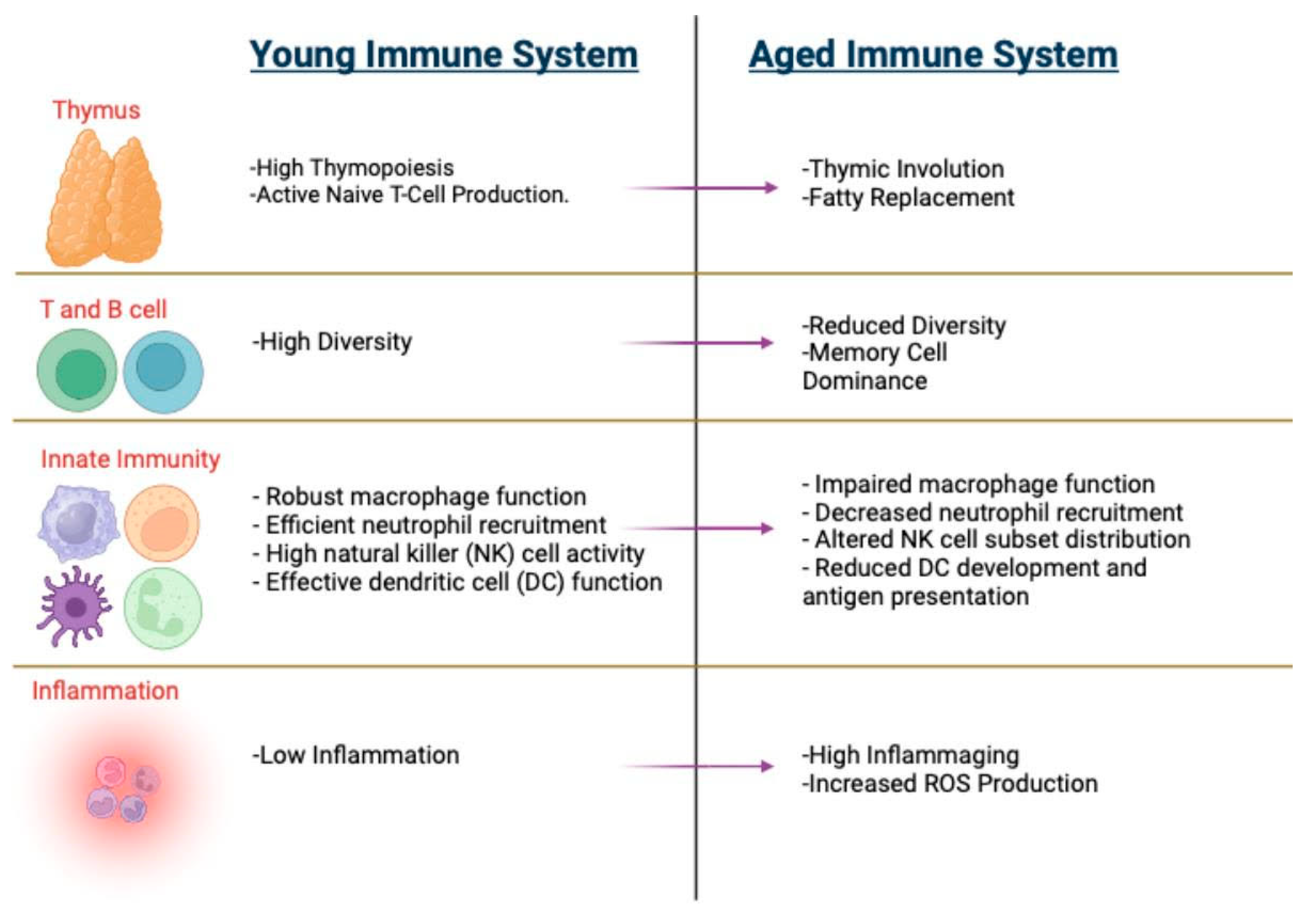
2. Hallmarks of Immunosenescence
2.1. Thymic Involution
2.2. Inflammaging
2.3. Cellular Metabolic Adaptations
2.4. Hematopoietic Changes
3. Impact of Aging on Immune Cells
3.1. Macrophages
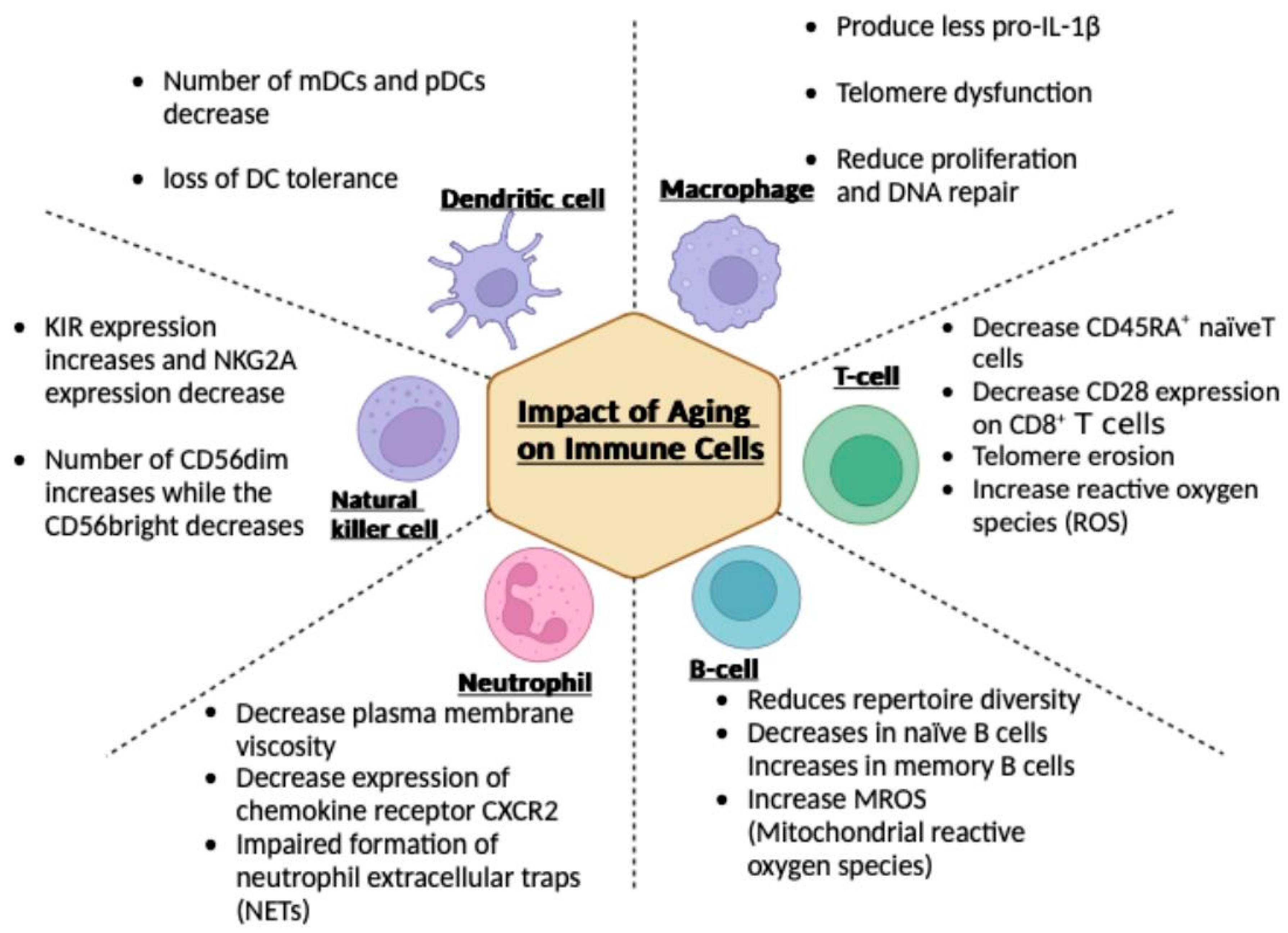
3.2. T Cells
3.3. B Cells
3.4. Neutrophils
3.5. Natural Killer (NK) Cells
3.6. Dendritic Cells
4. Comprehensive Approaches for Managing Age-Related Immune Decline

5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bar-Dayan, Y.; Afek, A.; Goldberg, I.; Kopolovic, J. Proliferation, apoptosis and thymic involution. Tissue Cell 1999, 31, 391–396. [Google Scholar] [CrossRef]
- Wu, H.; Qin, X.; Dai, H.; Zhang, Y. Time-course transcriptome analysis of medullary thymic epithelial cells in the early phase of thymic involution. Mol. Immunol. 2018, 99, 87–94. [Google Scholar] [CrossRef]
- Reis, M.D.; Csomos, K.; Dias, L.P.; Prodan, Z.; Szerafin, T.; Savino, W.; Takacs, L. Decline of FOXN1 gene expression in human thymus correlates with age: Possible epigenetic regulation. Immun. Ageing 2015, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Flores, K.G.; Li, J.; Sempowski, G.D.; Haynes, B.F.; Hale, L.P. Analysis of the human thymic perivascular space during aging. J. Clin. Investig. 1999, 104, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Sauce, D.; Larsen, M.; Fastenackels, S.; Duperrier, A.; Keller, M.; Grubeck-Loebenstein, B.; Ferrand, C.; Debré, P.; Sidi, D.; Appay, V. Evidence of premature immune aging in patients thymectomized during early childhood. J. Clin. Investig. 2009, 119, 3070–3078. [Google Scholar] [CrossRef] [PubMed]
- Andrew, D.; Aspinall, R. Age-associated thymic atrophy is linked to a decline in IL-7 production. Exp. Gerontol. 2002, 37, 455–463. [Google Scholar] [CrossRef]
- Hussein, S.A.; Sabri, Y.Y.; Fouad, M.A.; Al-Zawam, H.H.; Mohamed, N.M. Role of different imaging modalities in the evaluation of normal and diseased thymus. Egypt. J. Bronchol. 2020, 14, 5. [Google Scholar] [CrossRef]
- Araki, T.; Nishino, M.; Gao, W.; Dupuis, J.; Hunninghake, G.M.; Murakami, T.; Washko, G.R.; O’Connor, G.T.; Hatabu, H. Normal thymus in adults: Appearance on CT and associations with age, sex, BMI and smoking. Eur. Radiol. 2016, 26, 15–24. [Google Scholar] [CrossRef]
- Moore, A.V.; Korobkin, M.; Olanow, W.; Heaston, D.K.; Ram, P.C.; Dunnick, N.R.; Silverman, P.M. Age-related changes in the thymus gland: CT-pathologic correlation. AJR Am. J. Roentgenol. 1983, 141, 241–246. [Google Scholar] [CrossRef]
- Francis, I.R.; Glazer, G.M.; Bookstein, F.L.; Gross, B.H. The thymus: Reexamination of age-related changes in size and shape. AJR Am. J. Roentgenol. 1985, 145, 249–254. [Google Scholar] [CrossRef]
