Intranasal Prime–Boost with Spike Vectors Generates Antibody and T-Cell Responses at the Site of SARS-CoV-2 Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Viral Vector Design
2.3. Vesicular Stomatitis Virus Pseudotyped with SARS-CoV-2 Spike
2.4. Single-Cycle Adenovirus Expressing SARS-CoV-2 Spike
2.5. Virus Titration by TCID50
2.6. Western Blotting
2.7. Anti-Viral Protein Assay
2.8. In Vivo Studies
2.9. Sample Collection
2.10. Anti-Spike Antibody ELISA
2.11. Flow Cytometry
2.12. Statistical Analysis
3. Results
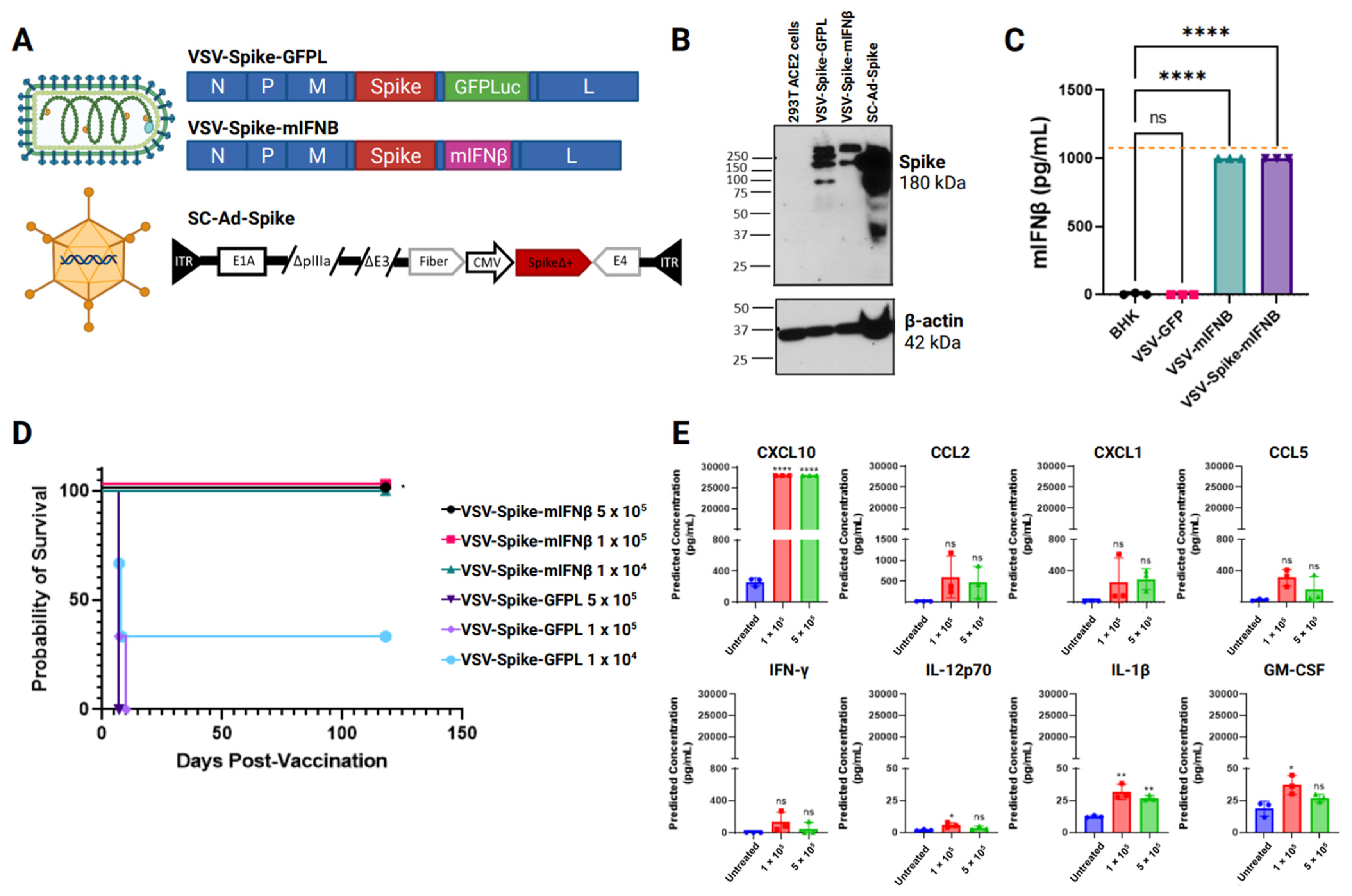
3.1. Safety and Immunogenicity of Intranasal Delivery of Viral Vaccines Expressing SARS-CoV-2 Spike
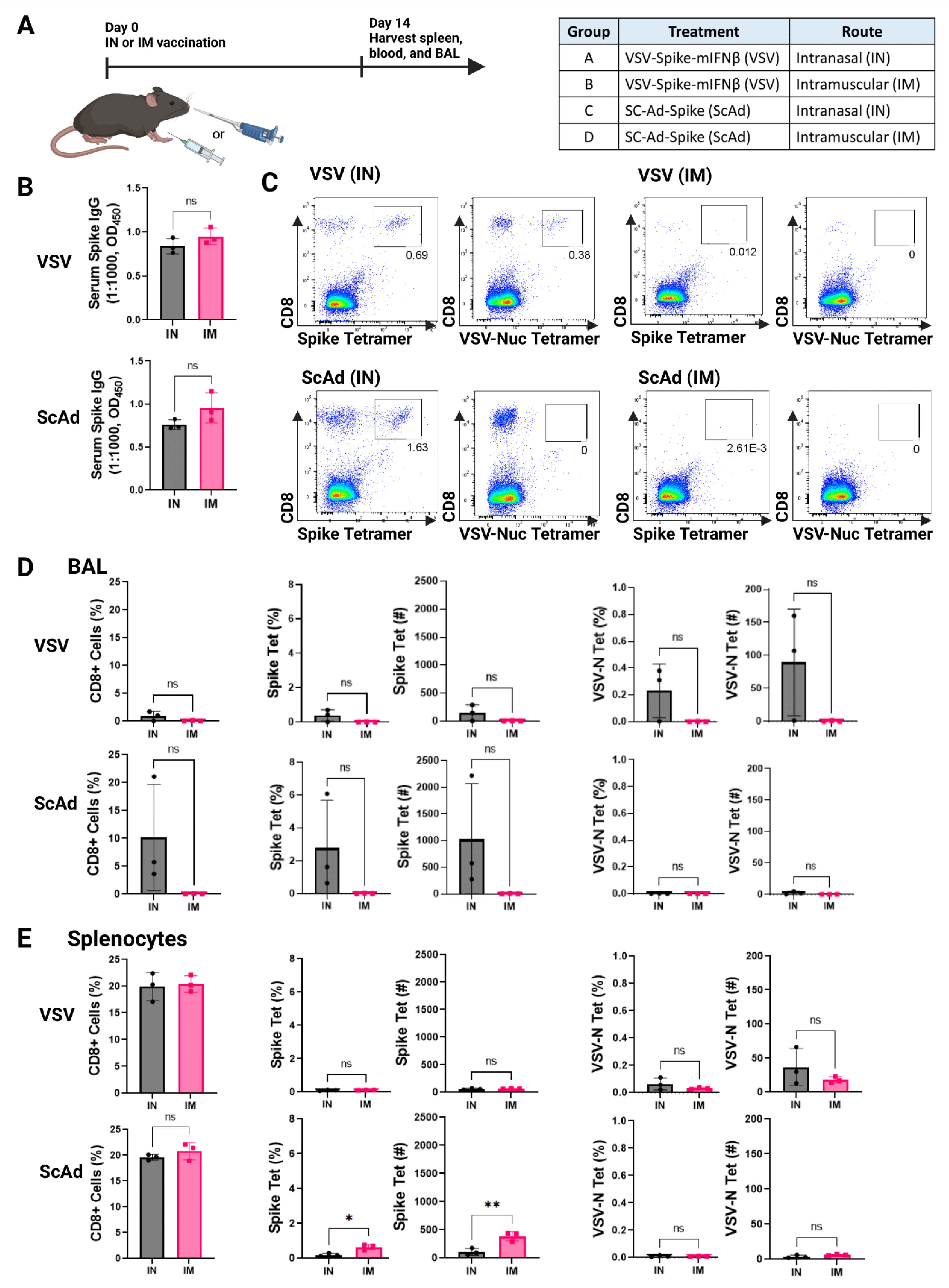
3.2. Intranasal, but Not Intramuscular, Delivery of Viruses Induces T-Cell Responses in the Lung Airways
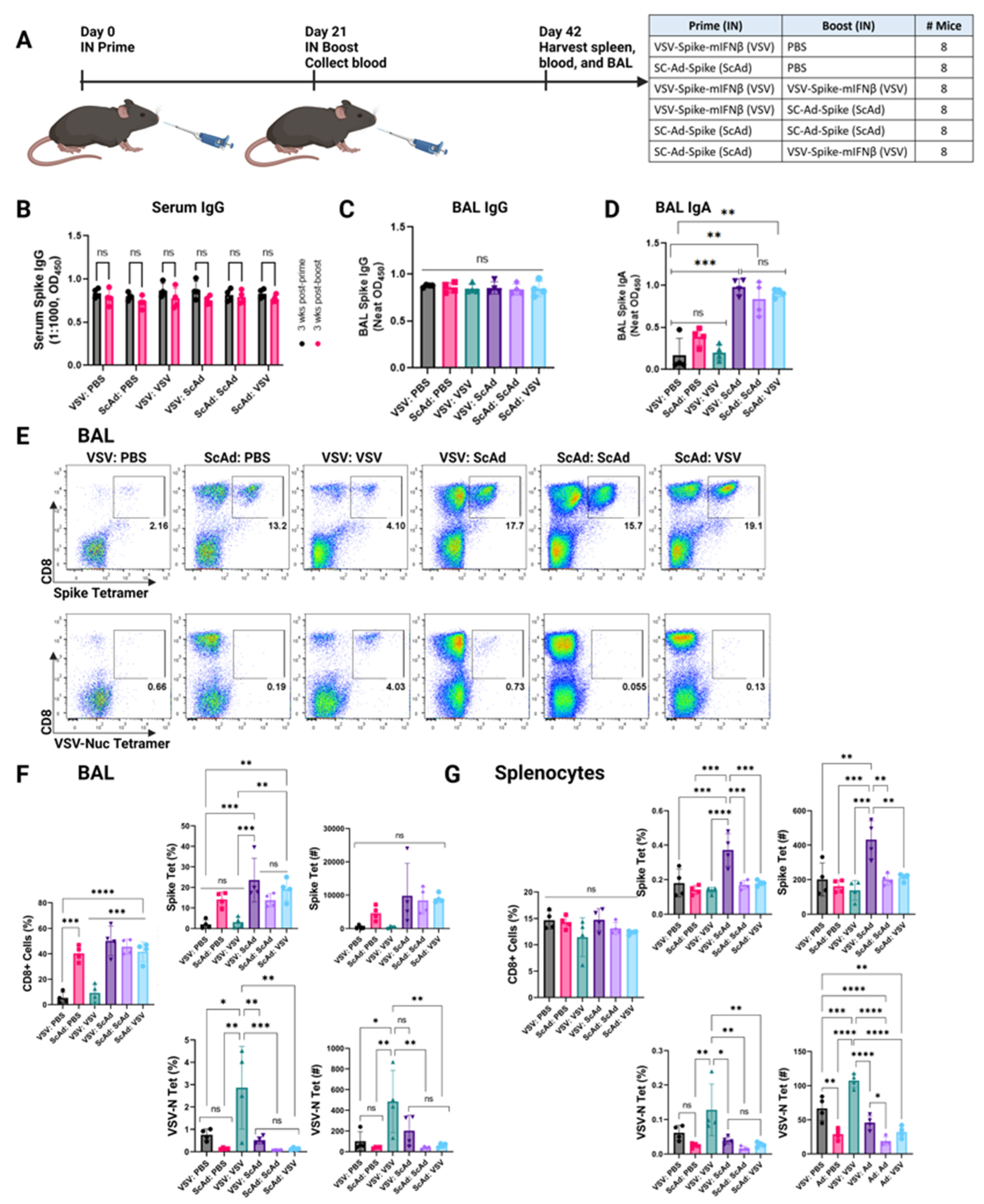
3.3. SC-Ad-Spike Induces Anti-Spike IgA Response in a Homologous Prime and Boost Regimen
3.4. SC-Ad-Spike Induces Anti-Spike CD8+ T-Cell Responses in the Lungs Independent of Prime and Boost Regimens
3.5. Anti-Spike Ab and T-Cell Responses Decrease with the Age of the Host
3.6. In Boosting Significantly Enhances IgA and CD8+ T-Cell Responses Compared to Intramuscular Boosting
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GeurtsvanKessel, C.H.; Geers, D.; Schmitz, K.S.; Mykytyn, A.Z.; Lamers, M.M.; Bogers, S.; Scherbeijn, S.; Gommers, L.; Sablerolles, R.S.G.; Nieuwkoop, N.N.; et al. Divergent SARS-CoV-2 Omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci. Immunol. 2022, 7, eabo2202. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Nathan, A.; Kaseke, C.; Berrios, C.; Khatri, A.; Choi, S.; Getz, M.A.; Tano-Menka, R.; Ofoman, O.; Gayton, A.; et al. T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals. Cell 2022, 185, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, H.; Jiménez, V.C.; Sitaras, I.; Bar-Zeev, N.; Čičin-Šain, L.; Higdon, M.M.; Deloria-Knoll, M. Post-vaccination T cell immunity to omicron. Front. Immunol. 2022, 13, 944713. [Google Scholar] [CrossRef]
