Detection of PCV2d in Vaccinated Pigs in Colombia and Prediction of Vaccine T Cell Epitope Coverage against Circulating Strains Using EpiCC Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Detection of PCV2 and Sequencing
2.3. Sequencing and Phylogenetic Analysis of PCV2-Capsid Protein (ORF2)
2.4. PCV2 Real-Time PCR in Serum and Tissue Samples
2.5. PCVAD Categorization
2.6. Histopathology
2.7. T Cell Epitope Content Comparison (EpiCC) Analysis
2.8. Statistical Analysis
3. Results
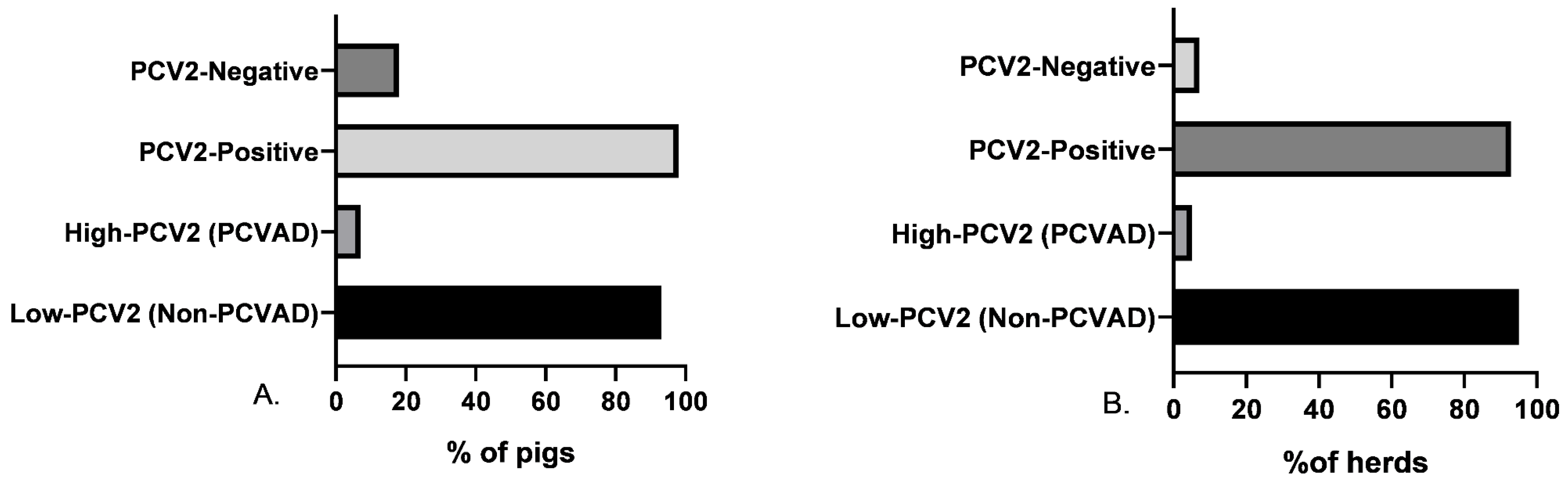
3.1. PCV2 Detection
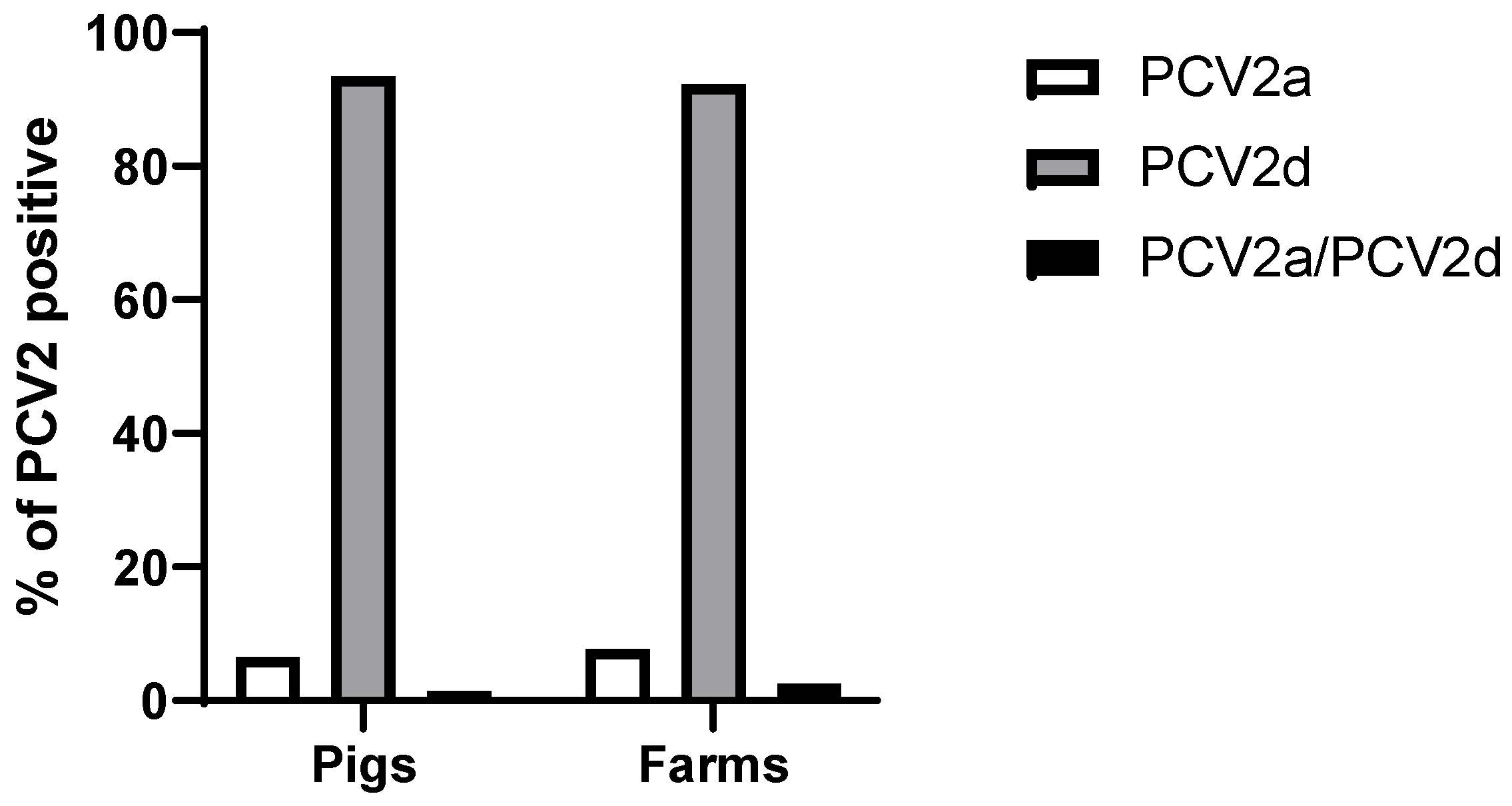
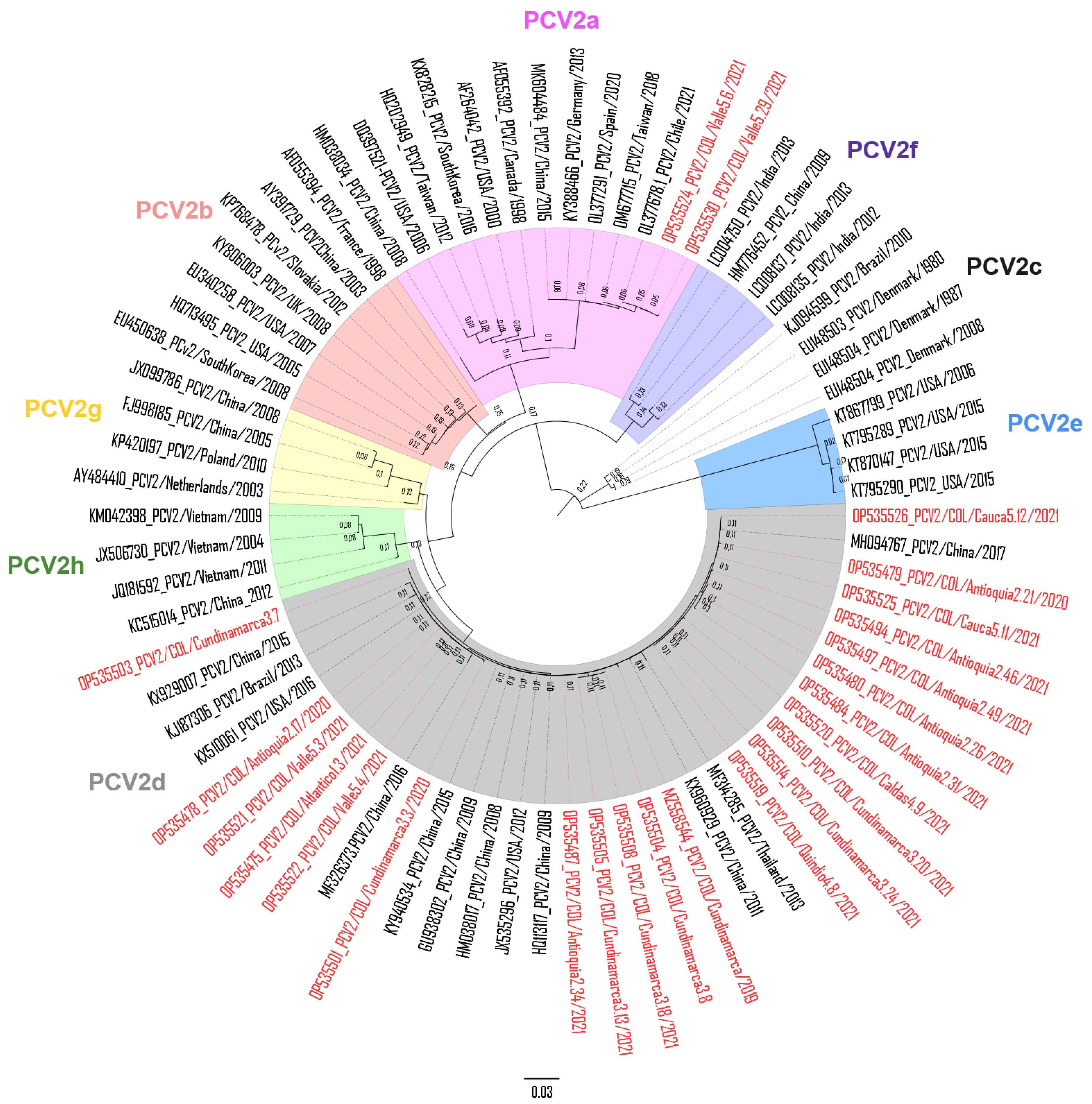
3.2. Sequence Analysis of PCV2
3.3. Histopathology
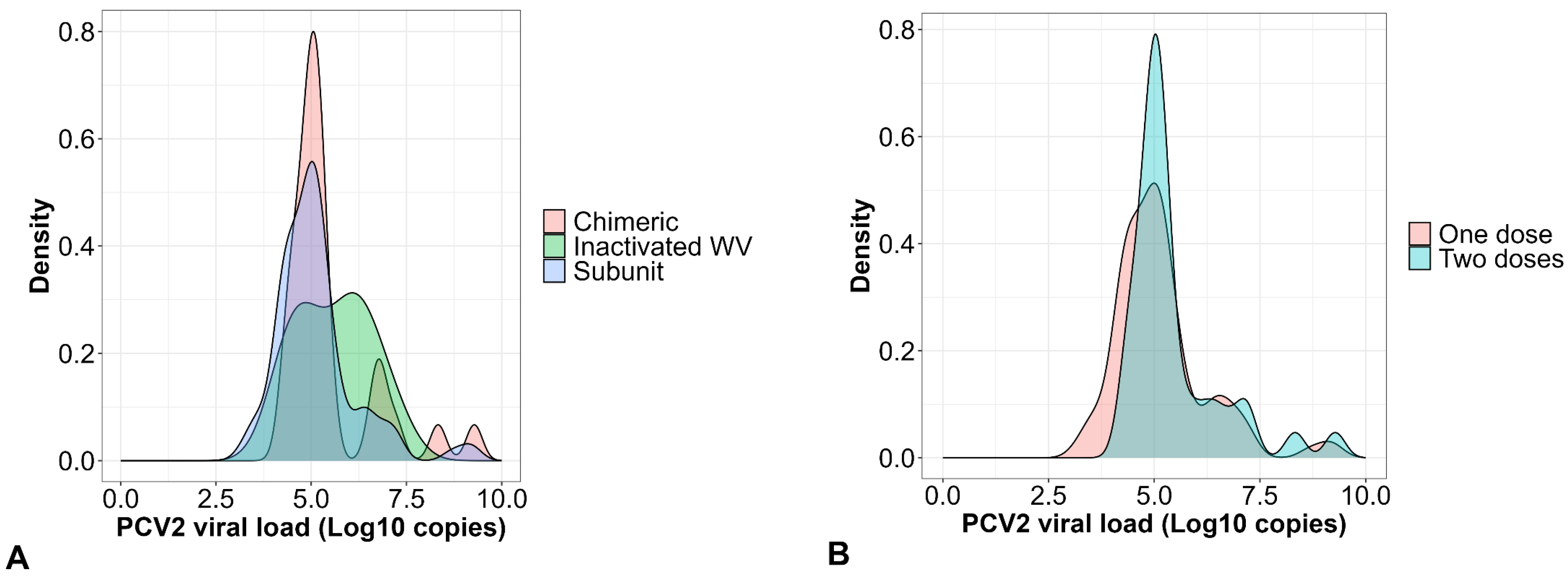
3.4. EpiCC Analysis of T Cell Epitope Relatedness and Coverage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosario, K.; Breitbart, M.; Harrach, B.; Segalés, J.; Delwart, E.; Biagini, P.; Varsani, A. Revisiting the taxonomy of the family Circoviridae: Establishment of the genus Cyclovirus and removal of the genus Gyrovirus. Arch. Virol. 2017, 162, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Palinski, R.; Piñeyro, P.; Shang, P.; Yuan, F.; Guo, R.; Fang, Y.; Byers, E.; Hause, B.M. A Novel Porcine Circovirus Distantly Related to Known Circoviruses Is Associated with Porcine Dermatitis and Nephropathy Syndrome and Reproductive Failure. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-H.; Hu, W.-Q.; Li, J.-Y.; Liu, T.-N.; Zhou, J.-Y.; Opriessnig, T.; Xiao, C.-T. Novel circovirus species identified in farmed pigs designated as Porcine circovirus 4, Hunan province, China. Transbound. Emerg. Dis. 2020, 67, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Karuppannan, A.K.; Castro, A.M.M.G.; Xiao, C.-T. Porcine circoviruses: Current status, knowledge gaps and challenges. Virus Res. 2020, 286, 198044. [Google Scholar] [CrossRef]
- Hamel, A.L.; Lin, L.L.; Nayar, G.P. Nucleotide sequence of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. J. Virol. 1998, 72, 5262–5267. [Google Scholar] [CrossRef]
- Mankertz, A.; Mankertz, J.; Wolf, K.; Buhk, H.J. Identification of a protein essential for replication of porcine circovirus. J. Gen. Virol. 1998, 79 Pt 2, 381–384. [Google Scholar] [CrossRef]
- Nawagitgul, P.; Morozov, I.; Bolin, S.R.; Harms, P.A.; Sorden, S.D.; Paul, P.S. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J. Gen. Virol. 2000, 81, 2281–2287. [Google Scholar] [CrossRef]
- Liu, J.; Chen, I.; Du, Q.; Chua, H.; Kwang, J. The ORF3 protein of porcine circovirus type 2 is involved in viral pathogenesis in vivo. J. Virol. 2006, 80, 5065–5073. [Google Scholar] [CrossRef]
- Ellis, J.; Hassard, L.; Clark, E.; Harding, J.; Allan, G.; Willson, P.; Strokappe, J.; Martin, K.; McNeilly, F.; Meehan, B.; et al. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can. Vet. J. 1998, 39, 44–51. [Google Scholar]