- Fishbein, K.W.; Makrogiannis, S.K.; Lukas, V.A.; Okine, M.; Ramachandran, R.; Ferrucci, L.; Egan, J.M.; Chia, C.W.; Spencer, R.G. Measurement of fat fraction in the human thymus by localized NMR and three-point Dixon MRI techniques. Magn. Reson. Imaging 2018, 50, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Ferdinand, B.; Gupta, P.; Kramer, E.L. Spectrum of thymic uptake at 18F-FDG PET. Radiographics 2004, 24, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Alberro, A.; Iribarren-Lopez, A.; Sáenz-Cuesta, M.; Matheu, A.; Vergara, I.; Otaegui, D. Inflammaging markers characteristic of advanced age show similar levels with frailty and dependency. Sci. Rep. 2021, 11, 4358. [Google Scholar] [CrossRef]
- Ferrucci, L.; Corsi, A.; Lauretani, F.; Bandinelli, S.; Bartali, B.; Taub, D.D.; Guralnik, J.M.; Longo, D.L. The origins of age-related proinflammatory state. Blood 2005, 105, 2294–2299. [Google Scholar] [CrossRef]
- Varadhan, R.; Yao, W.; Matteini, A.; Beamer, B.A.; Xue, Q.L.; Yang, H.; Manwani, B.; Reiner, A.; Jenny, N.; Parekh, N.; et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Puzianowska-Kuźnicka, M.; Owczarz, M.; Wieczorowska-Tobis, K.; Nadrowski, P.; Chudek, J.; Slusarczyk, P.; Skalska, A.; Jonas, M.; Franek, E.; Mossakowska, M. Interleukin-6 and C-reactive protein, successful aging, and mortality: The PolSenior study. Immun. Ageing 2016, 13, 21. [Google Scholar] [CrossRef]
- Di Iorio, A.; Ferrucci, L.; Sparvieri, E.; Cherubini, A.; Volpato, S.; Corsi, A.; Bonafè, M.; Franceschi, C.; Abate, G.; Paganelli, R. Serum IL-1beta levels in health and disease: A population-based study. ‘The InCHIANTI study’. Cytokine 2003, 22, 198–205. [Google Scholar] [CrossRef]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef]
- Kim, K.A.; Jeong, J.J.; Yoo, S.Y.; Kim, D.H. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol. 2016, 16, 9. [Google Scholar] [CrossRef]
- Libermann, T.A.; Baltimore, D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell Biol. 1990, 10, 2327–2334. [Google Scholar] [CrossRef]
- Ridker, P.M.; Lei, L.; Louie, M.J.; Haddad, T.; Nicholls, S.J.; Lincoff, A.M.; Libby, P.; Nissen, S.E. Inflammation and Cholesterol as Predictors of Cardiovascular Events Among 13 970 Contemporary High-Risk Patients with Statin Intolerance. Circulation 2024, 149, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Patsoukis, N.; Bardhan, K.; Chatterjee, P.; Sari, D.; Liu, B.; Bell, L.N.; Karoly, E.D.; Freeman, G.J.; Petkova, V.; Seth, P.; et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 2015, 6, 6692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Y.; Ye, K.; Picard, M.; Gu, Z. Independent impacts of aging on mitochondrial DNA quantity and quality in humans. BMC Genom. 2017, 18, 890. [Google Scholar] [CrossRef] [PubMed]
