Immunogenicity and Determinants of Antibody Response to the BNT162b2 mRNA Vaccine: A Longitudinal Study in a Cohort of People Living with HIV
Abstract
1. Introduction
2. Materials and Methods
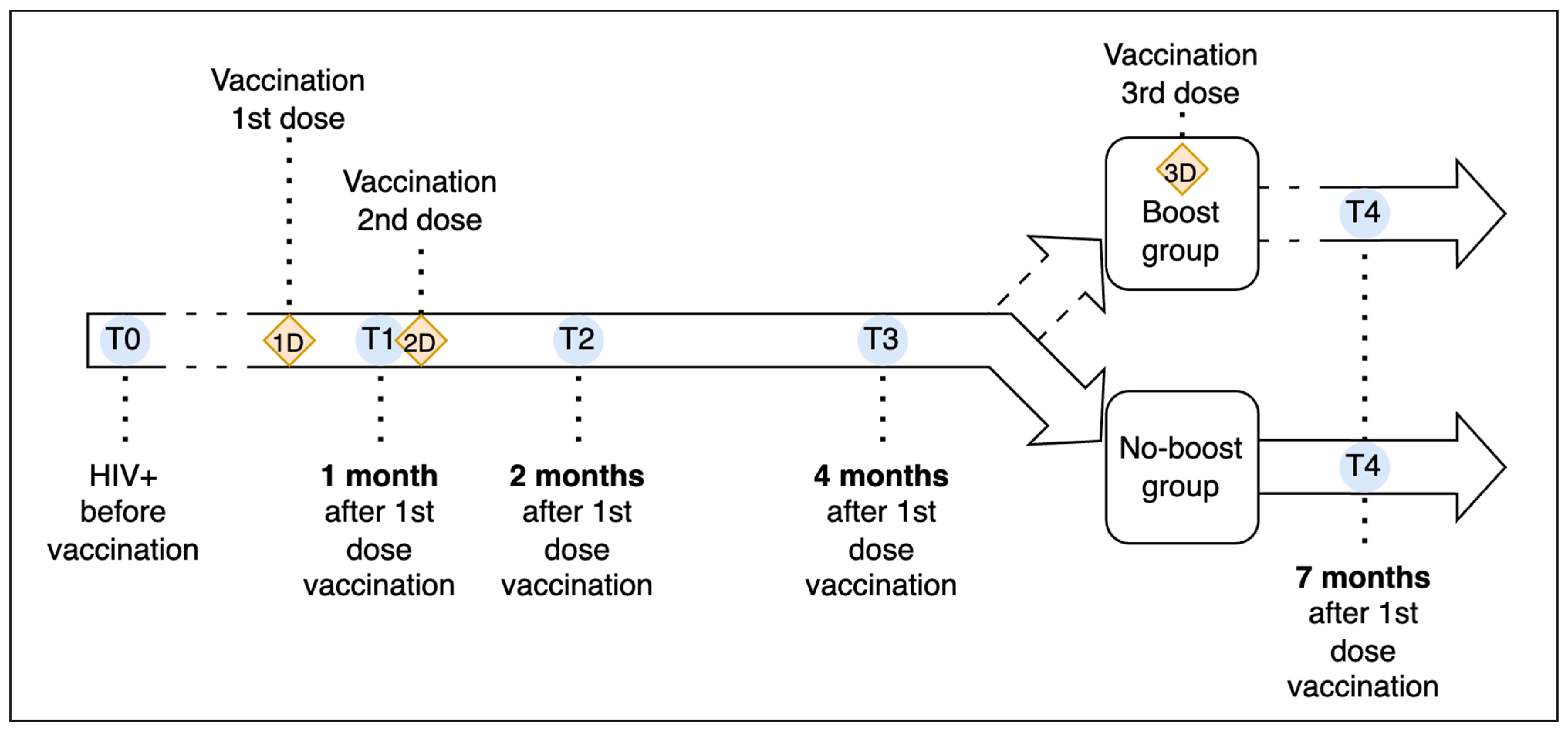
2.1. Study Design
2.2. Laboratory Methods
2.3. Data Analysis
3. Results
3.1. Study Population
3.2. Immunogenicity and Antibody Response
3.3. Impact of Booster Dose
3.4. CD4+ Count and HIV Viral Load
3.5. Predictors of Anti-S IgG Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- WHO. Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/ (accessed on 5 October 2024).