- Zhai, S.-L.; Chen, S.-N.; Xu, Z.-H.; Tang, M.-H.; Wang, F.-G.; Li, X.-J.; Sun, B.-B.; Deng, S.-F.; Hu, J.; Lv, D.-H.; et al. Porcine circovirus type 2 in China: An update on and insights to its prevalence and control. Virol. J. 2014, 11, 88. [Google Scholar] [CrossRef]
- Franzo, G.; Segalés, J. Porcine circovirus 2 (PCV-2) genotype update and proposal of a new genotyping methodology. PLoS ONE 2018, 13, e0208585. [Google Scholar] [CrossRef] [PubMed]
- Maity, H.K.; Samanta, K.; Deb, R.; Gupta, V.K. Revisiting porcine circovirus infection: Recent insights and its significance in the piggery sector. Vaccines 2023, 11, 1308. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.-T.; Halbur, P.G.; Opriessnig, T. Global molecular genetic analysis of porcine circovirus type 2 (PCV2) sequences confirms the presence of four main PCV2 genotypes and reveals a rapid increase of PCV2d. J. Gen. Virol. 2015, 96, 1830–1841. [Google Scholar] [CrossRef]
- Opriessnig, T.; Xiao, C.-T.; Gerber, P.F.; Halbur, P.G. Emergence of a novel mutant PCV2b variant associated with clinical PCVAD in two vaccinated pig farms in the U.S. concurrently infected with PPV2. Vet. Microbiol. 2013, 163, 177–183. [Google Scholar] [CrossRef]
- Franzo, G.; Cortey, M.; Segalés, J.; Hughes, J.; Drigo, M. Phylodynamic analysis of porcine circovirus type 2 reveals global waves of emerging genotypes and the circulation of recombinant forms. Mol. Phylogenet. Evol. 2016, 100, 269–280. [Google Scholar] [CrossRef]
- Rincón Monroy, M.A.; Ramirez-Nieto, G.C.; Vera, V.J.; Correa, J.J.; Mogollón-Galvis, J.D. Detection and molecular characterization of porcine circovirus type 2 from piglets with porcine circovirus associated diseases in Colombia. Virol. J. 2014, 11, 143. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vargas-Bermudez, D.S.; Mogollón, J.D.; Jaime, J. The Prevalence and Genetic Diversity of PCV3 and PCV2 in Colombia and PCV4 Survey during 2015-2016 and 2018-2019. Pathogens 2022, 11, 633. [Google Scholar] [CrossRef]
- Segalés, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef]
- Opriessnig, T.; Xiao, C.-T.; Halbur, P.G.; Gerber, P.F.; Matzinger, S.R.; Meng, X.-J. A commercial porcine circovirus (PCV) type 2a-based vaccine reduces PCV2d viremia and shedding and prevents PCV2d transmission to naïve pigs under experimental conditions. Vaccine 2017, 35, 248–254. [Google Scholar] [CrossRef]
- Seo, H.W.; Han, K.; Park, C.; Chae, C. Clinical, virological, immunological and pathological evaluation of four porcine circovirus type 2 vaccines. Vet. J. 2014, 200, 65–70. [Google Scholar] [CrossRef]