- Meissner, C.; Bruse, P.; Mohamed, S.A.; Schulz, A.; Warnk, H.; Storm, T.; Oehmichen, M. The 4977 bp deletion of mitochondrial DNA in human skeletal muscle, heart and different areas of the brain: A useful biomarker or more? Exp. Gerontol. 2008, 43, 645–652. [Google Scholar] [CrossRef]
- Henson, S.M.; Lanna, A.; Riddell, N.E.; Franzese, O.; Macaulay, R.; Griffiths, S.J.; Puleston, D.J.; Watson, A.S.; Simon, A.K.; Tooze, S.A.; et al. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8⁺ T cells. J. Clin. Investig. 2014, 124, 4004–4016. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, C.; Liu, X.; Gu, C.; Liu, Y.; Gao, Y.; Huang, Z.; Jiang, Q.; Chen, B.; He, D.; et al. An aging-related immune landscape in the hematopoietic immune system. Immun. Ageing 2024, 21, 3. [Google Scholar] [CrossRef]
- Beerman, I.; Bhattacharya, D.; Zandi, S.; Sigvardsson, M.; Weissman, I.L.; Bryder, D.; Rossi, D.J. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. USA 2010, 107, 5465–5470. [Google Scholar] [CrossRef]
- Sudo, K.; Ema, H.; Morita, Y.; Nakauchi, H. Age-associated characteristics of murine hematopoietic stem cells. J. Exp. Med. 2000, 192, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.J.; Bryder, D.; Zahn, J.M.; Ahlenius, H.; Sonu, R.; Wagers, A.J.; Weissman, I.L. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc. Natl. Acad. Sci. USA 2005, 102, 9194–9199. [Google Scholar] [CrossRef]
- Cho, R.H.; Sieburg, H.B.; Muller-Sieburg, C.E. A new mechanism for the aging of hematopoietic stem cells: Aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood 2008, 111, 5553–5561. [Google Scholar] [CrossRef]
- Beerman, I.; Bock, C.; Garrison, B.S.; Smith, Z.D.; Gu, H.; Meissner, A.; Rossi, D.J. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell 2013, 12, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.; Mehta, A.; de Boer, C.G.; Kowalczyk, M.S.; Lee, K.; Haldeman, P.; Rogel, N.; Knecht, A.R.; Farouq, D.; Regev, A.; et al. Heterogeneous Responses of Hematopoietic Stem Cells to Inflammatory Stimuli Are Altered with Age. Cell Rep. 2018, 25, 2992–3005.e2995. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.; Rathinam, V.; Fitzgerald, K.A.; Golenbock, D.T.; Mathew, A. Defective pro-IL-1β responses in macrophages from aged mice. Immun. Ageing 2012, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cheng, N.; Qiao, B.; Zhang, F.; Wu, J.; Liu, C.; Li, Y.; Du, J. Age-related decline of interferon-gamma responses in macrophage impairs satellite cell proliferation and regeneration. J. Cachexia Sarcopenia Muscle 2020, 11, 1291–1305. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, H.; Zhao, Y.; Wang, Y.; Wang, W.; He, Y.; Zhang, W.; Zhu, X.; Zhou, Y.; Zhang, L.; et al. Telomere Dysfunction Disturbs Macrophage Mitochondrial Metabolism and the NLRP3 Inflammasome through the PGC-1α/TNFAIP3 Axis. Cell Rep. 2018, 22, 3493–3506. [Google Scholar] [CrossRef]