- Lu, S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009, 21, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, N.; Ndiaye, B.P.; Bowyer, G.; Wade, D.; Sridhar, S.; Wright, D.; Powlson, J.; Ndiaye, I.; Dièye, S.; Thompson, C.; et al. Safety and Immunogenicity of a Heterologous Prime-Boost Ebola Virus Vaccine Regimen in Healthy Adults in the United Kingdom and Senegal. J. Infect. Dis. 2019, 219, 1187–1197. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Cauley, L.S.; Lefrançois, L. Guarding the perimeter: Protection of the mucosa by tissue-resident memory T cells. Mucosal Immunol. 2012, 6, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Matchett, W.E.; Anguiano-Zarate, S.S.; Nehete, P.N.; Shelton, K.; Nehete, B.P.; Yang, G.; Dorta-Estremera, S.; Barnette, P.; Xiao, P.; Byrareddy, S.N.; et al. Divergent HIV-1-Directed Immune Responses Generated by Systemic and Mucosal Immunization with Replicating Single-Cycle Adenoviruses in Rhesus Macaques. J. Virol. 2019, 93, 10–1128. [Google Scholar] [CrossRef]
- Matchett, W.E.; Malewana, G.B.R.; Mudrick, H.; Medlyn, M.J.; Barry, M.A. Genetic Adjuvants in Replicating Single-Cycle Adenovirus Vectors Amplify Systemic and Mucosal Immune Responses against HIV-1 Envelope. Vaccines 2020, 8, 64. [Google Scholar] [CrossRef]
- Weaver, E.A.; Nehete, P.N.; Nehete, B.P.; Yang, G.; Buchl, S.J.; Hanley, P.W.; Palmer, D.; Montefiori, D.C.; Ferrari, G.; Ng, P.; et al. Comparison of systemic and mucosal immunization with helper-dependent adenoviruses for vaccination against mucosal challenge with SHIV. PLoS ONE 2013, 8, e67574. [Google Scholar] [CrossRef]
- Correa, V.A.; Portilho, A.I.; De Gaspari, E. Vaccines, adjuvants and key factors for mucosal immune response. Immunology 2022, 167, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Vanaparthy, R.; Mohan, G.; Vasireddy, D.; Atluri, P. Review of COVID-19 viral vector-based vaccines and COVID-19 variants. Le Infez. Med. 2021, 29, 328–338. [Google Scholar] [CrossRef]