- Kixmöller, M.; Ritzmann, M.; Eddicks, M.; Saalmüller, A.; Elbers, K.; Fachinger, V. Reduction of PMWS-associated clinical signs and co-infections by vaccination against PCV2. Vaccine 2008, 26, 3443–3451. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Huang, L.; Bussalleu, E.; Lefebvre, D.J.; Fort, M.; Van Doorsselaere, J.; Nauwynck, H.J. Antigenic subtyping and epitopes’ competition analysis of porcine circovirus type 2 using monoclonal antibodies. Vet. Microbiol. 2012, 157, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Reiner, G.; Hofmeister, R.; Willems, H. Genetic variability of porcine circovirus 2 (PCV2) field isolates from vaccinated and non-vaccinated pig herds in Germany. Vet. Microbiol. 2015, 180, 41–48. [Google Scholar] [CrossRef]
- Gava, D.; Serrão, V.H.B.; Fernandes, L.T.; Cantão, M.E.; Ciacci-Zanella, J.R.; Morés, N.; Schaefer, R. Structure analysis of capsid protein of Porcine circovirus type 2 from pigs with systemic disease. Braz. J. Microbiol 2018, 49, 351–357. [Google Scholar] [CrossRef]
- Firth, C.; Charleston, M.A.; Duffy, S.; Shapiro, B.; Holmes, E.C. Insights into the evolutionary history of an emerging livestock pathogen: Porcine circovirus 2. J. Virol. 2009, 83, 12813–12821. [Google Scholar] [CrossRef]
- Bandrick, M.; Gutiérrez, A.H.; Desai, P.; Rincon, G.; Martin, W.D.; Terry, F.E.; De Groot, A.S.; Foss, D.L. T cell epitope content comparison (EpiCC) analysis demonstrates a bivalent PCV2 vaccine has greater T cell epitope overlap with field strains than monovalent PCV2 vaccines. Vet. Immunol. Immunopathol. 2020, 223, 110034. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Grau-Roma, L.; Cortey, M.; Fort, M.; Rodríguez, F.; Sibila, M.; Segalés, J. Pigs naturally exposed to porcine circovirus type 2 (PCV2) generate antibody responses capable to neutralise PCV2 isolates of different genotypes and geographic origins. Vet. Res. 2014, 45, 29. [Google Scholar] [CrossRef]
- De Groot, A.S.; Martin, W. From immunome to vaccine: Epitope mapping and vaccine design tools. Novartis Found. Symp. 2003, 254, 57–72; discussion 72. [Google Scholar] [PubMed]
- Moise, L.; Gutiérrez, A.H.; Khan, S.; Tan, S.; Ardito, M.; Martin, W.D.; De Groot, A.S. New Immunoinformatics Tools for Swine: Designing Epitope-Driven Vaccines, Predicting Vaccine Efficacy, and Making Vaccines on Demand. Front. Immunol. 2020, 11, 563362. [Google Scholar] [CrossRef]
- Oli, A.N.; Obialor, W.O.; Ifeanyichukwu, M.O.; Odimegwu, D.C.; Okoyeh, J.N.; Emechebe, G.O.; Adejumo, S.A.; Ibeanu, G.C. Immunoinformatics and vaccine development: An overview. Immunotargets Ther. 2020, 9, 13–30. [Google Scholar] [CrossRef]
- Fenaux, M.; Opriessnig, T.; Halbur, P.G.; Elvinger, F.; Meng, X.J. A chimeric porcine circovirus (PCV) with the immunogenic capsid gene of the pathogenic PCV type 2 (PCV2) cloned into the genomic backbone of the nonpathogenic PCV1 induces protective immunity against PCV2 infection in pigs. J. Virol. 2004, 78, 6297–6303. [Google Scholar] [CrossRef]
