Immunogenicity and Safety of Omicron-Containing Multivalent COVID-19 Vaccines in Unvaccinated and Previously Vaccinated Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
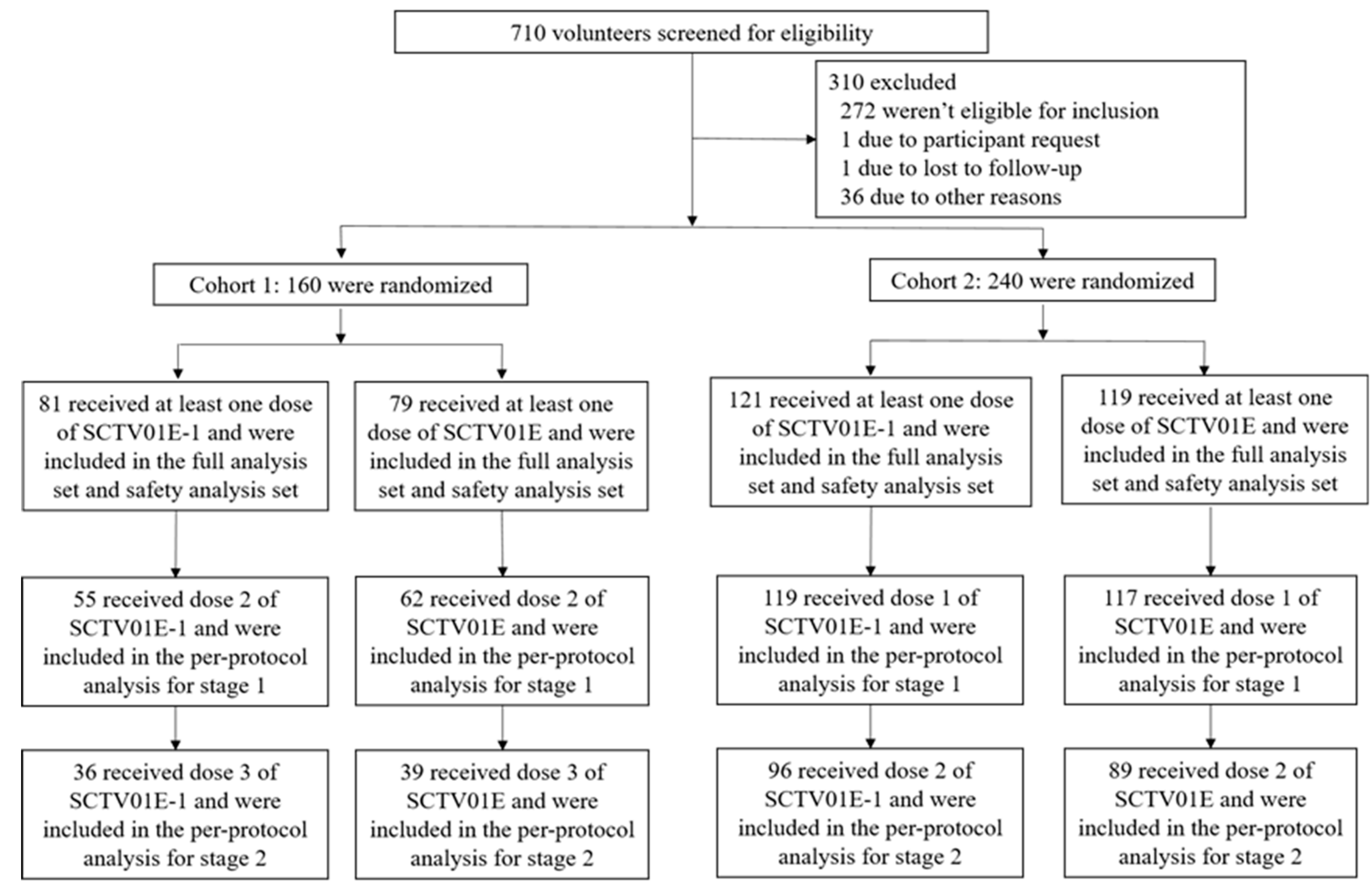
2.2. Study Participants
2.3. Randomization and Masking
2.4. Procedures
2.5. Immunogenicity and Safety Assessments
2.6. Study Outcomes
2.7. Statistical Analysis
3. Results
3.1. Study Participants, Demographics and Baseline Characteristics
3.2. Immunogenicity
