Does the Vaccination against Tick-Borne Encephalitis Offer Good Value for Money for Incidence Rates below the WHO Threshold for Endemicity? A Case Study for Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. General Model Settings
2.2. Model Structure
2.3. Base Case Inputs
2.4. Vaccination
2.5. Health Utility (HU) Estimates
2.6. Cost Estimates
2.7. Analysis
3. Results
3.1. Base Case Results
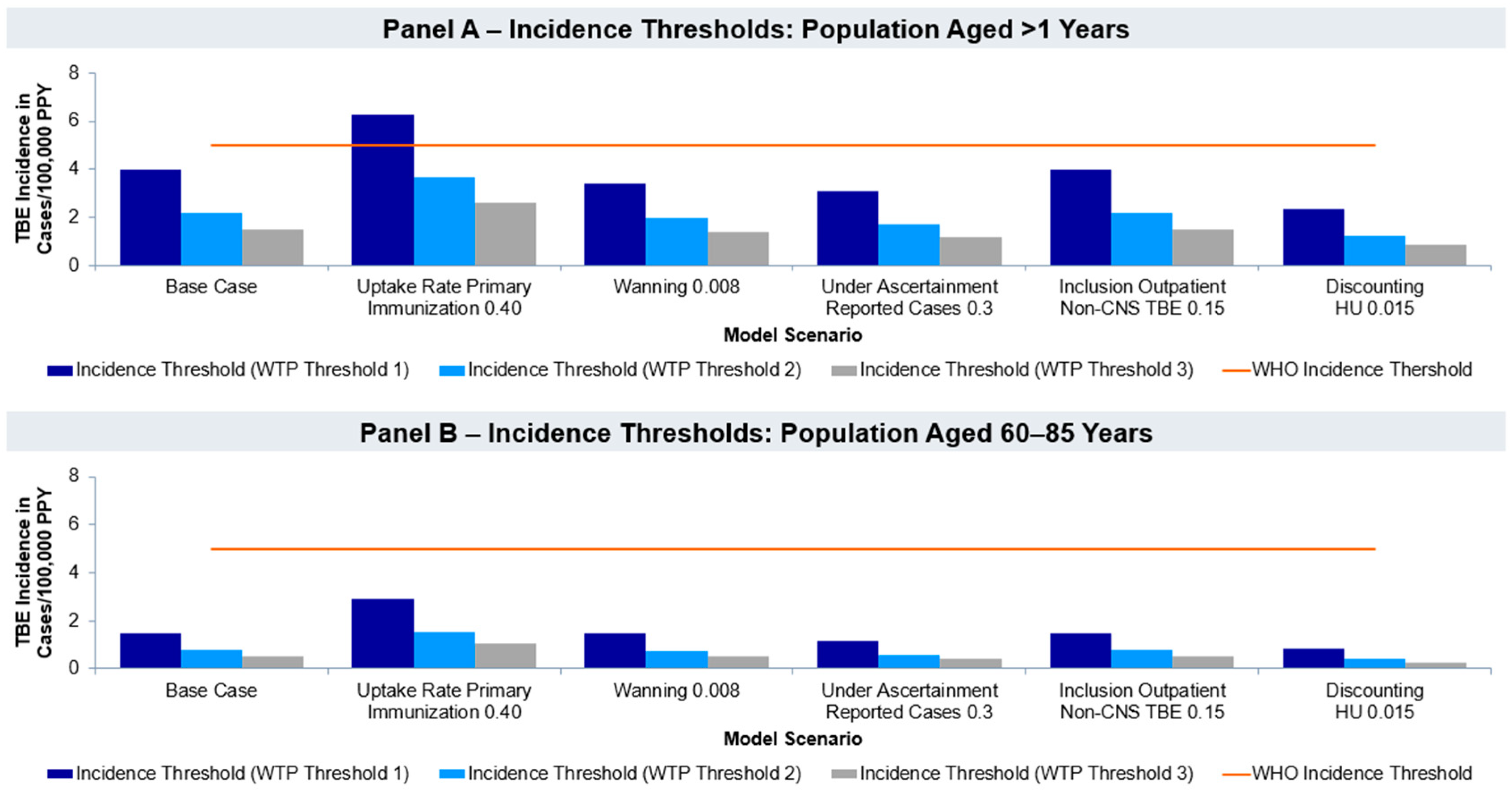
3.2. Incidence Threshold Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schley, K.; Malerczyk, C.; Beier, D.; Schiffner-Rohe, J.; von Eiff, C.; Häckl, D.; Süß, J. Vaccination rate and adherence of tick-borne encephalitis vaccination in Germany. Vaccine 2021, 39, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, N.; Antonello, R.M.; Luzzati, R.; Zajkowska, J.; Di Bella, S.; Giacobbe, D.R. Tick-borne encephalitis in Europe: A brief update on epidemiology, diagnosis, prevention, and treatment. Eur. J. Intern. Med. 2019, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rubel, F.; Brugger, K. Tick-borne encephalitis incidence forecasts for Austria, Germany, and Switzerland. Ticks Tick-Borne Dis. 2020, 11, 101437. [Google Scholar] [CrossRef] [PubMed]
- Shedrawy, J.; Henriksson, M.; Hergens, M.-P.; Askling, H.H. Estimating costs and health outcomes of publicly funded tick-born encephalitis vaccination: A cost-effectiveness analysis. Vaccine 2018, 36, 7659–7665. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Tick-Borne Encephalitis; World Health Organization (WHO): Geneva, Switzerland, 2023; Available online: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/norms-and-standards/vaccine-standardization/tick-borne-encephalitis (accessed on 3 June 2024).