- Gutiérrez, A.H.; Rapp-Gabrielson, V.J.; Terry, F.E.; Loving, C.L.; Moise, L.; Martin, W.D.; De Groot, A.S. T-cell epitope content comparison (EpiCC) of swine H1 influenza A virus hemagglutinin. Influenza Other Respir. Viruses 2017, 11, 531–542. [Google Scholar] [CrossRef]
- Foss, D.L.; Gutiérrez, A.H.; Bandrick, M.; Perumbakkam, S.; De Groot, A.S.; Martin, W.D.; Terry, F.E.; Aldaz, A.; Allison, J.R.D.; Angulo, J. Comparison of predicted T cell epitopes in porcine circovirus type 2 isolates from 2017 to 2021 and selected vaccines (EpiCC analysis) confirms the global relevance of a bivalent vaccine approach. Vet. Vaccine 2023, 2, 100028. [Google Scholar] [CrossRef]
- Yang, K.; Jiao, Z.; Zhou, D.; Guo, R.; Duan, Z.; Tian, Y. Development of a multiplex PCR to detect and discriminate porcine circoviruses in clinical specimens. BMC Infect. Dis. 2019, 19, 778. [Google Scholar] [CrossRef]
- Oliver-Ferrando, S.; Segalés, J.; López-Soria, S.; Callén, A.; Merdy, O.; Joisel, F.; Sibila, M. Evaluation of natural porcine circovirus type 2 (PCV2) subclinical infection and seroconversion dynamics in piglets vaccinated at different ages. Vet. Res. 2016, 47, 121. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Opriessnig, T.; Prickett, J.R.; Madson, D.M.; Shen, H.-G.; Juhan, N.M.; Pogranichniy, R.R.; Meng, X.-J.; Halbur, P.G. Porcine circovirus type 2 (PCV2)-infection and re-inoculation with homologous or heterologous strains: Virological, serological, pathological and clinical effects in growing pigs. Vet. Res. 2010, 41, 31. [Google Scholar] [CrossRef]
- Opriessnig, T.; Meng, X.-J.; Halbur, P.G. Porcine circovirus type 2 associated disease: Update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J. Vet. Diagn. Investig. 2007, 19, 591–615. [Google Scholar] [CrossRef]
- Pallarés, F.J.; Añón, J.A.; Rodríguez-Gómez, I.M.; Gómez-Laguna, J.; Fabré, R.; Sánchez-Carvajal, J.M.; Ruedas-Torres, I.; Carrasco, L. Prevalence of mycoplasma-like lung lesions in pigs from commercial farms from Spain and Portugal. Porc. Health Manag. 2021, 7, 26. [Google Scholar] [CrossRef]
- Gutiérrez, A.H.; Loving, C.; Moise, L.; Terry, F.E.; Brockmeier, S.L.; Hughes, H.R.; Martin, W.D.; De Groot, A.S. In vivo validation of predicted and conserved T cell epitopes in a swine influenza model. PLoS ONE 2016, 11, e0159237. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Kim, D.R. pcr: An R package for quality assessment, analysis and testing of qPCR data. PeerJ 2018, 6, e4473. [Google Scholar] [CrossRef]
- Kekarainen, T.; Gonzalez, A.; Llorens, A.; Segalés, J. Genetic variability of porcine circovirus 2 in vaccinating and non-vaccinating commercial farms. J. Gen. Virol. 2014, 95, 1734–1742. [Google Scholar] [CrossRef]