- Mudrick, H.E.; Massey, S.; McGlinch, E.B.; Parrett, B.J.; Hemsath, J.R.; Barry, M.E.; Rubin, J.D.; Uzendu, C.; Hansen, M.J.; Erskine, C.L.; et al. Comparison of replicating and nonreplicating vaccines against SARS-CoV-2. Sci. Adv. 2022, 8, eabm8563. [Google Scholar] [CrossRef] [PubMed]
- Crosby, C.M.; Barry, M.A. IIIa deleted adenovirus as a single-cycle genome replicating vector. Virology 2014, 462, 158–165. [Google Scholar] [CrossRef]
- Crosby, C.M.; Barry, M.A. Transgene Expression and Host Cell Responses to Replication-Defective, Single-Cycle, and Replication-Competent Adenovirus Vectors. Genes 2017, 8, 79. [Google Scholar] [CrossRef]
- Crosby, C.M.; Nehete, P.; Sastry, K.J.; Barry, M.A. Amplified and persistent immune responses generated by single-cycle replicating adenovirus vaccines. J. Virol. 2015, 89, 669–675. [Google Scholar] [CrossRef]
- Matchett, W.E.; Anguiano-Zarate, S.S.; Barry, M.A. Comparison of systemic and mucosal immunization with replicating Single cycle Adenoviruses. Glob. Vaccines Immunol. 2018, 3. [Google Scholar] [CrossRef][Green Version]
- Regules, J.A.; Beigel, J.H.; Paolino, K.M.; Voell, J.; Castellano, A.R.; Hu, Z.; Muñoz, P.; Moon, J.E.; Ruck, R.C.; Bennett, J.W.; et al. A Recombinant Vesicular Stomatitis Virus Ebola Vaccine. N. Engl. J. Med. 2017, 376, 330–341. [Google Scholar] [CrossRef]
- Woolsey, C.; Geisbert, T.W. Current state of Ebola virus vaccines: A snapshot. PLoS Pathog. 2021, 17, e1010078. [Google Scholar] [CrossRef]
- Suder, E.; Furuyama, W.; Feldmann, H.; Marzi, A.; de Wit, E. The vesicular stomatitis virus-based Ebola virus vaccine: From concept to clinical trials. Hum. Vaccines Immunother. 2018, 14, 2107–2113. [Google Scholar] [CrossRef]