- Bogovic, P. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. WJCC 2015, 3, 430. [Google Scholar] [CrossRef]
- Schley, K.; Friedrich, J.; Pilz, A.; Huang, L.; Balkaran, B.L.; Maculaitis, M.C.; Malerczyk, C. Evaluation of under-testing and under-diagnosis of tick-borne encephalitis in Germany. BMC Infect. Dis. 2023, 23, 139. [Google Scholar] [CrossRef]
- Beauté, J.; Spiteri, G.; Warns-Petit, E.; Zeller, H. Tick-borne encephalitis in Europe, 2012 to 2016. Eurosurveillance 2018, 23, 1800201. [Google Scholar] [CrossRef]
- Rampa, J.E.; Askling, H.H.; Lang, P.; Zens, K.D.; Gültekin, N.; Stanga, Z.; Schlagenhauf, P. Immunogenicity and safety of the tick-borne encephalitis vaccination (2009–2019): A systematic review. Travel. Med. Infect. Dis. 2020, 37, 101876. [Google Scholar] [CrossRef]
- Nygren, T.M.; Pilic, A.; Böhmer, M.M.; Wagner-Wiening, C.; Wichmann, O.; Harder, T.; Hellenbrand, W. Tick-borne encephalitis vaccine effectiveness and barriers to vaccination in Germany. Sci. Rep. 2022, 12, 11706. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Vaccines against Tick-Borne Encephalitis WHO Position Paper. 2011. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/policies/position-papers/tick-borne-encephalitis (accessed on 3 June 2024).
- Erber, W.; Khan, F.; Zavadska, D.; Freimane, Z.; Dobler, G.; Böhmer, M.M.; Jodar, L.; Schmitt, H.-J. Effectiveness of TBE vaccination in southern Germany and Latvia. Vaccine 2022, 40, 819–825. [Google Scholar] [CrossRef]
- Pilz, A.; Erber, W.; Schmitt, H.-J. Vaccine uptake in 20 countries in Europe 2020: Focus on tick-borne encephalitis (TBE). Ticks Tick-Borne Dis. 2023, 14, 102059. [Google Scholar] [CrossRef] [PubMed]
- Šmit, R. Cost-effectiveness of tick-borne encephalitis vaccination in Slovenian adults. Vaccine 2012, 30, 6301–6306. [Google Scholar] [CrossRef] [PubMed]
- Šmit, R.; Postma, M.J. Vaccines for tick-borne diseases and cost-effectiveness of vaccination: A public health challenge to reduce the diseases’ burden. Expert. Rev. Vaccines 2016, 15, 5–7. [Google Scholar] [CrossRef]
- Folkhälsomyndigheten [The Public Health Agency of Sweden] (Ed.) Health Economic Analysis of TBE Vaccination at SLL (Stockholms läns landsting [Stockholm County Council]). Compiled on Behalf of SLL; Folkhälsomyndigheten [The Public Health Agency of Sweden]: Solna, Sweden, 2018. [Google Scholar]
- Jürisson, M.; Taba, P.; Võrno, T.; Abram, M.; Eiche, I.-E.; Uusküla, A. Cost-Effectiveness of Tick-Borne Encephalitis Vaccination in Estonia; Institute of Health Care, University of Tartu: Tartu, Estonia, 2015; ISBN 978-9985-4-0879-7. [Google Scholar]
- Mihajlović, J.; Hovius, J.W.R.; Sprong, H.; Bogovič, P.; Postma, M.J.; Strle, F. Cost-effectiveness of a potential anti-tick vaccine with combined protection against Lyme borreliosis and tick-borne encephalitis in Slovenia. Ticks Tick-Borne Dis. 2019, 10, 63–71. [Google Scholar] [CrossRef]
