Evaluation of a Low-Temperature Immersion Immunization Strategy for the Infectious Spleen and Kidney Necrosis Virus orf037l Gene-Deleted Attenuated Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Cells, and Viruses
2.2. Construction of the ISKNV ∆orf037l Recombinant Transfer Vector
2.3. Construction of ISKNV Δorf037l Deletion Recombinant Viral Strain
2.4. Assessment of the Expression of Genes Flanking orf037l
2.5. Assessment of Δorf037l Virulence
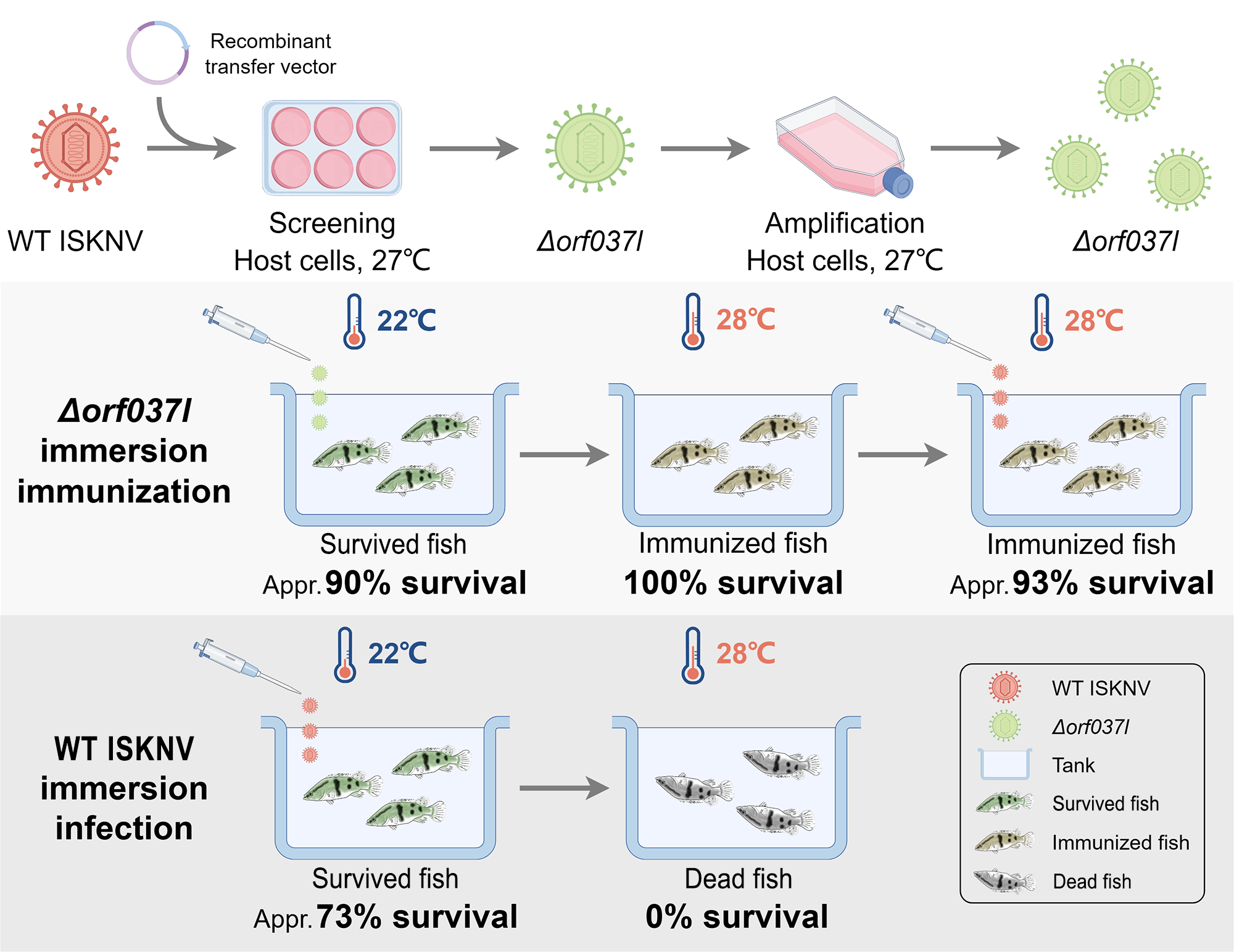
2.6. Immunization Procedure with Δorf037l
2.7. Histopathological Sections and Hematoxylin–Eosin (H&E) Staining
2.8. Expression Levels of Immune-Related Genes in Mandarin Fish Induced by Δorf037l
2.9. Neutralization Assay
2.10. Determination of ISKNV-Specific IgM via Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Statistical Analysis
3. Results
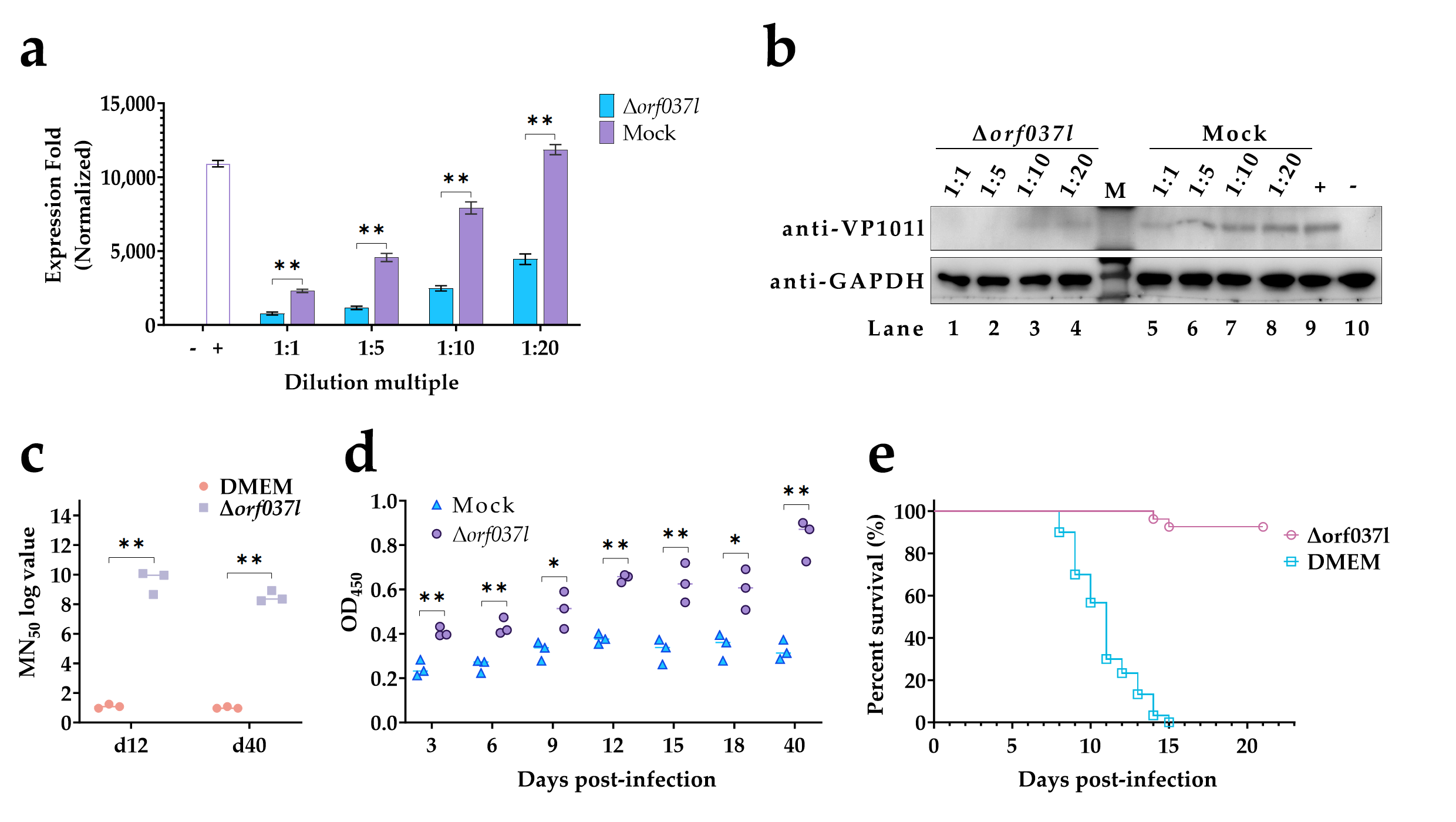
3.1. Construction and Identification of Δorf037l
3.2. The Challenge of Immersion Immunization with Δorf037l
3.3. Evaluation of the Protective Efficacy Induced by Low-Temperature Immersion Immunization with Δorf037l
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, J.G.; Zeng, K.; Weng, S.P.; Chan, S.M. Experimental Transmission, Pathogenicity and Physical–Chemical Properties of Infectious Spleen and Kidney Necrosis Virus (Isknv). Aquaculture 2002, 204, 11–24. [Google Scholar] [CrossRef]
- Zerbini, F.M.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; Hendrickson, R.C.; et al. Changes to Virus Taxonomy and the ICTV Statutes Ratified by the International Committee on Taxonomy of Viruses (2023). Arch. Virol. 2023, 168, 175. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G.; Hick, P.; Ince, I.A.; Jancovich, J.K.; Marschang, R.; Qin, Q.; Subramaniam, K.; Waltzek, T.B.; Whittington, R.; Williams, T.; et al. Ictv Virus Taxonomy Profile: Iridoviridae. J. Gen. Virol. 2017, 98, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Li, Y.; Fu, Y.; Zhang, W.; Luo, P.; Sun, Q.; Yu, F.; Weng, S.; Li, W.; He, J.; et al. The Inactivated Isknv-I Vaccine Confers Highly Effective Cross-Protection against Epidemic Rsiv-I and Rsiv-II from Cultured Spotted Sea Bass Lateolabrax maculatus. Microbiol. Spectr. 2023, 11, e0449522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Lü, L.; Weng, S.P.; Huang, J.N.; Chan, S.M.; He, J.G. Molecular Epidemiology and Phylogenetic Analysis of a Marine Fish Infectious Spleen and Kidney Necrosis Virus-Like (Isknv-Like) Virus. Arch. Virol. 2007, 152, 763–773. [Google Scholar] [CrossRef]