- Dube, C.T.; Ong, Y.H.B.; Wemyss, K.; Krishnan, S.; Tan, T.J.; Janela, B.; Grainger, J.R.; Ronshaugen, M.; Mace, K.A.; Lim, C.Y. Age-Related Alterations in Macrophage Distribution and Function Are Associated with Delayed Cutaneous Wound Healing. Front. Immunol. 2022, 13, 943159. [Google Scholar] [CrossRef]
- Lazuardi, L.; Jenewein, B.; Wolf, A.M.; Pfister, G.; Tzankov, A.; Grubeck-Loebenstein, B. Age-related loss of naïve T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology 2005, 114, 37–43. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Lei, L.; Sun, L.; Jiao, A.; Zhu, K.; Xie, T.; Liu, H.; Zhang, X.; Su, Y.; et al. Age-Related Gene Alteration in Naïve and Memory T cells Using Precise Age-Tracking Model. Front. Cell Dev. Biol. 2020, 8, 624380. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.Q.; Ruan, L.; Zhou, H.R.; Gao, W.L.; Zhang, Q.; Zhang, C.T. Age-related changes in peripheral T-cell subpopulations in elderly individuals: An observational study. Open Life Sci. 2023, 18, 20220557. [Google Scholar] [CrossRef]
- Sun, X.; Nguyen, T.; Achour, A.; Ko, A.; Cifello, J.; Ling, C.; Sharma, J.; Hiroi, T.; Zhang, Y.; Chia, C.W.; et al. Longitudinal analysis reveals age-related changes in the T cell receptor repertoire of human T cell subsets. J. Clin. Investig. 2022, 132, e158122. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Karandikar, N.J.; Betts, M.R.; Ambrozak, D.R.; Hill, B.J.; Crotty, L.E.; Casazza, J.P.; Kuruppu, J.; Migueles, S.A.; Connors, M.; et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 2003, 101, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.M.; Horikawa, I.; Pine, S.R.; Fujita, K.; Morgan, K.M.; Vera, E.; Mazur, S.J.; Appella, E.; Vojtesek, B.; Blasco, M.A.; et al. p53 isoforms regulate aging- and tumor-associated replicative senescence in T lymphocytes. J. Clin. Investig. 2013, 123, 5247–5257. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Damjanovic, A.; Metter, E.J.; Nguyen, H.; Truong, T.; Najarro, K.; Morris, C.; Longo, D.L.; Zhan, M.; Ferrucci, L.; et al. Age-associated telomere attrition of lymphocytes in vivo is co-ordinated with changes in telomerase activity, composition of lymphocyte subsets and health conditions. Clin. Sci. 2015, 128, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, F.J.; Franzese, O.; Finney, H.M.; Fletcher, J.M.; Belaramani, L.L.; Salmon, M.; Dokal, I.; Webster, D.; Lawson, A.D.; Akbar, A.N. The loss of telomerase activity in highly differentiated CD8+CD28-CD27- T cells is associated with decreased Akt (Ser473) phosphorylation. J. Immunol. 2007, 178, 7710–7719. [Google Scholar] [CrossRef]