- Vargas-Bermudez, D.S.; Diaz, A.; Polo, G.; Mogollon, J.D.; Jaime, J. Infection and Coinfection of Porcine-Selected Viruses (PPV1 to PPV8, PCV2 to PCV4, and PRRSV) in Gilts and Their Associations with Reproductive Performance. Vet. Sci. 2024, 11, 185. [Google Scholar] [CrossRef]
- Gerber, P.F.; Johnson, J.; Shen, H.; Striegel, D.; Xiao, C.-T.; Halbur, P.G.; Opriessnig, T. Association of concurrent porcine circovirus (PCV) 2a and 2b infection with PCV associated disease in vaccinated pigs. Res. Vet. Sci. 2013, 95, 775–781. [Google Scholar] [CrossRef]
- Park, K.H.; Oh, T.; Yang, S.; Cho, H.; Kang, I.; Chae, C. Evaluation of a porcine circovirus type 2a (PCV2a) vaccine efficacy against experimental PCV2a, PCV2b, and PCV2d challenge. Vet. Microbiol. 2019, 231, 87–92. [Google Scholar] [CrossRef]
- Woźniak, A.; Miłek, D.; Matyba, P.; Stadejek, T. Real-Time PCR Detection Patterns of Porcine Circovirus Type 2 (PCV2) in Polish Farms with Different Statuses of Vaccination against PCV2. Viruses 2019, 11, 1135. [Google Scholar] [CrossRef]
- Cho, H.; Oh, T.; Suh, J.; Chae, C. A Comparative Field Evaluation of the Effect of Growth Performance Between Porcine Circovirus Type 2a (PCV2a)- and PCV2b-Based Bivalent Vaccines Containing PCV2 and Mycoplasma hyopneumoniae. Front. Vet. Sci. 2022, 9, 859344. [Google Scholar] [CrossRef]
- Fan, M.; Bian, L.; Tian, X.; Hu, Z.; Wu, W.; Sun, L.; Yuan, G.; Li, S.; Yue, L.; Wang, Y.; et al. Infection characteristics of porcine circovirus type 2 in different herds from intensive farms in China, 2022. Front. Vet. Sci. 2023, 10, 1187753. [Google Scholar] [CrossRef]
- Nauwynck, H.J.; Sanchez, R.; Meerts, P.; Lefebvre, D.J.; Saha, D.; Huang, L.; Misinzo, G. Cell tropism and entry of porcine circovirus 2. Virus Res. 2012, 164, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Van Renne, N.; Wei, R.; Pochet, N.; Nauwynck, H.J. Dissecting clinical outcome of porcine circovirus type 2 with in vivo derived transcriptomic signatures of host tissue responses. BMC Genom. 2018, 19, 831. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Van Renne, N.; Nauwynck, H.J. Strain-Dependent Porcine Circovirus Type 2 (PCV2) Entry and Replication in T-Lymphoblasts. Viruses 2019, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Wahaab, A.; Shi, K.; Mustafa, B.E.; Zhang, Y.; Zhang, J.; Li, Z.; Qiu, Y.; Li, B.; Liu, K.; et al. Molecular epidemic characteristics and genetic evolution of porcine circovirus type 2 (PCV2) in swine herds of shanghai, china. Viruses 2022, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.-L.; Chen, S.-N.; Wei, Z.-Z.; Zhang, J.-W.; Huang, L.; Lin, T.; Yue, C.; Ran, D.-L.; Yuan, S.-S.; Wei, W.-K.; et al. Co-existence of multiple strains of porcine circovirus type 2 in the same pig from China. Virol. J. 2011, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhan, Y.; Wang, D.; Xie, X.; Liu, T.; Liu, W.; Wang, N.; Deng, Z.; Lei, H.; Yang, Y.; et al. Evidence of natural co-infection with PCV2b subtypes in vivo. Arch. Virol. 