- Jeong, J.B.; Kim, H.Y.; Jun, L.J.; Lyu, J.H.; Park, N.G.; Kim, J.K.; Jeong, H.D. Outbreaks and Risks of Infectious Spleen and Kidney Necrosis Virus Disease in Freshwater Ornamental Fishes. Dis. Aquat. Org. 2008, 78, 209–215. [Google Scholar] [CrossRef]
- Johnson, S.J.; Hick, P.M.; Robinson, A.P.; Rimmer, A.E.; Tweedie, A.; Becker, J.A. The Impact of Pooling Samples on Surveillance Sensitivity for the Megalocytivirus Infectious Spleen and Kidney Necrosis Virus. Transbound. Emerg. Dis. 2019, 66, 2318–2328. [Google Scholar] [CrossRef]
- Fu, X.; Li, N.; Liu, L.; Lin, Q.; Wang, F.; Lai, Y.; Jiang, H.; Pan, H.; Shi, C.; Wu, S. Genotype and Host Range Analysis of Infectious Spleen and Kidney Necrosis Virus (Isknv). Virus Genes 2011, 42, 97–109. [Google Scholar] [CrossRef]
- Fonseca, A.A., Jr.; Laguardia-Nascimento, M.; Scotá Ferreira, A.P.; Pinto, C.A.; Pereira Freitas, T.R.; Rivetti Júnior, A.V.; Ferreira Homem, V.S.; Camargos, M.F. Detection of Megalocytivirus in Oreochromis niloticus and Pseudoplatystoma corruscans in Brazil. Dis. Aquat. Org. 2022, 149, 25–32. [Google Scholar] [CrossRef]
- Girisha, S.K.; Kushala, K.B.; Nithin, M.S.; Puneeth, T.G.; Naveen Kumar, B.T.; Vinay, T.N.; Suresh, T.; Ajay, S.K.; Venugopal, M.N.; Ramesh, K.S. First Report of the Infectious Spleen and Kidney Necrosis Virus (Isknv) Infection in Ornamental Fishes in India. Transbound. Emerg. Dis. 2021, 68, 964–972. [Google Scholar] [CrossRef]
- Alathari, S.; Chaput, D.L.; Bolaños, L.M.; Joseph, A.; Jackson, V.L.N.; Verner-Jeffreys, D.; Paley, R.; Tyler, C.R.; Temperton, B. A Multiplexed, Tiled Pcr Method for Rapid Whole-Genome Sequencing of Infectious Spleen and Kidney Necrosis Virus (Isknv) in Tilapia. Viruses 2023, 15, 965. [Google Scholar] [CrossRef] [PubMed]
- Bøgwald, J.; Dalmo, R.A. Review on Immersion Vaccines for Fish: An Update 2019. Microorganisms 2019, 7, 627. [Google Scholar] [CrossRef] [PubMed]
- Rifai, A.B. Megalocytivirus Infection in Marine and Freshwater Fishes in Several Regions in Indonesia. J. Vet. 2020, 21, 423–434. [Google Scholar]
- Bondad-Reantaso, M.G.; Subasinghe, R.P.; Arthur, J.R.; Ogawa, K.; Chinabut, S.; Adlard, R.; Tan, Z.; Shariff, M. Disease and Health Management in Asian Aquaculture. Vet. Parasitol. 2005, 132, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Thanasaksiri, K.; Takano, R.; Fukuda, K.; Chaweepack, T.; Wongtavatchai, J. Identification of Infectious Spleen and Kidney Necrosis Virus from Farmed Barramundi Lates Calcarifer in Thailand and Study of Its Pathogenicity. Aquaculture 2019, 500, 188–191. [Google Scholar] [CrossRef]