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A Comprehensive Review of SARS-CoV-2 Vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccin. Immunother. 2022, 18, 2002083. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.Y.; Li, J.X.; Hu, Y.M.; Hu, Y.S.; Zeng, G.; Zhu, F.C. Quadrivalent Influenza Vaccine (Sinovac Biotech) for Seasonal Influenza Prophylaxis. Expert. Rev. Vaccines 2021, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Frater, J.; Ewer, K.J.; Ogbe, A.; Pace, M.; Adele, S.; Adland, E.; Alagaratnam, J.; Aley, P.K.; Ali, M.; Ansari, M.A.; et al. Safety and Immunogenicity of the ChAdOx1 NCoV-19 (AZD1222) Vaccine against SARS-CoV-2 in HIV Infection: A Single-Arm Substudy of a Phase 2/3 Clinical Trial. Lancet HIV 2021, 8, e474–e485. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Koen, A.L.; Izu, A.; Fairlie, L.; Cutland, C.L.; Baillie, V.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Immunogenicity of the ChAdOx1 NCoV-19 (AZD1222) Vaccine against SARS-CoV-2 in People Living with and without HIV in South Africa: An Interim Analysis of a Randomised, Double-Blind, Placebo-Controlled, Phase 1B/2A Trial. Lancet HIV 2021, 8, e568–e580. [Google Scholar] [CrossRef] [PubMed]
- Woldemeskel, B.A.; Karaba, A.H.; Garliss, C.C.; Beck, E.J.; Wang, K.H.; Laeyendecker, O.; Cox, A.L.; Blankson, J.N. The BNT162b2 MRNA Vaccine Elicits Robust Humoral and Cellular Immune Responses in People Living With Human Immunodeficiency Virus (HIV). Clin. Infect. Dis. 2022, 74, 1268–1270. [Google Scholar] [CrossRef]
- Jakharia, N.; Subramanian, A.K.; Shapiro, A.E. COVID-19 in the Immunocompromised Host, Including People with Human Immunodeficiency Virus. Infect. Dis. Clin. N. Am. 2022, 36, 397–421. [Google Scholar] [CrossRef]
- Napuri, N.I.; Curcio, D.; Swerdlow, D.L.; Srivastava, A. Immune Response to COVID-19 and MRNA Vaccination in Immunocompromised Individuals: A Narrative Review. Infect. Dis. Ther. 2022, 11, 1391–1414. [Google Scholar] [CrossRef]
- Bin Lee, A.R.Y.; Wong, S.Y.; Chai, L.Y.A.; Lee, S.C.; Lee, M.X.; Muthiah, M.D.; Tay, S.H.; Teo, C.B.; Tan, B.K.J.; Chan, Y.H.; et al. Efficacy of Covid-19 Vaccines in Immunocompromised Patients: Systematic Review and Meta-Analysis. BMJ 2022, 376, e068632. [Google Scholar] [CrossRef]
- Marra, A.R.; Kobayashi, T.; Suzuki, H.; Alsuhaibani, M.; Hasegawa, S.; Tholany, J.; Perencevich, E.; Maezato, A.M.; Ricardo, V.C.V.; Salinas, J.L.; et al. The Effectiveness of Coronavirus Disease 2019 (COVID-19) Vaccine in the Prevention of Post–COVID-19 Conditions: A Systematic Literature Review and Meta-Analysis. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e192. [Google Scholar] [CrossRef]
- Song, Q.; Bates, B.; Shao, Y.R.; Hsu, F.C.; Liu, F.; Madhira, V.; Mitra, A.K.; Bergquist, T.; Kavuluru, R.; Li, X.; et al. Risk and Outcome of Breakthrough COVID-19 Infections in Vaccinated Patients With Cancer: Real-World Evidence From the National COVID Cohort Collaborative. J. Clin. Oncol. 2022, 40, 1414–1427. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, M.; Trunfio, M.; Sasset, L.; Leoni, D.; Castelli, E.; Lo Menzo, S.; Gardin, S.; Putaggio, C.; Brundu, M.; Garzotto, P.; et al. Factors Associated with Severe COVID-19 and Post-Acute COVID-19 Syndrome in a Cohort of People Living with HIV on Antiretroviral Treatment and with Undetectable HIV RNA. Viruses 2022, 14, 493. [Google Scholar] [CrossRef] [PubMed]