- Desjeux, G.; Galoisy-Guibal, L.; Colin, C. Cost-benefit analysis of tick-borne encephalitis vaccinaion in French troops based in Kosovo. PharmacoEconomics 2005, 23, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.; Schwarz, M.; Beck, E.; Meszaros, K.; Schneider, M.; Ultsch, B.; Greiner, W. Public Health Impact and Cost-Effectiveness Analysis of Routine Infant 4CMenB Vaccination in Germany to Prevent Serogroup B Invasive Meningococcal Disease. Infect. Dis. Ther. 2022, 11, 367–387. [Google Scholar] [CrossRef]
- Mickienė, A.; Laiškonis, A.; Günther, G.; Vene, S.; Lundkvist, Å.; Lindquist, L. Tickborne Encephalitis in an Area of High Endemicity in Lithuania: Disease Severity and Long-Term Prognosis. Clin. Infect. Dis. 2002, 35, 650–658. [Google Scholar] [CrossRef]
- Veje, M.; Nolskog, P.; Petzold, M.; Bergström, T.; Lindén, T.; Peker, Y.; Studahl, M. Tick-Borne Encephalitis sequelae at long-term follow-up: A self-reported case-control study. Acta Neurol. Scand. 2016, 134, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Dobler, G.; Mackenstedt, U. TBE in Germany. In The TBE Book; Chapter 12b; Global Health Press: Singapore, 2022. [Google Scholar]
- Bohr, V.; Rasmussen, N.; Hansen, B.; Gade, A.; Kjersem, H.; Johnsen, N.; Paulson, O. Pneumococcal meningitis: An evaluation of prognostic factors in 164 cases based on mortality and on a study of lasting sequelae. J. Infect. 1985, 10, 143–157. [Google Scholar] [CrossRef]
- Robert Koch-Institut. “SurvStat@RKI 2.0 Individuelle Abfrage. Inzidenzwerte FSME,” Robert Koch-Institut. 2022. Available online: https://survstat.rki.de/Content/Query/Create.aspx (accessed on 3 February 2023).
- Bogovič, P.; Kastrin, A.; Lotrič-Furlan, S.; Ogrinc, K.; Županc, T.A.; Korva, M.; Knap, N.; Strle, F. Clinical and Laboratory Characteristics and Outcome of Illness Caused by Tick-Borne Encephalitis Virus without Central Nervous System Involvement. Emerg. Infect. Dis. 2022, 28, 291–301. [Google Scholar] [CrossRef]
- Eurostat. “Sterbetafel nach Alter und Geschlecht,” European Commission. 2020. Available online: https://ec.europa.eu/eurostat/databrowser/view/DEMO_MLIFETABLE/default/table?lang=de&category=demo.demo_mor (accessed on 3 February 2023).
- Eurostat. “Bevölkerung am 1. Januar nach Alter und Geschlecht,” European Commission. 2022. Available online: https://ec.europa.eu/eurostat/databrowser/view/DEMO_PJAN/default/table?lang=de&category=demo.demo_pop (accessed on 3 February 2023).
- Robert Koch-Institut. Impfquoten bei Erwachsenen in Deutschland; Robert Koch-Institut: Berlin, Germany, 2022. [Google Scholar]
- Lauer-Fischer LAUER-TAXE Online 4.0. Available online: https://portal.cgmlauer.cgm.com/LF/default.aspx?p=12000 (accessed on 7 June 2019).