- Subramaniam, K.; Shariff, M.; Omar, A.; Hair-Bejo, M.; Ong, B. Detection and Molecular Characterization of Infectious Spleen and Kidney Necrosis Virus from Major Ornamental Fish Breeding States in P eninsular M alaysia. J. Fish Dis. 2014, 37, 609–618. [Google Scholar] [CrossRef]
- Johan, C.A.C.; Abdullah, M.D.D.; Emilia, S.N.; Zainathan, S.C. Molecular Epidemiology of Megalocytivirus in Freshwater Angelfish (Pterophyllum scalare) from Johor, Malaysia. Vet World 2023, 16, 2158–2172. [Google Scholar] [CrossRef]
- Li, N.; Fu, X.; Guo, H.; Lin, Q.; Liu, L.; Zhang, D.; Fang, X.; Wu, S. Protein Encoded by Orf093 is an Effective Vaccine Candidate for Infectious Spleen and Kidney Necrosis Virus in Chinese perch Siniperca chuatsi. Fish Shellfish Immunol. 2015, 42, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Crim, M.J. Viral Diseases. In The Zebrafish in Biomedical Research; Cartner, S.C., Eisen, J.S., Farmer, S.C., Guillemin, K.J., Kent, M.L., Sanders, G.E., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 509–526. [Google Scholar]
- Liang, H.; Zhang, L.; Fu, X.; Lin, Q.; Liu, L.; Niu, Y.; Luo, X.; Huang, Z.; Li, N. Development of a Double-Antibody Sandwich Elisa for Rapid Detection of the Mcp Antigen Concentration in Inactivated Isknv Vaccines. Vaccines 2021, 9, 1264. [Google Scholar] [CrossRef]
- Du, Y.; Hu, X.; Miao, L.; Chen, J. Current Status and Development Prospects of Aquatic Vaccines. Front. Immunol. 2022, 13, 1040336. [Google Scholar] [CrossRef]
- Thanasaksiri, K.; Fukuda, K.; Takano, R.; Hich, T.V.; Wongtavatchai, J.; Hanggono, B.; Ak, U.K. Efficacy of a Commercial Vaccine PISCIVAC™ Irido Si against Iridoviral Disease and Streptococcosis in Asian Seabass (Lates calcarifer). Aquac. Int. 2024. [Google Scholar] [CrossRef]
- Debnath, S.C.; McMurtrie, J.; Temperton, B.; Delamare-Deboutteville, J.; Mohan, C.V.; Tyler, C.R. Tilapia Aquaculture, Emerging Diseases, and the Roles of the Skin Microbiomes in Health and Disease. Aquac. Int. 2023, 31, 2945–2976. [Google Scholar] [CrossRef]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef]