- Augello, M.; Bono, V.; Rovito, R.; Tincati, C.; Marchetti, G. Immunologic Interplay Between HIV/AIDS and COVID-19: Adding Fuel to the Flames? Curr. HIV/AIDS Rep. 1904, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Clinical Features and Prognostic Factors of COVID-19 in People Living with HIV Hospitalized with Suspected or Confirmed SARS-CoV-2 Infection; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Ssentongo, P.; Heilbrunn, E.S.; Ssentongo, A.E.; Advani, S.; Chinchilli, V.M.; Nunez, J.J.; Du, P. Epidemiology and Outcomes of COVID-19 in HIV-Infected Individuals: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 6283. [Google Scholar] [CrossRef]
- Boffito, M.; Waters, L. More Evidence for Worse COVID-19 Outcomes in People with HIV. Lancet HIV 2021, 8, e661. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors Associated with COVID-19-Related Death Using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Danwang, C.; Noubiap, J.J.; Robert, A.; Yombi, J.C. Outcomes of Patients with HIV and COVID-19 Co-Infection: A Systematic Review and Meta-Analysis. AIDS Res. Ther. 2022, 19, 3. [Google Scholar] [CrossRef]
- Meckiff, B.J.; Ramírez-Suástegui, C.; Fajardo, V.; Chee, S.J.; Kusnadi, A.; Simon, H.; Eschweiler, S.; Grifoni, A.; Pelosi, E.; Weiskopf, D.; et al. Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4+ T Cells in COVID-19. Cell 2020, 183, 1340–1353. [Google Scholar] [CrossRef]
- Ambrosioni, J.; Blanco, J.L.; Reyes-Urueña, J.M.; Davies, M.A.; Sued, O.; Marcos, M.A.; Martínez, E.; Bertagnolio, S.; Alcamí, J.; Miro, J.M.; et al. Overview of SARS-CoV-2 Infection in Adults Living with HIV. Lancet HIV 2021, 8, e294–e305. [Google Scholar] [CrossRef]
- Nomah, D.K.; Reyes-Urueña, J.; Díaz, Y.; Moreno, S.; Aceiton, J.; Bruguera, A.; Vivanco-Hidalgo, R.M.; Llibre, J.M.; Domingo, P.; Falcó, V.; et al. Sociodemographic, Clinical, and Immunological Factors Associated with SARS-CoV-2 Diagnosis and Severe COVID-19 Outcomes in People Living with HIV: A Retrospective Cohort Study. Lancet HIV 2021, 8, e701–e710. [Google Scholar] [CrossRef]
- Augello, M.; Bono, V.; Rovito, R.; Tincati, C.; D’Arminio Monforte, A.; Marchetti, G. Six-Month Immune Responses to MRNA-1273 Vaccine in Combination Antiretroviral Therapy Treated Late Presenter People with HIV According to Previous SARS-CoV-2 Infection. AIDS 2023, 37, 1503–1517. [Google Scholar] [CrossRef] [PubMed]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global Impact of the First Year of COVID-19 Vaccination: A Mathematical Modelling Study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Shoham, S.; Batista, C.; Ben Amor, Y.; Ergonul, O.; Hassanain, M.; Hotez, P.; Kang, G.; Kim, J.H.; Lall, B.; Larson, H.J.; et al. Vaccines and Therapeutics for Immunocompromised Patients with COVID-19. eClinicalMedicine 2023, 59, 101965. [Google Scholar] [CrossRef] [PubMed]
- El Chaer, F.; El Sahly, H.M. Vaccination in the Adult Patient Infected with HIV: A Review of Vaccine Efficacy and Immunogenicity. Am. J. Med. 2019, 132, 437–446. [Google Scholar] [CrossRef]