- Case, J.B.; Rothlauf, P.W.; Chen, R.E.; Liu, Z.; Zhao, H.; Kim, A.S.; Bloyet, L.M.; Zeng, Q.; Tahan, S.; Droit, L.; et al. Neutralizing Antibody and Soluble ACE2 Inhibition of a Replication-Competent VSV-SARS-CoV-2 and a Clinical Isolate of SARS-CoV-2. Cell Host Microbe 2020, 28, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, M.E.; Haslwanter, D.; Bortz, R.H., 3rd; Wirchnianski, A.S.; Lasso, G.; Vergnolle, O.; Abbasi, S.A.; Fels, J.M.; Laudermilch, E.; Florez, C.; et al. A Replication-Competent Vesicular Stomatitis Virus for Studies of SARS-CoV-2 Spike-Mediated Cell Entry and Its Inhibition. Cell Host Microbe 2020, 28, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Weisblum, Y.; Muecksch, F.; Hoffmann, H.H.; Michailidis, E.; Lorenzi, J.C.C.; Mendoza, P.; Rutkowska, M.; Bednarski, E.; Gaebler, C.; et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J. Exp. Med. 2020, 21, e20201181. [Google Scholar] [CrossRef]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef] [PubMed]
- Koutsakos, M.; Turner, J.S.; Guillamet, M.C.V.; Reynolds, D.; Lei, T.; Byers, D.; Ellebedy, A.H.; Mudd, P. Assessment of antibodies in the upper and lower human respiratory tract at steady state and after respiratory viral infection. Clin. Transl. Immunol. 2023, 12, e1460. [Google Scholar] [CrossRef]
- Adler, J.M.; Martin Vidal, R.; Langner, C.; Vladimirova, D.; Abdelgawad, A.; Kunecova, D.; Lin, X.; Nouailles, G.; Voss, A.; Kunder, S.; et al. An intranasal live-attenuated SARS-CoV-2 vaccine limits virus transmission. Nat. Commun. 2024, 15, 995. [Google Scholar] [CrossRef]
- Dhama, K.; Dhawan, M.; Tiwari, R.; Bin Emran, T.; Mitra, S.; Rabaan, A.A.; Alhumaid, S.; Al Alawi, Z.; Al Mutair, A. COVID-19 intranasal vaccines: Current progress, advantages, prospects, and challenges. Hum. Vaccines Immunother. 2022, 18, 2045853. [Google Scholar] [CrossRef] [PubMed]