- Adams, A. Progress, Challenges and Opportunities in Fish Vaccine Development. Fish Shellfish Immunol. 2019, 90, 210–214. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, W.; Xu, Z. Current Use and Development of Fish Vaccines in China. Fish Shellfish Immunol. 2020, 96, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Kwon, M.G.; Seo, J.S.; Hwang, S.D.; Jeong, J.M.; Lee, J.H.; Jeong, A.R.; Jee, B.Y. Current Use and Management of Commercial Fish Vaccines in Korea. Fish Shellfish Immunol. 2020, 102, 20–27. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Areechon, N.; Unajak, S. Development of Fish Vaccine in Southeast Asia: A Challenge for the Sustainability of Se Asia Aquaculture. Fish Shellfish Immunol. 2020, 103, 73–87. [Google Scholar] [CrossRef]
- Jeong, K.-H.; Kim, H.J.; Kim, H.-J. Current Status and Future Directions of Fish Vaccines Employing Virus-Like Particles. Fish Shellfish Immunol. 2020, 100, 49–57. [Google Scholar] [CrossRef]
- Adams, A.; Subasinghe, R. Use of Fish Vaccines in Aquaculture (Including Methods of Administration). In Veterinary Vaccines for Livestock, 1st ed.; The Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Du, Y.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. The Influence of Concentration of Inactivated Edwardsiella tarda Bacterin and Immersion Time on Antigen Uptake and Expression of Immune-Related Genes in Japanese Flounder (Paralichthys olivaceus). Microb. Pathog. 2017, 103, 19–28. [Google Scholar] [CrossRef]
- Bedekar, M.K.; Kole, S. Fundamentals of Fish Vaccination. In Vaccine Design: Methods and Protocols, Volume 2. Vaccines for Veterinary Diseases; Thomas, S., Ed.; Springer US: New York, NY, USA, 2022; pp. 147–173. [Google Scholar]
- Liu, G.; Zhu, J.; Chen, K.; Gao, T.; Yao, H.; Liu, Y.; Zhang, W.; Lu, C. Development of Streptococcus agalactiae Vaccines for Tilapia. Dis. Aquat. Org. 2016, 122, 163–170. [Google Scholar] [CrossRef]
- Zeng, R.; Fu, J.; Pan, W.; Zhan, Z.; Weng, S.; Guo, C.; He, J. Low-Temperature Immunization Attenuates the Residual Virulence of orf074r Gene-Deleted Infectious Spleen and Kidney Necrosis Virus: A Candidate Immersion Vaccine. J. Virol. 2023, 97, e0128923. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Pan, W.; Lin, Y.; He, J.; Luo, Z.; Li, Z.; Weng, S.; He, J.; Guo, C. Development of a Gene-Deleted Live Attenuated Candidate Vaccine against Fish Virus (Isknv) with Low Pathogenicity and High Protection. iScience 2021, 24, 102750. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-F.; He, J.; Zeng, R.-Y.; Li, Z.-M.; Luo, Z.-Y.; Pan, W.-Q.; Weng, S.-P.; Guo, C.-J.; He, J.-G. Deletion of the Infectious Spleen and Kidney Necrosis Virus Orf069l Reduces Virulence to Mandarin Fish Siniperca chuatsi. Fish Shellfish Immunol. 