- Levy, I.; Wieder-Finesod, A.; Litchevsky, V.; Biber, A.; Indenbaum, V.; Olmer, L.; Huppert, A.; Mor, O.; Goldstein, M.; Levin, E.G.; et al. Immunogenicity and Safety of the BNT162b2 MRNA COVID-19 Vaccine in People Living with HIV-1. Clin. Microbiol. Infect. 2021, 27, 1851–1855. [Google Scholar] [CrossRef]
- Kernéis, S.; Launay, O.; Turbelin, C.; Batteux, F.; Hanslik, T.; Boëlle, P.Y. Long-Term Immune Responses to Vaccination in HIV-Infected Patients: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2014, 58, 1130–1139. [Google Scholar] [CrossRef]
- Geretti, A.M.; Brook, G.; Cameron, C.; Chadwick, D.; French, N.; Heyderman, R.; Ho, A.; Hunter, M.; Ladhani, S.; Lawton, M.; et al. British HIV Association Guidelines on the Use of Vaccines in HIV-Positive Adults 2015. HIV Med. 2016, 17 (Suppl. S3), s2–s81. [Google Scholar] [CrossRef]
- Mena, G.; García-Basteiro, A.L.; Bayas, J.M. Hepatitis B and A Vaccination in HIV-Infected Adults: A Review. Hum. Vaccin. Immunother. 2015, 11, 2582–2598. [Google Scholar] [CrossRef]
- George, V.K.; Pallikkuth, S.; Parmigiani, A.; Alcaide, M.; Fischl, M.; Arheart, K.L.; Pahwa, S. HIV Infection Worsens Age-Associated Defects in Antibody Responses to Influenza Vaccine. J. Infect. Dis. 2015, 211, 1959–1968. [Google Scholar] [CrossRef]
- Noe, S.; Ochana, N.; Wiese, C.; Schabaz, F.; Von Krosigk, A.; Heldwein, S.; Rasshofer, R.; Wolf, E.; Jonsson-Oldenbuettel, C. Humoral Response to SARS-CoV-2 Vaccines in People Living with HIV. Infection 2022, 50, 617–623. [Google Scholar] [CrossRef]
- Brumme, Z.L.; Mwimanzi, F.; Lapointe, H.R.; Cheung, P.K.; Sang, Y.; Duncan, M.C.; Yaseen, F.; Agafitei, O.; Ennis, S.; Ng, K.; et al. Humoral Immune Responses to COVID-19 Vaccination in People Living with HIV Receiving Suppressive Antiretroviral Therapy. NPJ Vaccines 2022, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Vergori, A.; Cozzi-Lepri, A.; Matusali, G.; Cicalini, S.; Bordoni, V.; Meschi, S.; Mazzotta, V.; Colavita, F.; Fusto, M.; Cimini, E.; et al. Long Term Assessment of Anti-SARS-CoV-2 Immunogenicity after MRNA Vaccine in Persons Living with HIV. Vaccines 2023, 11, 1739. [Google Scholar] [CrossRef]
- Touizer, E.; Alrubayyi, A.; Ford, R.; Hussain, N.; Gerber, P.P.; Shum, H.L.; Rees-Spear, C.; Muir, L.; Gea-Mallorquí, E.; Kopycinski, J.; et al. Attenuated Humoral Responses in HIV after SARS-CoV-2 Vaccination Linked to B Cell Defects and Altered Immune Profiles. iScience 2023, 26, 105862. [Google Scholar] [CrossRef]
- Kang, L.; Shang, W.; Gao, P.; Wang, Y.; Liu, J.; Liu, M. Immunogenicity and Safety of COVID-19 Vaccines among People Living with HIV: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1569. [Google Scholar] [CrossRef]
- Milano, E.; Ricciardi, A.; Casciaro, R.; Pallara, E.; De Vita, E.; Bavaro, D.F.; Larocca, A.M.V.; Stefanizzi, P.; Tafuri, S.; Saracino, A. Immunogenicity and Safety of the BNT162b2 COVID-19 MRNA Vaccine in PLWH: A Monocentric Study in Bari, Italy. J. Med. Virol. 2022, 94, 2230–2236. [Google Scholar] [CrossRef]
- EACS—European AIDS Clinical Society. EACS Guidelines—Version 10.0 November 20; EACS: Brussels, Belgium, 2020. [Google Scholar]
- Pfizer Manufacturing Belgium. NV PFIZER-BIONTECH COVID-19 VACCINE—Bnt162b2 Injection, Suspension. Available online: https://labeling.pfizer.com/ShowLabeling.aspx?id=14471 (accessed on 13 December 2023).