- Patrick, M.S.; Cheng, N.L.; Kim, J.; An, J.; Dong, F.; Yang, Q.; Zou, I.; Weng, N.P. Human T Cell Differentiation Negatively Regulates Telomerase Expression Resulting in Reduced Activation-Induced Proliferation and Survival. Front. Immunol. 2019, 10, 1993. [Google Scholar] [CrossRef]
- Das, R.; Ponnappan, S.; Ponnappan, U. Redox regulation of the proteasome in T lymphocytes during aging. Free Radic. Biol. Med. 2007, 42, 541–551. [Google Scholar] [CrossRef]
- Gibson, K.L.; Wu, Y.C.; Barnett, Y.; Duggan, O.; Vaughan, R.; Kondeatis, E.; Nilsson, B.O.; Wikby, A.; Kipling, D.; Dunn-Walters, D.K. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell 2009, 8, 18–25. [Google Scholar] [CrossRef]
- Kurupati, R.K.; Haut, L.H.; Schmader, K.E.; Ertl, H.C. Age-related changes in B cell metabolism. Aging 2019, 11, 4367–4381. [Google Scholar] [CrossRef]
- Lee, J.L.; Fra-Bido, S.C.; Burton, A.R.; Innocentin, S.; Hill, D.L.; Linterman, M.A. B cell-intrinsic changes with age do not impact antibody-secreting cell formation but delay B cell participation in the germinal centre reaction. Aging Cell 2022, 21, e13692. [Google Scholar] [CrossRef]
- Rodriguez-Zhurbenko, N.; Quach, T.D.; Hopkins, T.J.; Rothstein, T.L.; Hernandez, A.M. Human B-1 Cells and B-1 Cell Antibodies Change with Advancing Age. Front. Immunol. 2019, 10, 483. [Google Scholar] [CrossRef]
- Perskin, M.H.; Cronstein, B.N. Age-related changes in neutrophil structure and function. Mech. Ageing Dev. 1992, 64, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Uhl, B.; Vadlau, Y.; Zuchtriegel, G.; Nekolla, K.; Sharaf, K.; Gaertner, F.; Massberg, S.; Krombach, F.; Reichel, C.A. Aged neutrophils contribute to the first line of defense in the acute inflammatory response. Blood 2016, 128, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Gullotta, G.S.; De Feo, D.; Friebel, E.; Semerano, A.; Scotti, G.M.; Bergamaschi, A.; Butti, E.; Brambilla, E.; Genchi, A.; Capotondo, A.; et al. Age-induced alterations of granulopoiesis generate atypical neutrophils that aggravate stroke pathology. Nat. Immunol. 2023, 24, 925–940. [Google Scholar] [CrossRef]
- Weisel, K.C.; Bautz, F.; Seitz, G.; Yildirim, S.; Kanz, L.; Möhle, R. Modulation of CXC chemokine receptor expression and function in human neutrophils during aging in vitro suggests a role in their clearance from circulation. Mediat. Inflamm. 2009, 2009, 790174. [Google Scholar] [CrossRef]
- Barkaway, A.; Rolas, L.; Joulia, R.; Bodkin, J.; Lenn, T.; Owen-Woods, C.; Reglero-Real, N.; Stein, M.; Vázquez-Martínez, L.; Girbl, T.; et al. Age-related changes in the local milieu of inflamed tissues cause aberrant neutrophil trafficking and subsequent remote organ damage. Immunity 2021, 54, 1494–1510.e1497. [Google Scholar] [CrossRef] [PubMed]