2019, 95, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Pan, W.; Lin, Y.; Liang, M.; Fu, J.; Weng, S.; He, J.; Guo, C. A Safe and Efficient Double-Gene-Deleted Live Attenuated Immersion Vaccine to Prevent the Disease Caused by the Infectious Spleen and Kidney Necrosis Virus. J. Virol. 2023, 97, e0085723. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Slater, M.J. Responses of Aquaculture Fish to Climate Change-Induced Extreme Temperatures: A Review. J. World Aquac. Soc. 2022, 53, 314–366. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature Increase and Its Effects on Fish Stress Physiology in the Context of Global Warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Reverter, M.; Sarter, S.; Caruso, D.; Avarre, J.-C.; Combe, M.; Pepey, E.; Pouyaud, L.; Vega-Heredía, S.; de Verdal, H.; Gozlan, R.E. Aquaculture at the Crossroads of Global Warming and Antimicrobial Resistance. Nat. Commun. 2020, 11, 1870. [Google Scholar] [CrossRef]
- Kim, S.-W.; Kim, S.-J.; Oh, M.-J. Efficacy of Live NNV Immersion Vaccine Immunized at Low Temperature in Sevenband Grouper, Epinephelus septemfasciatus. Virus Res. 2021, 292, 198227. [Google Scholar] [CrossRef]
- Nishizawa, T.; Takami, I.; Yang, M.; Oh, M.-J. Live Vaccine of Viral Hemorrhagic Septicemia Virus (VHSV) for Japanese Flounder at Fish Rearing Temperature of 21 °C Instead of Poly (I:C) Administration. Vaccine 2011, 29, 8397–8404. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Oh, M.-J.; Nishizawa, T. Potential for a Live Red Seabream Iridovirus (RSIV) Vaccine in Rock Bream Oplegnathus fasciatus at a Low Rearing Temperature. Vaccine 2014, 32, 363–368. [Google Scholar] [CrossRef]
- Do, J.W.; Moon, C.H.; Kim, H.J.; Ko, M.S.; Kim, S.B.; Son, J.H.; Kim, J.S.; An, E.J.; Kim, M.K.; Lee, S.K.; et al. Complete Genomic DNA Sequence of Rock Bream Iridovirus. Virology 2004, 325, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Lü, L.; Zhou, S.Y.; Chen, C.; Weng, S.P.; Chan, S.M.; He, J.G. Complete Genome Sequence Analysis of an Iridovirus Isolated from the Orange-Spotted Grouper, Epinephelus coioides. Virology 2005, 339, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Weng, S.; Shi, X.; Xu, X.; Shi, N.; He, J. Development of a Mandarin Fish Siniperca chuatsi Fry Cell Line Suitable for the Study of Infectious Spleen and Kidney Necrosis Virus (Isknv). Virus Res. 2008, 135, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, K.E.; Tan, C.S.; Walsh, S.R.; Hale, A.; Ansel, J.L.; Kanjilal, D.G.; Jaegle, K.; Peter, L.; Borducchi, E.N.; Nkolola, J.P.; et al. Safety and Immunogenicity of a Zika Purified Inactivated Virus Vaccine Given via Standard, Accelerated, or Shortened Schedules: A Single-Centre, Double-Blind, Sequential-Group, Randomised, Placebo-Controlled, Phase 1 Trial. Lancet Infect. Dis. 2020, 20, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liao, J.; Zhang, D.; Liu, S.; Zhang, L.; Kang, S.; Xu, L.; Chen, H.; Peng, W.; Zhou, S.; et al. Isolation, Characterization, and Transcriptome Analysis of an ISKNV-Like Virus from Largemouth Bass. Viruses 2023, 15, 398. [Google Scholar] [CrossRef]
- Kerddee, P.; Dinh-Hung, N.; Dong, H.T.; Hirono, I.; Soontara, C.; Areechon, N.; Srisapoome, P.; Kayansamruaj, P. Molecular Evidence for Homologous Strains of Infectious Spleen and Kidney Necrosis Virus (ISKNV) Genotype I Infecting Inland Freshwater Cultured Asian Sea Bass (Lates calcarifer) in Thailand. Arch. Virol. 2021, 166, 3061–3074. [Google Scholar] [CrossRef]
- Swaminathan, T.R.; Raj, N.S.; Preena, P.G.; Pradhan, P.K.; Sood, N.; Kumar, R.G.; Sudhagar, A.; Sood, N.K. Infectious Spleen and Kidney Necrosis Virus-Associated Large-Scale Mortality in Farmed Giant Gourami, Osphronemus goramy, in India. J. Fish Dis. 2021, 44, 2043–2053. [Google Scholar] [CrossRef]
- de Pádua Pereira, U.; Rocha, F.E.P.; Ferrari, N.A.; Favero, L.M.; Mainardi, R.M.; da Silva, M.B.; Alfieri, A.A.; Viadanna, P.H.O.; Waltzek, T.; Agnol, A.M.D. First Report of Infectious Spleen and Kidney Necrosis Virus (Isknv) in Two Native Cichlids Cultured in Brazil. Semin Ciências Agrárias 2024, 45, 239–250. [Google Scholar] [CrossRef]
- Ayiku, A.N.; Adelani, A.A.; Appenteng, P.; Nkansa, M.; Ngoi, J.M.; Morang’a, C.M.; Dzabeng, F.; Paley, R.; Cudjoe, K.S.; Verner-Jeffreys, D. Molecular Epidemiology and Current Management of Infectious Spleen and Kidney Necrosis Virus (Isknv) Infection in Ghanaian Cultured Tilapia. Aquaculture 2024, 581, 740330. [Google Scholar] [CrossRef]
- Staib, C.; Drexler, I.; Ohlmann, M.; Wintersperger, S.; Erfle, V.; Sutter, G. Transient Host Range Selection for Genetic Engineering of Modified Vaccinia Virus Ankara. Biotechniques 2000, 28, 1137–1148. [Google Scholar] [CrossRef]