- Scholz, S.; Damm, O.; Schneider, U.; Ultsch, B.; Wichmann, O.; Greiner, W. Epidemiology and cost of seasonal influenza in Germany—A claims data analysis. BMC Public Health 2019, 19, 1090. [Google Scholar] [CrossRef] [PubMed]
- IQWiG. Allgemeine Methoden. Entwurf für Version 7.0; Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG): Köln, Germany, 2022. [Google Scholar]
- Institute of Medicine (US) Committee on Assuring the Health of the Public in the 21st Century. Understanding Population Health and Its Determinants. In The Future of the Public’s Health in the 21st Century; National Academies Press (US): Washington, DC, USA, 2002. [Google Scholar]
- Hollmann, M.; Garin, O.; Galante, M.; Ferrer, M.; Dominguez, A.; Alonso, J. Impact of Influenza on Health-Related Quality of Life among Confirmed (H1N1)2009 Patients. PLoS ONE 2013, 8, e60477. [Google Scholar] [CrossRef]
- Livartowski, A.; Boucher, J.; Detournay, B.; Reinert, P. Cost-effectiveness evaluation of vaccination against Haemophilus influenzae invasive diseases in France. Vaccine 1996, 14, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Heinz, F.X.; Holzmann, H.; Essl, A.; Kundi, M. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine 2007, 25, 7559–7567. [Google Scholar] [CrossRef] [PubMed]
- Šmit, R.; Postma, M.J. The Burden of Tick-Borne Encephalitis in Disability-Adjusted Life Years (DALYs) for Slovenia. PLoS ONE 2015, 10, e0144988. [Google Scholar] [CrossRef]
- Tolley, K. “What Are Health Utilities?” Hayward Medical Communications. 2014. Available online: https://tolleyhealtheconomics.com/wp-content/uploads/2014/09/What-are-health-utilities-Final.pdf (accessed on 6 October 2024).
- Korves, C.T.; Goldie, S.J.; Murray, M.B. Cost-effectiveness of alternative blood-screening strategies for West Nile Virus in the United States. PLoS Med. 2006, 3, e21. [Google Scholar] [CrossRef] [PubMed]
- OECD. “OECD Brief May 2020. Health Care Prices”. Organisation for Economic Co-Operation and Development. 2020. Available online: https://www.oecd.org/health/health-systems/Health-Care-Prices-Brief-May-2020.pdf (accessed on 3 June 2024).
- Destatis-Statistisches Bundesamt. “Preise. Verbraucherpreisindex und Inflationsrate”. Statistisches Bundesamt. Available online: https://www.destatis.de/DE/Themen/Wirtschaft/Preise/Verbraucherpreisindex/_inhalt.html# (accessed on 23 March 2023).
- Bertram, M.Y.; Lauer, J.A.; De Joncheere, K.; Edejer, T.; Hutubessy, R.; Kieny, M.-P.; Hill, S.R. Cost–effectiveness thresholds: Pros and cons. Bull. World Health Organ. 2016, 94, 925–930. [Google Scholar] [CrossRef]
- Eurostat. “Reales BIP pro Kopf” European Commission. 2022. Available online: https://ec.europa.eu/eurostat/databrowser/view/sdg_08_10/default/table (accessed on 26 July 2023).
- Jit, M.; Mibei, W. Discounting in the evaluation of the cost-effectiveness of a vaccination programme: A critical review. Vaccine 2015, 33, 3788–3794. [Google Scholar] [CrossRef]