2017, 162, 2015–2020. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, S.; Sun, P.; Khan, A.; Guo, J.; Zheng, X.; Sun, Y.; Fan, K.; Yin, W.; Li, H.; et al. Matrine combined with Osthole inhibited the PERK apoptosis of splenic lymphocytes in PCV2-infected mice model. BMC Vet. Res. 2023, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Correa-Fiz, F.; Franzo, G.; Llorens, A.; Segalés, J.; Kekarainen, T. Porcine circovirus 2 (PCV-2) genetic variability under natural infection scenario reveals a complex network of viral quasispecies. Sci. Rep. 2018, 8, 15469. [Google Scholar] [CrossRef]
- Franzo, G.; Tucciarone, C.M.; Cecchinato, M.; Drigo, M. Porcine circovirus type 2 (PCV2) evolution before and after the vaccination introduction: A large scale epidemiological study. Sci. Rep. 2016, 6, 39458. [Google Scholar] [CrossRef]
- Noh, Y.-H.; Kim, S.-C.; Jeong, C.-G.; Lee, S.-C.; Lee, D.-U.; Yoon, I.-J.; Kim, W.-I. Pathological Evaluation of Porcine Circovirus 2d (PCV2d) Strain and Comparative Evaluation of PCV2d and PCV2b Inactivated Vaccines against PCV2d Infection in a Specific Pathogen-Free (SPF) Yucatan Miniature Pig Model. Vaccines 2022, 10, 1469. [Google Scholar] [CrossRef]
- Bandrick, M.; Balasch, M.; Heinz, A.; Taylor, L.; King, V.; Toepfer, J.; Foss, D. A bivalent porcine circovirus type 2 (PCV2), PCV2a-PCV2b, vaccine offers biologically superior protection compared to monovalent PCV2 vaccines. Vet. Res. 2022, 53, 12. [Google Scholar] [CrossRef]
- Oh, T.; Suh, J.; Park, K.H.; Yang, S.; Cho, H.; Chae, C. A Comparison of Pathogenicity and Virulence of Three Porcine Circovirus Type 2 (PCV2) Genotypes (a, b, and d) in Pigs Singularly Inoculated with PCV2 and Dually Inoculated with Mycoplasma hyopneumoniae and PCV2. Pathogens 2021, 10, 979. [Google Scholar] [CrossRef]
- Oh, T.; Park, K.H.; Yang, S.; Cho, H.; Suh, J.; Chae, C. Pathogenicity of Porcine Circovirus Type 2d (PCV2d) in Pigs Infected with PCV2d or Co-infected with Mycoplasma hyopneumoniae and PCV2d or with Porcine Reproductive and Respiratory Syndrome Virus and PCV2d. J. Comp. Pathol. 2021, 187, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Oh, T.; Park, K.; Yang, S.; Cho, H.; Chae, C. A Comparison of Virulence of Three Porcine Circovirus Type 2 (PCV2) Genotypes (a, b, and d) in Pigs Singularly Inoculated with PCV2 and Dually Inoculated with PCV2 and Porcine Reproductive and Respiratory Syndrome Virus. Pathogens 2021, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Meyers, L.M.; Gutiérrez, A.H.; Boyle, C.M.; Terry, F.; McGonnigal, B.G.; Salazar, A.; Princiotta, M.F.; Martin, W.D.; De Groot, A.S.; Moise, L. Highly conserved, non-human-like, and