- Ao, J.; Chen, X. Identification and Characterization of a Novel Gene Encoding an RGD-Containing Protein in Large Yellow Croaker Iridovirus. Virology 2006, 355, 213–222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scharsack, J.P.; Franke, F. Temperature Effects on Teleost Immunity in the Light of Climate Change. J. Fish Biol. 2022, 101, 780–796. [Google Scholar] [CrossRef] [PubMed]
- Barbarossa, V.; Bosmans, J.; Wanders, N.; King, H.; Bierkens, M.F.P.; Huijbregts, M.A.J.; Schipper, A.M. Threats of Global Warming to the World’s Freshwater Fishes. Nat. Commun. 2021, 12, 1701. [Google Scholar] [CrossRef] [PubMed]
- Ahne, W.; Bjorklund, H.; Essbauer, S.; Fijan, N.; Kurath, G.; Winton, J. Spring Viremia of Carp (Svc). Dis. Aquat. Org. 2002, 52, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, X.; Xing, J.; Sheng, X.; Chi, H.; Zhan, W. Vaccination with Live Hirame Novirhabdovirus (Hirrv) at Temperature-Controlled Condition Induced Protective Immunity In Flounder (Paralichthys olivaceus). Microb. Pathog. 2021, 157, 104993. [Google Scholar] [CrossRef]
- Saito, H.; Minami, S.; Yuguchi, M.; Shitara, A.; Kondo, H.; Kato, G.; Sano, M. Effect of Temperature on the Protective Efficacy of a Live Attenuated Vaccine against Herpesviral Haematopoietic necrosis in goldfish. J. Fish Dis. 2024, 47, e13906. [Google Scholar] [CrossRef]
- Tian, X.-L.; Dong, S.-L. Land-Based Intensive Aquaculture Systems. In Aquaculture Ecology; Dong, S.-L., Tian, X.-L., Gao, Q.-F., Dong, Y.-W., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 369–402. [Google Scholar]
- Rajesh, M.; Kamalam, B.S.; Sarma, D. Recirculating Aquaculture System for Intensive Fish Farming in Indian Himalayan Region: An Overview. In Fisheries and Aquaculture of the Temperate Himalayas; Pandey, P.K., Pandey, N., Akhtar, M.S., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 173–204. [Google Scholar]
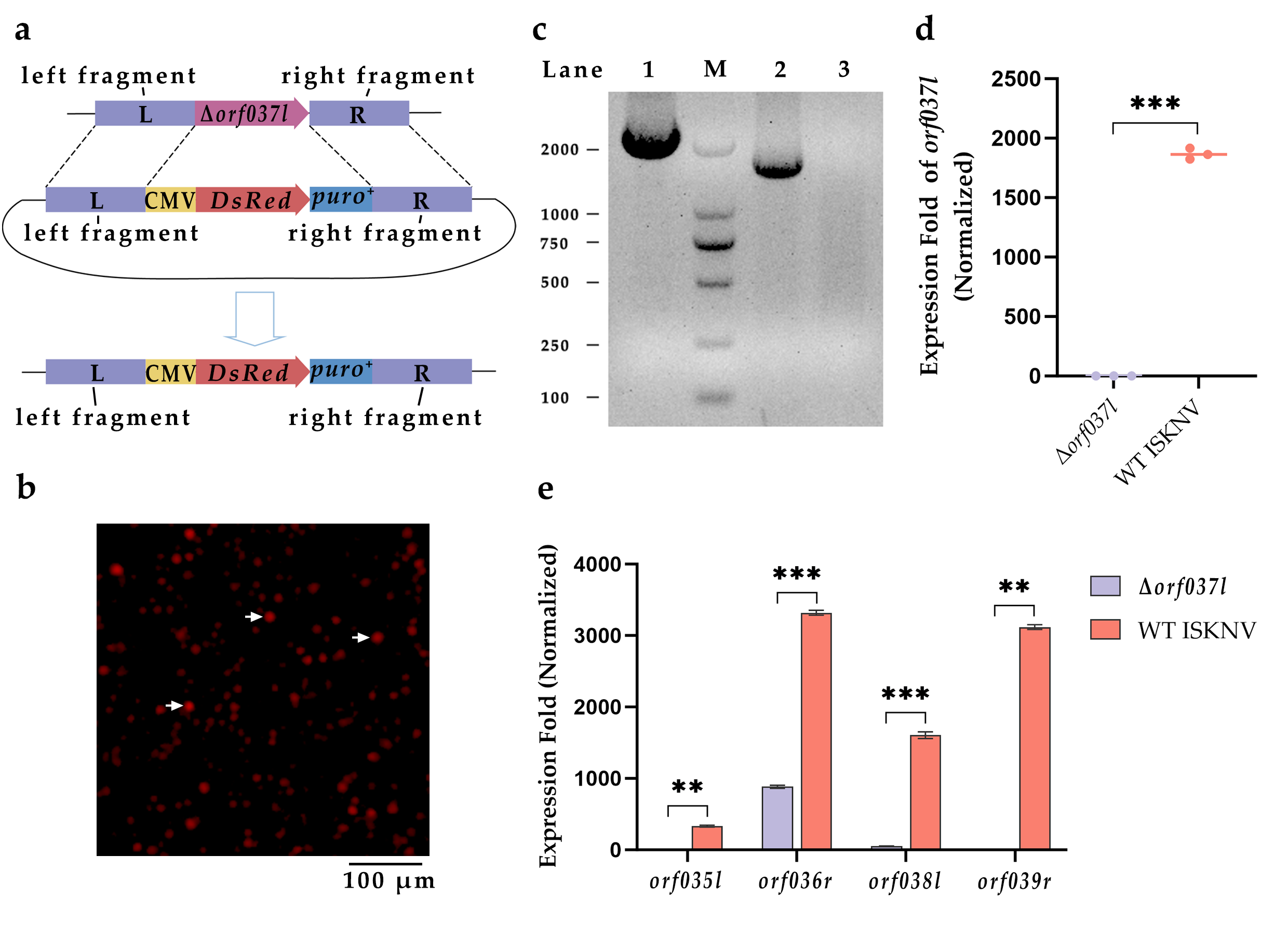
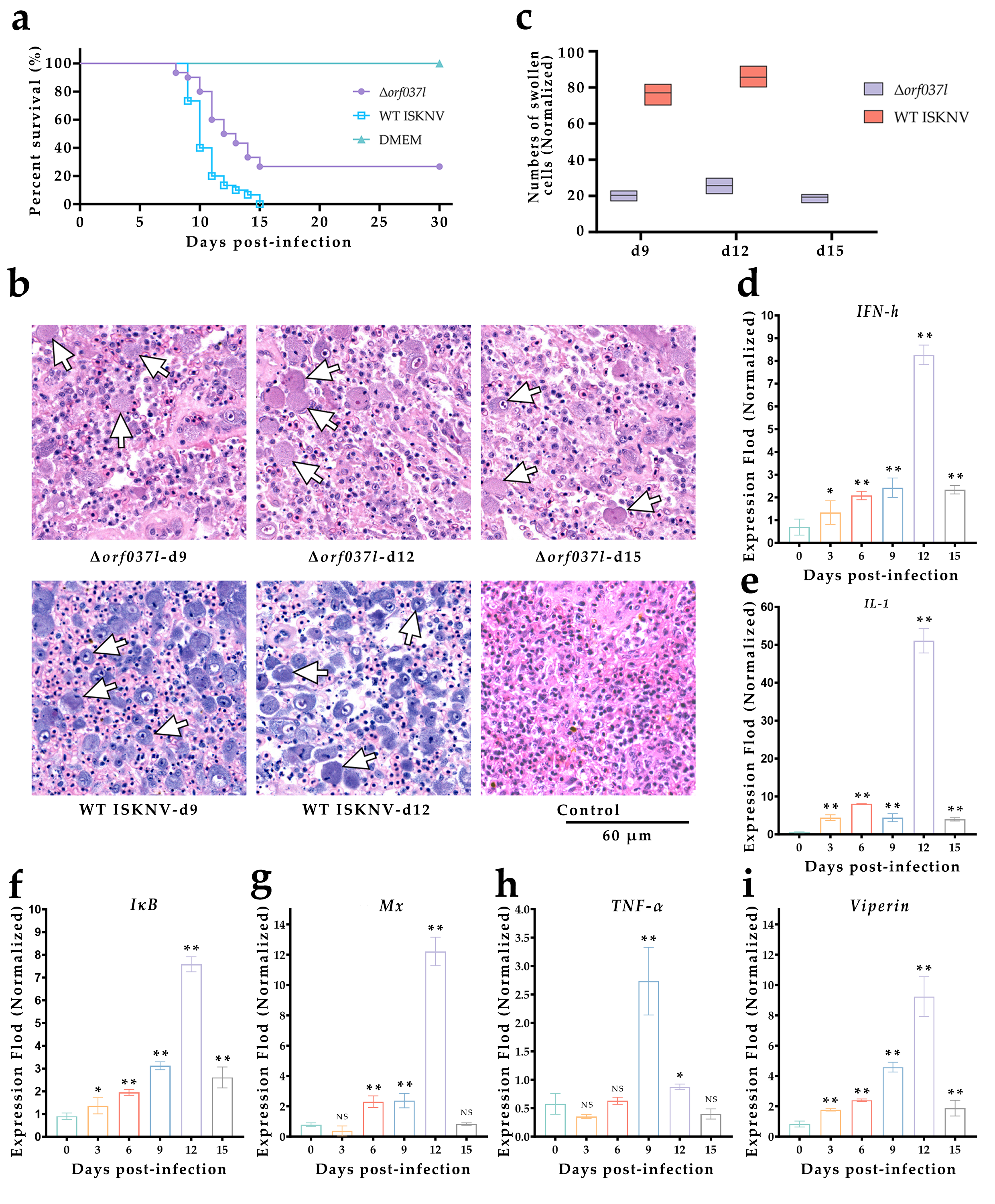
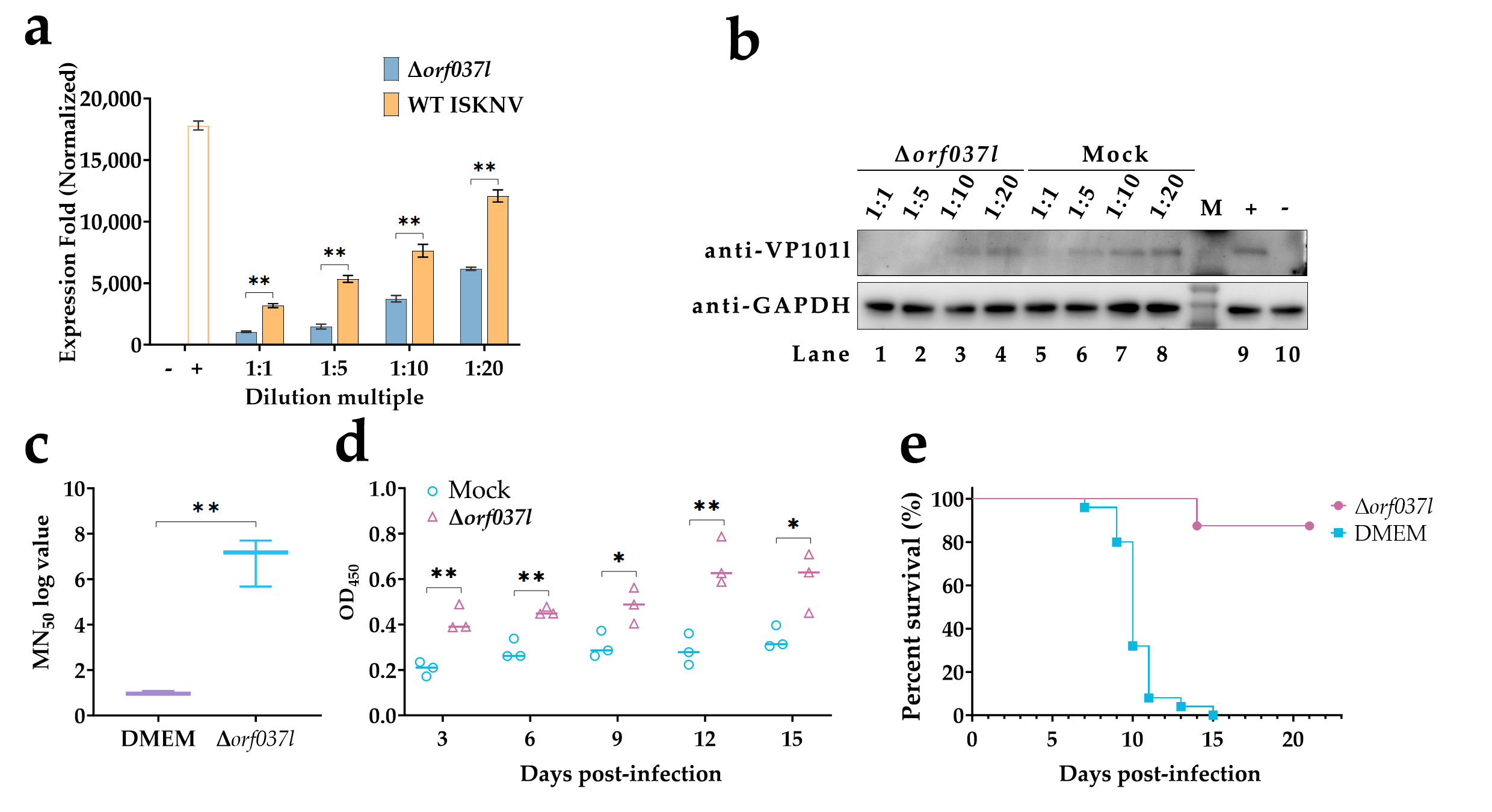
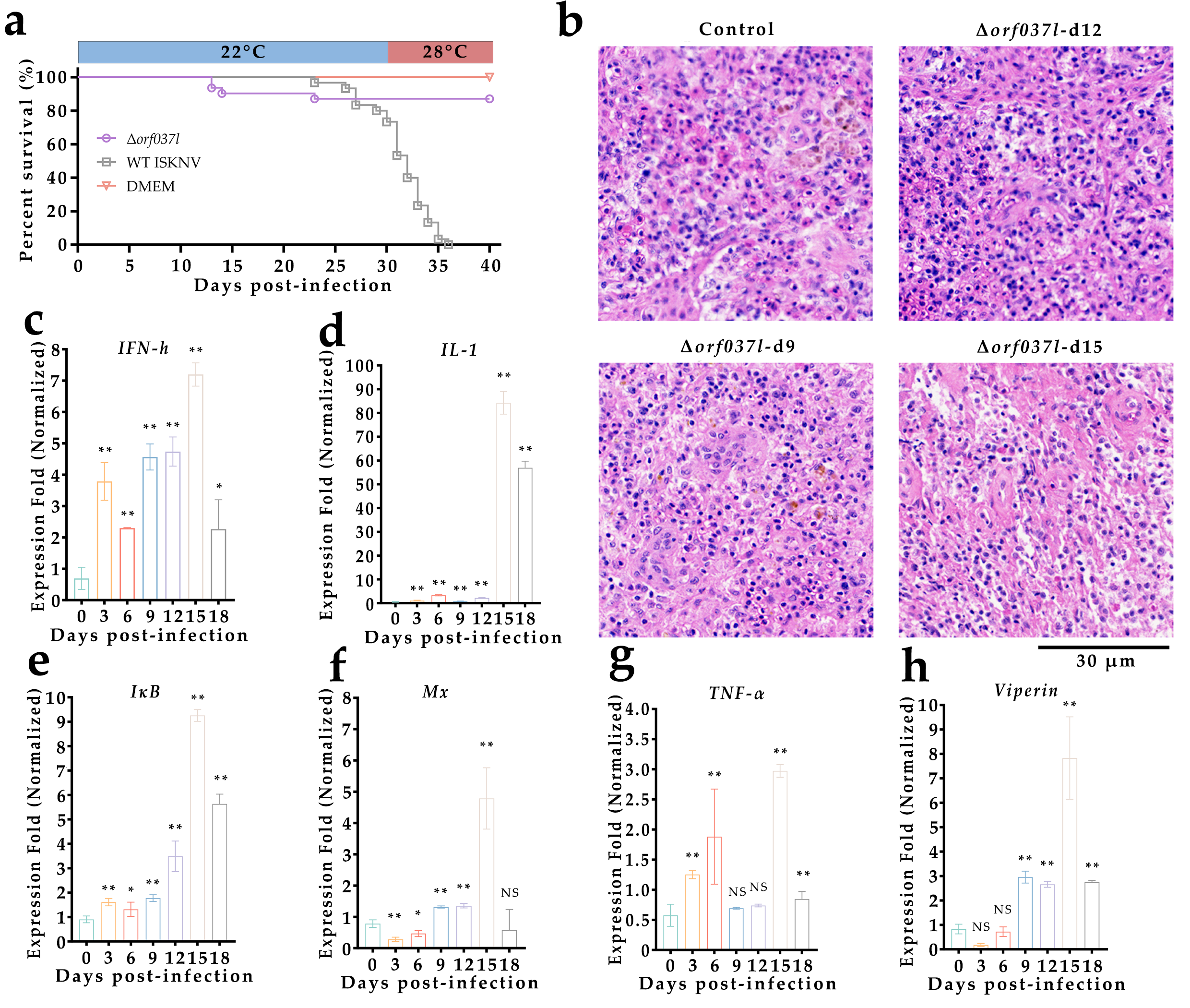
| Name | Experiment | Sequences (from 5′ to 3′) |
|---|---|---|
| orf037l-left-F | PCR | CCCAAGCTTTGCCACATCCATCCTTACAG |
| orf037l-left-R | PCR | CGGGATCCCGGAAAAGATGATAGGCGTG |
| orf037l-right-F | PCR | GGGGTACCAGTCAGTTTTATATCATCAA |
| orf037l-right-R | PCR | CGGAATTCGTTGACCTGGGTTCAGGCCA |
| orf037l-outer-F | PCR | CCCACCGCCACATTGCCAAGT |
| orf037l-outer-R | PCR | CCCCGGCCAAAAAGATATACAACG |
| isknvorf037l-qF | RT-qPCR | AGACACGAGCCACAAACTGT |
| isknvorf037l-qR | RT-qPCR | CTCACGCAGCTTATTGACGC |
| isknvmcp-qF | RT-qPCR | CAATGTAGCACCCGCACTGACC |
| isknvmcp-qR | RT-qPCR | ACCTCACGCTCCTCACTTGTC |