- Ghiani, M.; Hagemann, C.; Friedrich, J.; Maywald, U.; Wilke, T.; Von Eiff, C.; Malerczyk, C. Can risk area designation help increase vaccination coverage for Tick-Borne Encephalitis? Evidence from German claims data. Vaccine 2022, 40, 7335–7342. [Google Scholar] [CrossRef]
- Robert Koch-Institut. FSME: Risikogebiete in Deutschland. Epidemiol. Bull. 2007, 15, 129–133. [Google Scholar]
- Nygren, T.M.; Pilic, A.; Böhmer, M.M.; Wagner-Wiening, C.; Wichmann, O.; Hellenbrand, W. Recovery and sequelae in 523 adults and children with tick-borne encephalitis in Germany. Infection 2023, 51, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Zens, K.D.; Altpeter, E.S.; Wymann, M.N.; Mack, A.; Baer, N.B.; Haile, S.R.; Steffen, R.; Fehr, J.S.; Lang, P. ACombined Cross-Sectional Analysis and Case-Control Study Evaluating Tick-Borne Encephalitis Vaccination Coverage, Disease and Vaccine Effectiveness in Children 0–17 in Switzerland, 2005–2022. Eurosurveillance 2024, 29, 2300558. [Google Scholar] [CrossRef] [PubMed]
- Nygren, T.M.; Pilic, A.; Böhmer, M.M.; Wagner-Wiening, C.; Went, S.-B.; Wichmann, O.; Hellenbrand, W. Tick-borne encephalitis: Acute clinical manifestations and severity in 581 cases from Germany, 2018–2020. J. Infect. 2023, 86, 369–375. [Google Scholar] [CrossRef]
- Daniel Mullins, C.; Onwudiwe, N.C.; Branco de Araújo, G.T.; Chen, W.; Xuan, J.; Tichopád, A.; Hu, S. Guidance Document: Global Pharmacoeconomic Model Adaption Strategies. Value Health Reg. Issues 2014, 5, 7–13. [Google Scholar] [CrossRef] [PubMed]
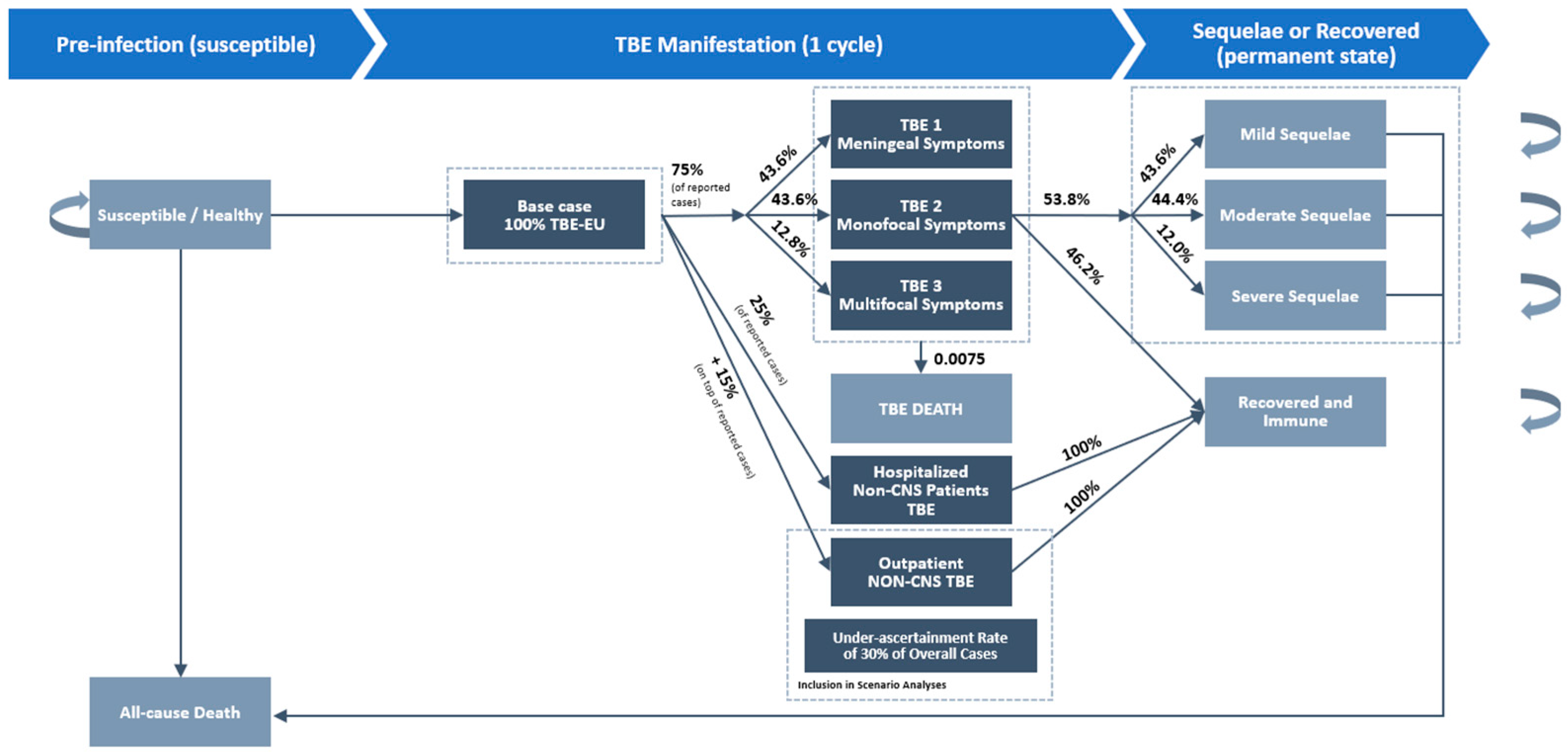
| Health State | Definition | Source |
|---|---|---|
| Susceptible | No present TBE infection. | |
| TBE 1 | Primarily meningeal symptoms including fever, headache, rigidity of the neck, and nausea. | [21,22] |
| TBE 2 | Disease with monofocal symptoms of the CNS and/or moderate diffuse brain dysfunction. | [21,22] |
| TBE 3 | Disease with multifocal symptoms of the CNS and/or severe diffuse brain dysfunction. | [21,22] |
| Inpatient non-CNS TBEv | TBEv cases without CNS manifestation in inpatient care. These infections are usually accompanied by unspecific, flu-like symptoms. | [6,21] |
| Outpatient non-CNS TBEv | TBEv cases without CNS manifestation in outpatient care. These infections are usually accompanied by unspecific, flu-like symptoms. | [6] |
| TBE death | Death due to TBE | [4,15,17,18] |
| Mild sequelae | Presence of one or more mild symptoms, including dizziness, memory deficits, headache, tiredness, slight hearing impairment, minor psychological problems, or unsteady gait. Daily life and working abilities are not markedly affected. | [24] |
| Moderate sequelae | Presence of many or more severe symptoms, ataxia of gait, paresis of the extremities, pronounced dementia, or severe deafness. Patient affected in daily life and working ability. | [24] |