- Ministero della Salute. Direzione Generale Della Prevenzione Sanitaria Circolare, Ministero Della Salute n. 43604 Del 27-09-2021. 2021. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=82953&parte=1%20&serie=null (accessed on 10 October 2024).
- Parodi, E.; Jones, G.; Maclean, W. Italy Approves Booster COVID-19 Shots for Vulnerable Groups|Reuters. Available online: https://www.reuters.com/world/europe/italy-approves-booster-covid-19-shots-vulnerable-groups-2021-09-09/ (accessed on 13 December 2023).
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody Responses to the BNT162b2 MRNA Vaccine in Individuals Previously Infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Speich, B.; Chammartin, F.; Abela, I.A.; Amico, P.; Stoeckle, M.P.; Eichenberger, A.L.; Hasse, B.; Braun, D.L.; Schuurmans, M.M.; Müller, T.F.; et al. Antibody Response in Immunocompromised Patients after the Administration of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine BNT162b2 or MRNA-1273: A Randomized Controlled Trial. Clin. Infect. Dis. 2022, 75, E585–E593. [Google Scholar] [CrossRef]
- Yin, J.; Chen, Y.; Li, Y.; Wang, C.; Zhang, X. Immunogenicity and Efficacy of COVID-19 Vaccines in People Living with HIV: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2022, 124, 212–223. [Google Scholar] [CrossRef]
- Portillo, V.; Fedeli, C.; Ustero Alonso, P.; Petignat, I.; Mereles Costa, E.C.; Sulstarova, A.; Jaksic, C.; Yerly, S.; Calmy, A. Impact on HIV-1 RNA Levels and Antibody Responses Following SARS-CoV-2 Vaccination in HIV-Infected Individuals. Front. Immunol. 2022, 12, 820126. [Google Scholar] [CrossRef]
- Vergori, A.; Cozzi Lepri, A.; Cicalini, S.; Matusali, G.; Bordoni, V.; Lanini, S.; Meschi, S.; Iannazzo, R.; Mazzotta, V.; Colavita, F.; et al. Immunogenicity to COVID-19 MRNA Vaccine Third Dose in People Living with HIV. Nat. Commun. 2022, 13, 4922. [Google Scholar] [CrossRef] [PubMed]
- Gianserra, L.; Donà, M.G.; Giuliani, E.; Stingone, C.; Pontone, M.; Buonomini, A.R.; Giuliani, M.; Pimpinelli, F.; Morrone, A.; Latini, A. Immunogenicity and Safety of BNT162b2 Homologous Booster Vaccination in People Living with HIV under Effective CART. Vaccines 2022, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- Fowokan, A.; Samji, H.; Puyat, J.H.; Janjua, N.Z.; Wilton, J.; Wong, J.; Grennan, T.; Chambers, C.; Kroch, A.; Costiniuk, C.T.; et al. Effectiveness of COVID-19 Vaccines in People Living with HIV in British Columbia and Comparisons with a Matched HIV-Negative Cohort: A Test-Negative Design. Int. J. Infect. Dis. 2023, 127, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Lamacchia, G.; Salvati, L.; Kiros, S.T.; Mazzoni, A.; Vanni, A.; Capone, M.; Carnasciali, A.; Farahvachi, P.; Lagi, F.; Di Lauria, N.; et al. Fourth Dose of MRNA COVID-19 Vaccine Transiently Reactivates Spike-Specific Immunological Memory in People Living with HIV (PLWH). Biomedicines 2022, 10, 3261. [Google Scholar] [CrossRef]