- Willmon, C.; Diaz, R.M.; Wongthida, P.; Galivo, F.; Kottke, T.; Thompson, J.; Albelda, S.; Harrington, K.; Melcher, A.; Vile, R. Vesicular stomatitis virus-induced immune suppressor cells generate antagonism between intratumoral oncolytic virus and cyclophosphamide. Mol. Ther. 2011, 19, 140–149. [Google Scholar] [CrossRef]
- Willmon, C.L.; Saloura, V.; Fridlender, Z.G.; Wongthida, P.; Diaz, R.M.; Thompson, J.; Kottke, T.; Federspiel, M.; Barber, G.; Albelda, S.M.; et al. Expression of IFN-beta enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma. Caner Res. 2009, 69, 7713–7720. [Google Scholar] [CrossRef]
- Wongthida, P.; Diaz, R.M.; Galivo, F.; Kottke, T.; Thompson, J.; Melcher, A.; Vile, R. VSV oncolytic virotherapy in the B16 model depends upon intact MyD88 signaling. Mol. Ther. 2011, 19, 150–158. [Google Scholar] [CrossRef]
- Stojdl, D.F.; Lichty, B.; Knowles, S.; Marius, R.; Atkins, H.; Sonenberg, N.; Bell, J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000, 6, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Stojdl, D.F.; Lichty, B.D.; Tenoever, B.R.; Paterson, J.M.; Power, A.T.; Knowles, S.; Marius, R.; Reynard, J.; Poliquin, L.; Atkins, H.; et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 2003, 4, 263–275. [Google Scholar] [CrossRef]
- Obuchi, M.; Fernandez, M.; Barber, G.N. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J. Virol. 2003, 77, 8843–8856. [Google Scholar] [CrossRef] [PubMed]
- Jenks, N.; Myers, R.; Greiner, S.M.; Thompson, J.; Mader, E.K.; Greenslade, A.; Griesmann, G.E.; Federspiel, M.J.; Rakela, J.; Borad, M.J.; et al. Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-beta in rodents and nonhuman primates. Hum. Gene Ther. 2010, 21, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Durham, N.M.; Mulgrew, K.; McGlinchey, K.; Monks, N.R.; Ji, H.; Herbst, R.; Suzich, J.; Hammond, S.A.; Kelly, E.J. Oncolytic VSV Primes Differential Responses to Immuno-oncology Therapy. Mol. Ther. 2017, 25, 1917–1932. [Google Scholar] [CrossRef]