| Severe sequelae | More pronounced clinical disabilities, often seriously affecting social life and working capabilities, and in a few cases, requiring institutional care. | [24] |
| Recovered and immune | Recovered from TBE without any sequelae. Immunity persists for the remainder of the model. | |
| All-cause death | All-cause death, based on age- and gender-stratified data extracted from national life and death tables. |
| Input Parameter | Base Case Value | Reference |
|---|---|---|
| Population | ||
| Population by age and gender 2022 | Age- and gender-specific | [28] |
| Epidemiology | ||
| Age-specific incidence rate—average from 2018 to 2022 | Age- and gender-specific | [25] |
| Uptake | ||
| Proportion of people receiving primary immunization: completion of three doses | 0.19 | [29] |
| Vaccine effectiveness | ||
| VE for first three years | 0.966 | [10] |
| Annual waning rate starting in year four | 0.05 | Expert assumption |
| Transition rates | ||
| Probability of TBE death | 0.008 | [21] |
| Probability of all-cause death—age-specific lifetables 2021 | Age- and gender-specific | [27] |
| Proportion of patients suffering from non-CNS TBEv (inpatient setting), among reported cases | 0.25 | [21] |
| Additional non-CNS TBEv (outpatient setting), as a proportion of reported cases | 0.15 | Expert assumption |
| Probability of TBE 1 | 0.436 | [21] |
| Probability of TBE 2 | 0.436 | [21] |
| Probability of TBE 3 | 0.128 | [21] |
| Probability of developing lifelong sequelae (Sequelae were classified as “mild”, “moderate”, or “severe”, depending on their influence on the patient’s quality of life, following [24]). | 0.538 | [21] |
| Mild sequelae | 0.436 | [21] |
| Moderate sequelae | 0.444 | [21] |
| Severe sequelae | 0.120 | [21] |
| Cost parameter Country-adjusted cost value (value in original publication) | ||
| Cost of vaccination (per dose) | EUR 50.12 | [30] |
| Administration costs | EUR 8.62 (EUR 7.90) | [20] |
| Direct medical annual costs per TBE 1 case | EUR 1627.58 (EUR 1235.00) | [18] |
| Direct medical annual costs per TBE 2 case | EUR 3841.62 (EUR 2915.00) | [18] |
| Direct medical annual costs per TBE 3 case | EUR 14,628.48 (EUR 11,100.00) | [18] |
| Direct medical annual costs, mild sequelae | EUR 98.69 (EUR 70.00) | [18] |
| Direct medical annual costs, moderate sequelae | EUR 172.00 (EUR 122.00) | [18] |
| Direct medical annual costs, severe sequelae | EUR 41,589.27 (EUR 28,952.00) | [18] |
| Direct medical costs, non-CNS TBE (inpatient setting) | EUR 2229.81 (EUR 2033.00) | [31] |
| Direct medical costs, non-CNS TBE (outpatient setting) | EUR 284.90 (EUR 259.75) | [31] |
| Discounting | ||
| Discount rate (costs) | 0.030 | [32] |
| Discount rate (health utility) | 0.030 | [32] |
| Utility values | ||
| Utility, TBE 1 | 0.39 × 0.0137 years (duration of 5 days) | [33] |
| Utility, TBE 2 | 0.24 × 0.0055 years + 0.28 × 0.0137 years (duration of 7 days) | [33] |
| Utility, TBE 3 | 0.24 × 0.0055 years + 0.28 × 0.0137 years (duration of 7 days) | [33] |