- Hornigold, K.; Chu, J.Y.; Chetwynd, S.A.; Machin, P.A.; Crossland, L.; Pantarelli, C.; Anderson, K.E.; Hawkins, P.T.; Segonds-Pichon, A.; Oxley, D.; et al. Age-related decline in the resistance of mice to bacterial infection and in LPS/TLR4 pathway-dependent neutrophil responses. Front. Immunol. 2022, 13, 888415. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, C.; Zou, Z.; Fan, E.K.Y.; Chen, L.; Li, Y.; Billiar, T.R.; Wilson, M.A.; Shi, X.; Fan, J. Aging-related Atg5 defect impairs neutrophil extracellular traps formation. Immunology 2017, 151, 417–432. [Google Scholar] [CrossRef]
- Lutz, C.T.; Moore, M.B.; Bradley, S.; Shelton, B.J.; Lutgendorf, S.K. Reciprocal age related change in natural killer cell receptors for MHC class I. Mech. Ageing Dev. 2005, 126, 722–731. [Google Scholar] [CrossRef]
- Björkström, N.K.; Riese, P.; Heuts, F.; Andersson, S.; Fauriat, C.; Ivarsson, M.A.; Björklund, A.T.; Flodström-Tullberg, M.; Michaëlsson, J.; Rottenberg, M.E.; et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood 2010, 116, 3853–3864. [Google Scholar] [CrossRef]
- Mariani, E.; Pulsatelli, L.; Meneghetti, A.; Dolzani, P.; Mazzetti, I.; Neri, S.; Ravaglia, G.; Forti, P.; Facchini, A. Different IL-8 production by T and NK lymphocytes in elderly subjects. Mech. Ageing Dev. 2001, 122, 1383–1395. [Google Scholar] [CrossRef]
- Fionda, C.; Ruggeri, S.; Sciumè, G.; Laffranchi, M.; Quinti, I.; Milito, C.; Palange, P.; Menichini, I.; Sozzani, S.; Frati, L.; et al. Age-dependent NK cell dysfunctions in severe COVID-19 patients. Front. Immunol. 2022, 13, 1039120. [Google Scholar] [CrossRef]
- Chidrawar, S.M.; Khan, N.; Chan, Y.L.; Nayak, L.; Moss, P.A. Ageing is associated with a decline in peripheral blood CD56bright NK cells. Immun. Ageing 2006, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Le Garff-Tavernier, M.; Béziat, V.; Decocq, J.; Siguret, V.; Gandjbakhch, F.; Pautas, E.; Debré, P.; Merle-Beral, H.; Vieillard, V. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell 2010, 9, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Gounder, S.S.; Abdullah, B.J.J.; Radzuanb, N.; Zain, F.; Sait, N.B.M.; Chua, C.; Subramani, B. Effect of Aging on NK Cell Population and Their Proliferation at Ex Vivo Culture Condition. Anal. Cell. Pathol. 2018, 2018, 7871814. [Google Scholar] [CrossRef] [PubMed]
- ElGindi, M.; Sapudom, J.; Garcia Sabate, A.; Chesney Quartey, B.; Alatoom, A.; Al-Sayegh, M.; Li, R.; Chen, W.; Teo, J. Effects of an aged tissue niche on the immune potency of dendritic cells using simulated microgravity. NPJ Aging 2023, 9, 14. [Google Scholar] [CrossRef]