- Anguiano-Zarate, S.S.; Matchett, W.; Nehete, P.N.; Sastry, J.K.; Marzi, A.; Barry, M. A Replicating Single-Cycle Adenovirus Vaccine Against Ebola Virus. J. Infect. Dis. 2018, 218, 1883–1889. [Google Scholar] [CrossRef]
- Boyaka, P.N. Inducing Mucosal IgA: A Challenge for Vaccine Adjuvants and Delivery Systems. J. Immunol. 2017, 199, 9–16. [Google Scholar] [CrossRef]
- McBride, C.E.; Li, J.; Machamer, C.E. The cytoplasmic tail of the severe acute respiratory syndrome coronavirus spike protein contains a novel endoplasmic reticulum retrieval signal that binds COPI and promotes interaction with membrane protein. J. Virol. 2007, 81, 2418–2428. [Google Scholar] [CrossRef]
- Whelan, S.P.; Ball, L.; Barr, J.N.; Wertz, G.T. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc. Natl. Acad. Sci. USA 1995, 92, 8388–8392. [Google Scholar] [CrossRef]
- Schnell, M.J.; Buonocore, L.; Whitt, M.; Rose, J.K. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J. Virol. 1996, 70, 2318–2323. [Google Scholar] [CrossRef]
- Mahy, B.W.J.; Kangro, H.O. Virology Methods Manual; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Spearman, C. The method of “Right and Wrong Cases” (Constant Stimuli) without Gauss’s formulae. Br. J. Psychol. 1908, 2, 227–242. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Pathol. Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Kurisetty, V.V.S.; Heiber, J.; Myers, R.; Pereira, G.S.; Goodwin, J.W.; Federspiel, M.J.; Russell, S.J.; Peng, K.W.; Barber, G.; Merchan, J.R. Preclinical safety and activity of recombinant VSV-IFN-β in an immunocompetent model of squamous cell carcinoma of the head and neck. Head Neck 2014, 36, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Saloura, V.; Wang, L.-C.S.; Fridlender, Z.G.; Sun, J.; Cheng, G.; Kapoor, V.; Sterman, D.H.; Harty, R.N.; Okumura, A.; Barber, G.N.; et al. Evaluation of an attenuated vesicular stomatitis virus vector expressing interferon-beta for use in malignant pleural mesothelioma: Heterogeneity in interferon responsiveness defines potential efficacy. Hum. Gene Ther. 2010, 21, 51–64. [Google Scholar] [CrossRef]