- Pérez-Alós, L.; Armenteros, J.J.A.; Madsen, J.R.; Hansen, C.B.; Jarlhelt, I.; Hamm, S.R.; Heftdal, L.D.; Pries-Heje, M.M.; Møller, D.L.; Fogh, K.; et al. Modeling of waning immunity after SARS-CoV-2 vaccination and influencing factors. Nat. Commun. 2022, 13, 1614. [Google Scholar] [CrossRef]
- Tuan, J.J.; Zapata, H.; Critch-Gilfillan, T.; Ryall, L.; Turcotte, B.; Mutic, S.; Andrews, L.; Roh, M.E.; Friedland, G.; Barakat, L.; et al. Qualitative Assessment of Anti-SARS-CoV-2 Spike Protein Immunogenicity (QUASI) after COVID-19 Vaccination in Older People Living with HIV. HIV Med. 2022, 23, 178–185. [Google Scholar] [CrossRef]
- Kim, D.; Chung, H.; Lee, J.E.; Kim, J.; Hwang, J.; Chung, Y. Immunologic Aspects of Dyslipidemia: A Critical Regulator of Adaptive Immunity and Immune Disorders. J. Lipid Atheroscler. 2021, 10, 184. [Google Scholar] [CrossRef]
- Koh, G.C.K.W.; Peacock, S.J.; Van Der Poll, T.; Wiersinga, W.J. The Impact of Diabetes on the Pathogenesis of Sepsis. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 31, 379–388. [Google Scholar] [CrossRef]
- Tran, L.; Tam, D.N.H.; Elhadad, H.; Hien, N.M.; Huy, N.T. Evaluation of COVID-19 Protease and HIV Inhibitors Interactions. Acta Pharm. 2021, 72, 1–8. [Google Scholar] [CrossRef]
- Ma, L.; Li, Q.; Xie, Y.; Jianyuan, Z.; Yi, D.; Guo, S.; Guo, F.; Wang, J.; Yang, L.; Cen, S. Repurposing of HIV/HCV Protease Inhibitors against SARS-CoV-2 3CLpro. Antivir. Res. 2022, 207, 105419. [Google Scholar] [CrossRef]
- Kouznetsova, V.L.; Huang, D.Z.; Tsigelny, I.F. Potential SARS-CoV-2 Protease Mpro Inhibitors: Repurposing FDA-Approved Drugs. Phys. Biol. 2021, 18, 025001. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, M.; Mótyán, J.A.; Szojka, Z.I.; Golda, M.; Miczi, M.; Tőzsér, J. Analysis of the Efficacy of HIV Protease Inhibitors against SARS-CoV-2’s Main Protease. Virol. J. 2020, 17, 190. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, S.; Bojkova, D.; Cinatl, J.; Van Damme, E.; Buyck, C.; Van Loock, M.; Woodfall, B.; Ciesek, S. Lack of Antiviral Activity of Darunavir against SARS-CoV-2. Int. J. Infect. Dis. 2020, 97, 7–10. [Google Scholar] [CrossRef]
- Baazim, H.; Antonio-Herrera, L.; Bergthaler, A. The Interplay of Immunology and Cachexia in Infection and Cancer. Nat. Rev. Immunol. 2022, 22, 309–321. [Google Scholar] [CrossRef]
- Tortellini, E.; Zingaropoli, M.A.; Mancarella, G.; Marocco, R.; Carraro, A.; Jamhour, M.; Barbato, C.; Guardiani, M.; Dominelli, F.; Pasculli, P.; et al. Quality of T-Cell Response to SARS-CoV-2 MRNA Vaccine in ART-Treated PLWH. Int. J. Mol. Sci. 2022, 23, 14988. [Google Scholar] [CrossRef]


| Sample Characteristics | n = 165 | % | Median | P25 | P75 |
|---|---|---|---|---|---|
| Age | 55.0 | 47.0 | 62.0 | ||
| Male | 137 | 83.0% | |||
| Italian | 150 | 90.9% | |||
| BMI | 25.2 | 22.9 | 27.8 | ||
| Years lived with HIV | 16.0 | 8.0 | 24.0 | ||
| Initiated cART within one year of diagnosis | 130 | 78.8% | |||