- Clements, S.J.; Maijo, M.; Ivory, K.; Nicoletti, C.; Carding, S.R. Age-Associated Decline in Dendritic Cell Function and the Impact of Mediterranean Diet Intervention in Elderly Subjects. Front. Nutr. 2017, 4, 65. [Google Scholar] [CrossRef]
- Sridharan, A.; Esposo, M.; Kaushal, K.; Tay, J.; Osann, K.; Agrawal, S.; Gupta, S.; Agrawal, A. Age-associated impaired plasmacytoid dendritic cell functions lead to decreased CD4 and CD8 T cell immunity. Age 2011, 33, 363–376. [Google Scholar] [CrossRef]
- Jing, Y.; Shaheen, E.; Drake, R.R.; Chen, N.; Gravenstein, S.; Deng, Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum. Immunol. 2009, 70, 777–784. [Google Scholar] [CrossRef]
- Bashir, H.; Singh, S.; Singh, R.P.; Agrewala, J.N.; Kumar, R. Age-mediated gut microbiota dysbiosis promotes the loss of dendritic cells tolerance. Aging Cell 2023, 22, e13838. [Google Scholar] [CrossRef]
- Couch, R.B.; Winokur, P.; Brady, R.; Belshe, R.; Chen, W.H.; Cate, T.R.; Sigurdardottir, B.; Hoeper, A.; Graham, I.L.; Edelman, R.; et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007, 25, 7656–7663. [Google Scholar] [CrossRef]
- Lawrence, H.; Pick, H.; Baskaran, V.; Daniel, P.; Rodrigo, C.; Ashton, D.; Edwards-Pritchard, R.C.; Sheppard, C.; Eletu, S.D.; Litt, D.; et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: A case-control test-negative design study. PLoS Med. 2020, 17, e1003326. [Google Scholar] [CrossRef] [PubMed]
- Lewnard, J.A.; Bruxvoort, K.J.; Fischer, H.; Hong, V.X.; Grant, L.R.; Jódar, L.; Cané, A.; Gessner, B.D.; Tartof, S.Y. Effectiveness of 13-Valent Pneumococcal Conjugate Vaccine Against Medically Attended Lower Respiratory Tract Infection and Pneumonia Among Older Adults. Clin. Infect. Dis. 2022, 75, 832–841. [Google Scholar] [CrossRef] [PubMed]
- McMahan, R.H.; Hulsebus, H.J.; Najarro, K.M.; Giesy, L.E.; Frank, D.N.; Orlicky, D.J.; Kovacs, E.J. Age-Related Intestinal Dysbiosis and Enrichment of Gut-specific Bacteria in the Lung Are Associated with Increased Susceptibility to Streptococcus pneumoniae Infection in Mice. Front. Aging 2022, 3, 859991. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, G.; Banerjee, N.; Liang, Y.; Du, X.; Boor, P.J.; Hoffman, K.L.; Khan, M.F. Aberrant Gut Microbiome Contributes to Intestinal Oxidative Stress, Barrier Dysfunction, Inflammation and Systemic Autoimmune Responses in MRL/lpr Mice. Front. Immunol. 2021, 12, 651191. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, Z.; Tan, Y.; Guo, Z.; Liu, Y.; Wang, Y.; Yuan, Y.; Yang, R.; Bi, Y.; Bai, Y.; et al. A novel strain of Bacteroides fragilis enhances phagocytosis and polarises M1 macrophages. Sci. Rep. 2016, 6, 29401. [Google Scholar] [CrossRef]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e437. [Google Scholar] [CrossRef]
- Hopkins, M.J.; Macfarlane, G.T. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J. Med. Microbiol. 2002, 51, 448–454. [Google Scholar] [CrossRef]