| Utility, non-CNS TBE (inpatient setting) | 0.495 × 0.0137 years (duration of 5 days) | [34] |
| Utility, non-CNS TBE (outpatient setting) | 0.495 × 0.0137 years (duration of 5 days) | [34] |
| Utility, mild sequelae | 0.023 | [35] |
| Utility, moderate sequelae | 0.160 | [35] |
| Utility, severe sequelae | 0.629 | [35] |
| Base Case Assumptions Strategies 1 + 2 | Variation in Scenario Analysis | |
|---|---|---|
| Uptake of primary vaccination | 0.19 | 0.40 |
| Yearly waning rate | 0.05 | 0.008 |
| Inclusion of outpatient non-CNS cases | 0 | 0.15 |
| Multiplier to account for under-ascertainment | No | 0.3 |
| Discount rate HU | 0.03 | 0.015 |
| Vaccination Strategy | Vaccination Strategy 1 | Vaccination Strategy 2 |
|---|---|---|
| Target group | Population of ≥1–85 years | Population of ≥60–85 years |
| Base case averted TBE cases (hospitalized, CNS involvement) | 1842 | 310 |
| Base case gained QALYs | 10,318 | 9125 |
| Base case cost per QALY gained in EUR | EUR 253,529 | EUR 82,358 |
| VE for first three years 0.937 | EUR 254,891 | EUR 82,499 |
| Uptake rate primary immunization 0.40 | EUR 459,805 | EUR 167,155 |
| Waning 0.008 | EUR 230,970 | EUR 81,475 |
| Under ascertainment 0.3 | EUR 193,144 | EUR 62,918 |
| Inclusion of non-CNS TBEv cases (outpatient setting)/rate 0.15 | EUR 253,502 | EUR 82,355 |
| Discounting HU 0.015 | EUR 136,337 | EUR 43,981 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, M.; Lintener, H.; Henkel, V.; Pilz, A.; Halsby, K.; Malerczyk, C.; Madhava, H.; Moïsi, J.C.; Yu, H.; Schley, K. Does the Vaccination against Tick-Borne Encephalitis Offer Good Value for Money for Incidence Rates below the WHO Threshold for Endemicity? A Case Study for Germany. Vaccines 2024, 12, 1165. https://doi.org/10.3390/vaccines12101165
Müller M, Lintener H, Henkel V, Pilz A, Halsby K, Malerczyk C, Madhava H, Moïsi JC, Yu H, Schley K. Does the Vaccination against Tick-Borne Encephalitis Offer Good Value for Money for Incidence Rates below the WHO Threshold for Endemicity? A Case Study for Germany. Vaccines. 2024; 12(10):1165. https://doi.org/10.3390/vaccines12101165
Chicago/Turabian StyleMüller, Malina, Hannah Lintener, Vivien Henkel, Andreas Pilz, Kate Halsby, Claudius Malerczyk, Harish Madhava, Jennifer C. Moïsi, Holly Yu, and Katharina Schley. 2024. "Does the Vaccination against Tick-Borne Encephalitis Offer Good Value for Money for Incidence Rates below the WHO Threshold for Endemicity? A Case Study for Germany" Vaccines 12, no. 10: 1165. https://doi.org/10.3390/vaccines12101165
APA StyleMüller, M., Lintener, H., Henkel, V., Pilz, A., Halsby, K., Malerczyk, C., Madhava, H., Moïsi, J. C., Yu, H., & Schley, K. (2024). Does the Vaccination against Tick-Borne Encephalitis Offer Good Value for Money for Incidence Rates below the WHO Threshold for Endemicity? A Case Study for Germany. Vaccines, 12(10), 1165. https://doi.org/10.3390/vaccines12101165