cross-reactive SARS-CoV-2 T cell epitopes for COVID-19 vaccine design and validation. npj Vaccines 2021, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Kotraiah, V.; Phares, T.W.; Terry, F.E.; Hindocha, P.; Silk, S.E.; Nielsen, C.M.; Moise, L.; Tucker, K.D.; Ashfield, R.; Martin, W.D.; et al. Identification and Immune Assessment of T Cell Epitopes in Five Plasmodium falciparum Blood Stage Antigens to Facilitate Vaccine Candidate Selection and Optimization. Front. Immunol. 2021, 12, 690348. [Google Scholar] [CrossRef]
- Cao, Q.M.; Tian, D.; Heffron, C.L.; Subramaniam, S.; Opriessnig, T.; Foss, D.L.; Calvert, J.G.; Meng, X.-J. Cytotoxic T lymphocyte epitopes identified from a contemporary strain of porcine reproductive and respiratory syndrome virus enhance CD4+CD8+ T, CD8+ T, and γδ T cell responses. Virology 2019, 538, 35–44. [Google Scholar] [CrossRef]
- Franzo, G.; Legnardi, M.; Tucciarone, C.M.; Drigo, M.; Martini, M.; Cecchinato, M. Evolution of infectious bronchitis virus in the field after homologous vaccination introduction. Vet. Res. 2019, 50, 92. [Google Scholar] [CrossRef]


| Province | Sow Density Population | Number of Herds | Number of Pigs Scrutinized | Serum | Lung | Lymph Node | Spleen |
|---|---|---|---|---|---|---|---|
| Cundinamarca | 34,646 | 11 | 25 | 22 | 25 | 23 | 23 |
| Antioquia | 143,821 | 38 | 69 | 57 | 63 | 58 | 58 |
| Atlántico | 18,910 | 5 | 10 | 8 | 9 | 8 | 8 |
| Valle del Cauca | 56,883 | 20 | 39 | 37 | 39 | 37 | 37 |
| Eje Cafetero | 30,175 | 10 | 24 | 23 | 24 | 24 | 24 |
| Total | 284,435 | 84 | 167 | 147 | 160 | 150 | 150 |
| Province | % Positive Samples (Positive/All Tested) | % Positive Herds (Positive/All Tested) | Viral Loads (log10 Copies/mL or g for Serum and the Tissues, Respectively) Minimum–Maximum, Mean |
|---|---|---|---|
| Cundinamarca | 100 (25/25) | 100 (11/11) | 4.3–9.28, 5.68 |
| Valle | 82.1 (32/39) | 90 (18/20) | 3.2–8.68, 5.08 |
| Antioquia | 82.6 (57/69) | 92.1 (35/38) | 3.57–9.07, 5.2 |
| Eje Cafetero | 66.6 (16/24) | 80 (8/10) | 3.99–5.5, 4.75 |
| Atlántico | 70 (7/10) | 100 (5/5) | 4.41–5.17, 4.9 |
| Total | 82 (137/167) | 92.8 (78/84) | 3.2–9.28, 5.19 |
| PCV2a (AF055392) | PCV2b (AF055394) | PCV2c (EU148503) | PCV2d (HM038017) | PCV2e (KT795289) | PCV2f (LC008135) | PCV2g (AY484410) | PCV2h (JX506730) | ||
|---|---|---|---|---|---|---|---|---|---|
| Identity ORF2 (nucleotide) | Colombian PCV2a sequences | 93.7–100 | 90–90.2 | 85.8–86.2 | 88.1–89 | 80.9–81.1 | 90.2–90.3 | 90.3 | 91 |
| Colombian PCV2d sequences | 90.2–91 | 92.9–93.7 | 88.8–89.4 | 99.3–100 | 83–84.3 | 91.9–92.8 | 96.2–96.9 | 94.2–94.9 | |
| Identity ORF2 (aa) | Colombian PCV2a sequences | 90.8–100 | 85.4–86.4 | 79–80 | 84.7–84.8 | 76.4–76.6 | 85.1–86.2 | 86.1–87.8 | 86.7–86.9 |