- Gill, H.S.; Rutherfurd, K.J.; Cross, M.L.; Gopal, P.K. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am. J. Clin. Nutr. 2001, 74, 833–839. [Google Scholar] [CrossRef]
- Lefevre, M.; Racedo, S.M.; Ripert, G.; Housez, B.; Cazaubiel, M.; Maudet, C.; Jüsten, P.; Marteau, P.; Urdaci, M.C. Probiotic strain Bacillus subtilis CU1 stimulates immune system of elderly during common infectious disease period: A randomized, double-blind placebo-controlled study. Immun. Ageing 2015, 12, 24. [Google Scholar] [CrossRef]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef]
- Keren, Z.; Naor, S.; Nussbaum, S.; Golan, K.; Itkin, T.; Sasaki, Y.; Schmidt-Supprian, M.; Lapidot, T.; Melamed, D. B-cell depletion reactivates B lymphopoiesis in the BM and rejuvenates the B lineage in aging. Blood 2011, 117, 3104–3112. [Google Scholar] [CrossRef] [PubMed]
- Bharath, L.P.; Agrawal, M.; McCambridge, G.; Nicholas, D.A.; Hasturk, H.; Liu, J.; Jiang, K.; Liu, R.; Guo, Z.; Deeney, J.; et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 2020, 32, 44–55.e46. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Gusewitch, G.; Warren, B.J.; Dotson, R.C.; Butterworth, D.E.; Nehlsen-Cannarella, S.L. Physical activity and immune function in elderly women. Med. Sci. Sports Exerc. 1993, 25, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.B.; Fox, O.; McNulty, C.L.; Greenwood, H.L.; Murphy, L.; Sapey, E.; Goodman, M.; Crabtree, N.; Thøgersen-Ntoumani, C.; Fisher, J.P.; et al. Habitual physical activity is associated with the maintenance of neutrophil migratory dynamics in healthy older adults. Brain Behav. Immun. 2016, 56, 12–20. [Google Scholar] [CrossRef]
- Pallast, E.G.; Schouten, E.G.; de Waart, F.G.; Fonk, H.C.; Doekes, G.; von Blomberg, B.M.; Kok, F.J. Effect of 50- and 100-mg vitamin E supplements on cellular immune function in noninstitutionalized elderly persons. Am. J. Clin. Nutr. 1999, 69, 1273–1281. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyani, P.; Christodoulou, R.; Vassiliou, E. Immunosenescence: Aging and Immune System Decline. Vaccines 2024, 12, 1314. https://doi.org/10.3390/vaccines12121314
Goyani P, Christodoulou R, Vassiliou E. Immunosenescence: Aging and Immune System Decline. Vaccines. 2024; 12(12):1314. https://doi.org/10.3390/vaccines12121314
Chicago/Turabian StyleGoyani, Priyanka, Rafail Christodoulou, and Evros Vassiliou. 2024. "Immunosenescence: Aging and Immune System Decline" Vaccines 12, no. 12: 1314. https://doi.org/10.3390/vaccines12121314
APA StyleGoyani, P., Christodoulou, R., & Vassiliou, E. (2024). Immunosenescence: Aging and Immune System Decline. Vaccines, 12(12), 1314. https://doi.org/10.3390/vaccines12121314