| No AIDS event | 121 | 74.2% | |||
| Nadir CD4+ T cell count | 270.0 | 140.0 | 421.0 | ||
| CD4 > 500 cells/mm3 | 127 | 77.0% | |||
| CD4 351–500 cells/mm3 | 23 | 13.9% | |||
| CD4 200–350 cell/mm3 | 15 | 9.1% | |||
| CD4+ T-cell count (T0) | 687.0 | 508.0 | 875.0 | ||
| CD4/CD8 ratio (>0.5) | 135 | 81.8% | |||
| CD4/CD8 (T0) | 0.9 | 0.6 | 1.3 | ||
| HIV-RNA at T0 (copies/mm3) | 0.0 | 0.0 | 20.0 | ||
| HIV-RNA (<50 copies/mm3) | 161 | 97.6% | |||
| INI (integrase inhibitors)-based ART | 94 | 57.0% | |||
| PI (protease inhibitors)-based ART | 33 | 20.0% | |||
| NNRTI (non-nucleoside reverse transcriptase inhibitor)-based ART | 60 | 36.4% | |||
| Dual therapy | 49 | 29.7% | |||
| Triple therapy | 110 | 66.7% | |||
| Dyslipidemia | 54 | 32.7% | |||
| Cardiovascular disease | 60 | 36.4% | |||
| Depression | 11 | 6.7% | |||
| Hepatopathy | 25 | 15.2% | |||
| Pneumological diseases | 4 | 2.4% | |||
| Renal failure/dialysis | 3 | 1.8% | |||
| Diabetes | 22 | 13.3% | |||
| Solid tumours | 28 | 17.0% | |||
| Hematological neoplasms | 4 | 2.4% | |||
| Autoimmunity | 13 | 7.9% | |||
| Hepatitis C virus antibodies (positive) | 37 | 22.4% | |||
| Antibodies to hepatitis B core antigen (positive) | 65 | 40.6% | |||
| Hepatitis B surface antigen (positive) | 3 | 1.9% | |||
| Treponema pallidum antibodies (positive) | 59 | 36.6% | |||
| Venereal Disease Research Laboratory Test (positive) | 10 | 6.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldovin, T.; Leoni, D.; Geppini, R.; Miatton, A.; Amoruso, I.; Fonzo, M.; Bertoncello, C.; Finco, M.; Mazzitelli, M.; Sasset, L.; et al. Immunogenicity and Determinants of Antibody Response to the BNT162b2 mRNA Vaccine: A Longitudinal Study in a Cohort of People Living with HIV. Vaccines 2024, 12, 1172. https://doi.org/10.3390/vaccines12101172
Baldovin T, Leoni D, Geppini R, Miatton A, Amoruso I, Fonzo M, Bertoncello C, Finco M, Mazzitelli M, Sasset L, et al. Immunogenicity and Determinants of Antibody Response to the BNT162b2 mRNA Vaccine: A Longitudinal Study in a Cohort of People Living with HIV. Vaccines. 2024; 12(10):1172. https://doi.org/10.3390/vaccines12101172
Chicago/Turabian StyleBaldovin, Tatjana, Davide Leoni, Ruggero Geppini, Andrea Miatton, Irene Amoruso, Marco Fonzo, Chiara Bertoncello, Mascia Finco, Maria Mazzitelli, Lolita Sasset, and et al. 2024. "Immunogenicity and Determinants of Antibody Response to the BNT162b2 mRNA Vaccine: A Longitudinal Study in a Cohort of People Living with HIV" Vaccines 12, no. 10: 1172. https://doi.org/10.3390/vaccines12101172
APA StyleBaldovin, T., Leoni, D., Geppini, R., Miatton, A., Amoruso, I., Fonzo, M., Bertoncello, C., Finco, M., Mazzitelli, M., Sasset, L., Cattelan, A., & Baldo, V. (2024). Immunogenicity and Determinants of Antibody Response to the BNT162b2 mRNA Vaccine: A Longitudinal Study in a Cohort of People Living with HIV. Vaccines, 12(10), 1172. https://doi.org/10.3390/vaccines12101172