| Colombian PCV2d sequences | 89–91.1 | 92.6–95 | 85.2–86.8 | 99.1–100 | 79.3–80.5 | 91–94.4 | 94–95.9 | 95.5–96.4 |
| Province | Number of Sequences | EpiCC Scores | T Cell Epitope Coverage % | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | VacAB | VacAlt-a I | VacAlt-a II | VacAB | VacAlt-a I | VacAlt-a II | ||
| All | 57 | 10.38 (10.16–10.59) | 8.36 (6.77–8.74) | 6.30 (6.28–7.13) | 6.63 (6.61–7.38) | 80.6 (66.62–83.24) | 60.8 (59.32–69.65) | 63.9 (62.42–72.05) |
| Atlántico | 1 | 10.35 | 8.40 | 6.28 | 6.61 | 81.1 | 60.7 | 63.9 |
| Antioquia | 23 | 10.35 (10.35–10.43) | 8.40 (8.40) | 6.28 (6.28) | 6.61 (6.61) | 81.1 (80.55–81.14) | 60.7 (60.25–60.7) | 63.9 (63.40–63.87) |
| Cundinamarca | 17 | 10.44 (10.35–10.59) | 8.42 (8.40–8.74) | 6.30 (6.28–6.63) | 6.63 (6.61–6.95) | 80.7 (79.31–83.24) | 60.4 (59.32–63.09) | 63.5 (62.42–66.21) |
| Eje Cafetero | 5 | 10.35 (10.16–10.43) | 8.40 (8.40) | 6.28 (6.28) | 6.61 (6.61) | 80.6 (80.55) | 60.3 (60.25) | 63.4 (63.40) |
| Valle del Cauca | 11 | 10.32 (10.16–10.35) | 8.19 (6.77–8.40) | 6.37 (6.28–7.13) | 6.68 (6.60–7.38) | 79.4 (66.62–81.14) | 61.7 (60.7–69.65) | 64.7 (63.87–72.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Bermudez, D.S.; Gil-Silva, A.C.; Naranjo-Ortíz, M.F.; Mogollón, J.D.; Gómez-Betancur, J.F.; Estrada, J.F.; Aldaz, Á.; Garzón-González, H.; Angulo, J.; Foss, D.; et al. Detection of PCV2d in Vaccinated Pigs in Colombia and Prediction of Vaccine T Cell Epitope Coverage against Circulating Strains Using EpiCC Analysis. Vaccines 2024, 12, 1119. https://doi.org/10.3390/vaccines12101119
Vargas-Bermudez DS, Gil-Silva AC, Naranjo-Ortíz MF, Mogollón JD, Gómez-Betancur JF, Estrada JF, Aldaz Á, Garzón-González H, Angulo J, Foss D, et al. Detection of PCV2d in Vaccinated Pigs in Colombia and Prediction of Vaccine T Cell Epitope Coverage against Circulating Strains Using EpiCC Analysis. Vaccines. 2024; 12(10):1119. https://doi.org/10.3390/vaccines12101119
Chicago/Turabian StyleVargas-Bermudez, Diana S., Alixs Constanza Gil-Silva, María F. Naranjo-Ortíz, José Darío Mogollón, Jair F. Gómez-Betancur, José F. Estrada, Álvaro Aldaz, Harold Garzón-González, José Angulo, Dennis Foss, and et al. 2024. "Detection of PCV2d in Vaccinated Pigs in Colombia and Prediction of Vaccine T Cell Epitope Coverage against Circulating Strains Using EpiCC Analysis" Vaccines 12, no. 10: 1119. https://doi.org/10.3390/vaccines12101119
APA StyleVargas-Bermudez, D. S., Gil-Silva, A. C., Naranjo-Ortíz, M. F., Mogollón, J. D., Gómez-Betancur, J. F., Estrada, J. F., Aldaz, Á., Garzón-González, H., Angulo, J., Foss, D., Gutierrez, A. H., & Jaime, J. (2024). Detection of PCV2d in Vaccinated Pigs in Colombia and Prediction of Vaccine T Cell Epitope Coverage against Circulating Strains Using EpiCC Analysis. Vaccines, 12(10), 1119. https://doi.org/10.3390/vaccines12101119