| scβ-actin-qF | RT-qPCR | CCCTCTGAACCCCAAAGCCA |
| scβ-actin-qR | RT-qPCR | CAGCCTGGATGGCAACGTACA |
| isknvorf035l-qF | RT-qPCR | CGTACTTGTGCAACACTGCC |
| isknvorf035l-qR | RT-qPCR | GTGGCCATGTATACCGAGGG |
| isknvorf036r-qF | RT-qPCR | TTACTGCGTGGAACGAGTCC |
| isknvorf036r-qR | RT-qPCR | TTGGCACGGAATGCCTGTAT |
| isknvorf038l-qF | RT-qPCR | CATGGTGACAGTCGAAGGCT |
| isknvorf038l-qR | RT-qPCR | CGAATGGCGTGAGGGTATGA |
| isknvorf039r-qF | RT-qPCR | CCGCAAACCTTTGATGCCAA |
| isknvorf039r-qR | RT-qPCR | GAAATGGCGCATAGCCACAG |
| scIFN-h-qF | RT-qPCR | CGCTCTGCTGTGATTGGC |
| scIFN-h-qR | RT-qPCR | GGGACTCCACCTCTGCCTTT |
| scMx-qF | RT-qPCR | GGATTCTGACATCGGGAGCAA |
| scMx-qR | RT-qPCR | GTGCAGTAGACTCATGCTGT |
| scIκb-qF | RT-qPCR | CAGACATCAACGCACAGGAA |
| scIκb-qR | RT-qPCR | CGTGAAGCCGCCATAGTTAA |
| scVIPERIN-qF | RT-qPCR | CCAAGAGGGGCCTCAAACTT |
| scVIPERIN-qR | RT-qPCR | CTGACACTTGGGAGCTGGAG |
| scIL-1-qF | RT-qPCR | GGACAGCGACATGGTGCGATT |
| scIL-1-qR | RT-qPCR | TTGAAGGTTCGGTGGCGTTGG |
| scTNF-α-qF | RT-qPCR | AGCCAGGCATCGTTCAGAGTCT |
| scTNF-α-qR | RT-qPCR | CTGTCCTCCTGAGCGGTGTCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, W.; Fu, J.; Zeng, R.; Liang, M.; You, Y.; Zhan, Z.; Lu, Z.; Weng, S.; Guo, C.; He, J. Evaluation of a Low-Temperature Immersion Immunization Strategy for the Infectious Spleen and Kidney Necrosis Virus orf037l Gene-Deleted Attenuated Vaccine. Vaccines 2024, 12, 1170. https://doi.org/10.3390/vaccines12101170
Pan W, Fu J, Zeng R, Liang M, You Y, Zhan Z, Lu Z, Weng S, Guo C, He J. Evaluation of a Low-Temperature Immersion Immunization Strategy for the Infectious Spleen and Kidney Necrosis Virus orf037l Gene-Deleted Attenuated Vaccine. Vaccines. 2024; 12(10):1170. https://doi.org/10.3390/vaccines12101170
Chicago/Turabian StylePan, Weiqiang, Jiajie Fu, Ruoyun Zeng, Mingcong Liang, Yanlin You, Zhipeng Zhan, Zhoutao Lu, Shaoping Weng, Changjun Guo, and Jianguo He. 2024. "Evaluation of a Low-Temperature Immersion Immunization Strategy for the Infectious Spleen and Kidney Necrosis Virus orf037l Gene-Deleted Attenuated Vaccine" Vaccines 12, no. 10: 1170. https://doi.org/10.3390/vaccines12101170
APA StylePan, W., Fu, J., Zeng, R., Liang, M., You, Y., Zhan, Z., Lu, Z., Weng, S., Guo, C., & He, J. (2024). Evaluation of a Low-Temperature Immersion Immunization Strategy for the Infectious Spleen and Kidney Necrosis Virus orf037l Gene-Deleted Attenuated Vaccine. Vaccines, 12(10), 1170. https://doi.org/10.3390/vaccines12101170