- Hastie, E.; Grdzelishvili, V.Z. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J. Gen. Virol. 2012, 93, 2529–2545. [Google Scholar] [CrossRef]
- Pol, J.G.; Workenhe, S.T.; Konda, P.; Gujar, S.; Kroemer, G. Cytokines in oncolytic virotherapy. Cytokine Growth Factor Rev. 2020, 56, 4–27. [Google Scholar] [CrossRef]
- Kramer, M.; Dennin, R.; Kramer, C.; Jones, G.; Connell, E.; Rolon, N.; Gruarin, A.; Kale, R.; Trown, P. Cell and virus sensitivity studies with recombinant human alpha interferons. J. Interf. Res. 1983, 3, 425–435. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metko, M.; Tonne, J.; Veliz Rios, A.; Thompson, J.; Mudrick, H.; Masopust, D.; Diaz, R.M.; Barry, M.A.; Vile, R.G. Intranasal Prime–Boost with Spike Vectors Generates Antibody and T-Cell Responses at the Site of SARS-CoV-2 Infection. Vaccines 2024, 12, 1191. https://doi.org/10.3390/vaccines12101191
Metko M, Tonne J, Veliz Rios A, Thompson J, Mudrick H, Masopust D, Diaz RM, Barry MA, Vile RG. Intranasal Prime–Boost with Spike Vectors Generates Antibody and T-Cell Responses at the Site of SARS-CoV-2 Infection. Vaccines. 2024; 12(10):1191. https://doi.org/10.3390/vaccines12101191
Chicago/Turabian StyleMetko, Muriel, Jason Tonne, Alexa Veliz Rios, Jill Thompson, Haley Mudrick, David Masopust, Rosa Maria Diaz, Michael A. Barry, and Richard G. Vile. 2024. "Intranasal Prime–Boost with Spike Vectors Generates Antibody and T-Cell Responses at the Site of SARS-CoV-2 Infection" Vaccines 12, no. 10: 1191. https://doi.org/10.3390/vaccines12101191
APA StyleMetko, M., Tonne, J., Veliz Rios, A., Thompson, J., Mudrick, H., Masopust, D., Diaz, R. M., Barry, M. A., & Vile, R. G. (2024). Intranasal Prime–Boost with Spike Vectors Generates Antibody and T-Cell Responses at the Site of SARS-CoV-2 Infection. Vaccines, 12(10), 1191. https://doi.org/10.3390/vaccines12101191