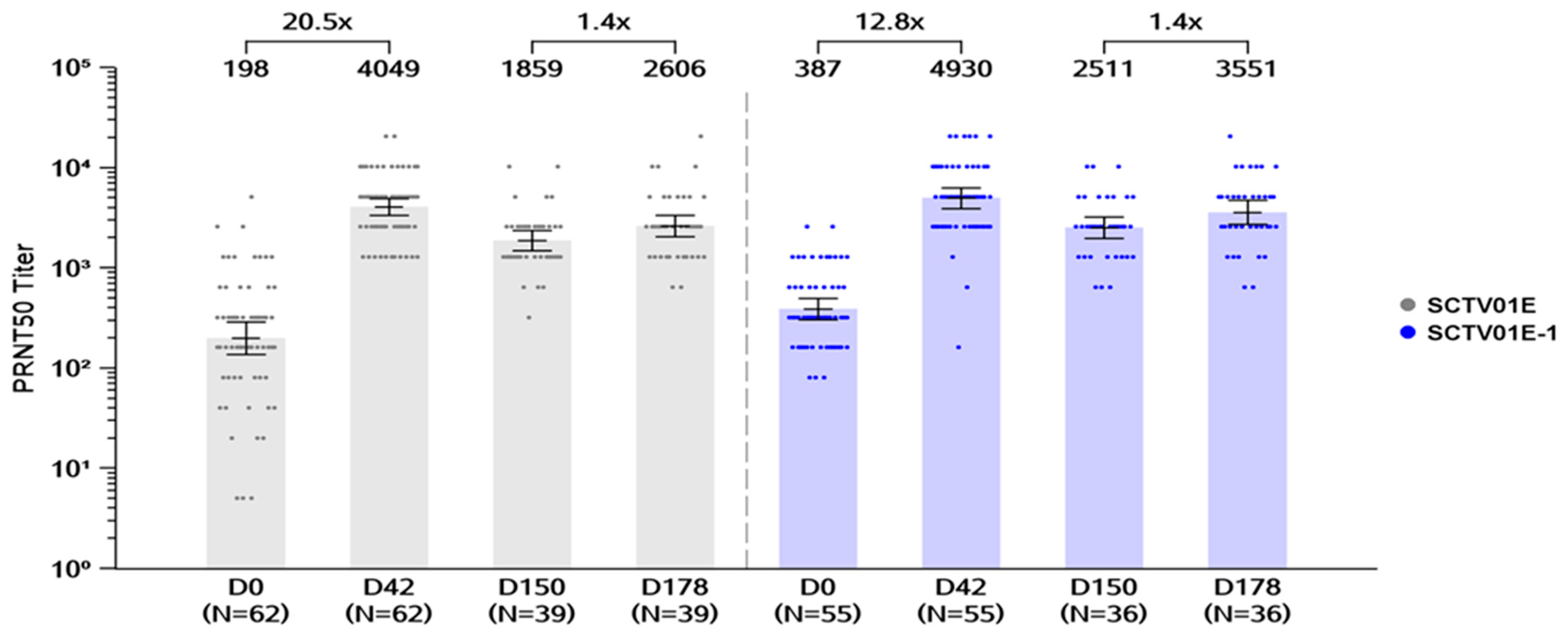
3.2.1. Cohort 1
Day 42 GMTs of Live Virus nAb and Seroresponse RATES against Omicron BA.5
Day 178 GMTs of Live Virus nAb and Seroresponse Rates against Omicron BA.5
Subgroup Analysis Based on Anti-SARS-CoV-2 Nucleocapsid Test
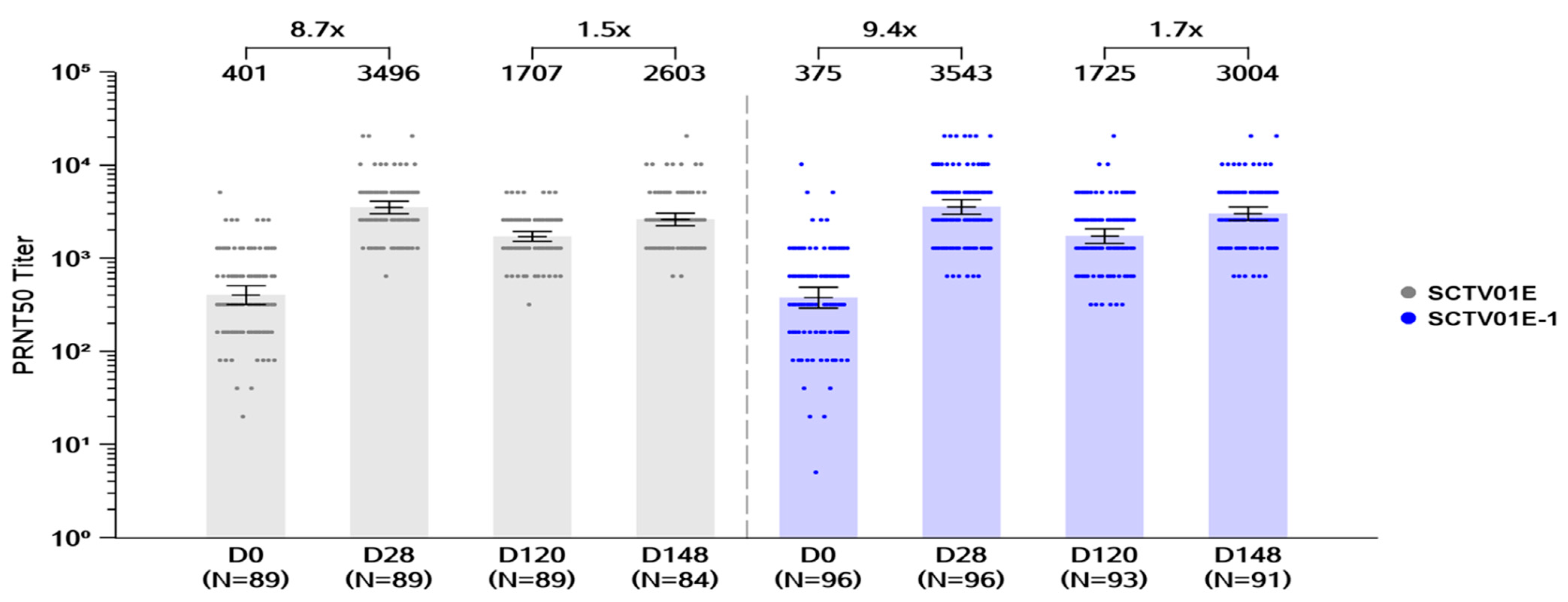
3.2.2. Cohort 2
Day 28 GMTs of Live Virus nAb and Seroresponse against Omicron BA.5
Day 148 GMTs of Live Virus nAb and Seroresponse Rates against Omicron BA.5
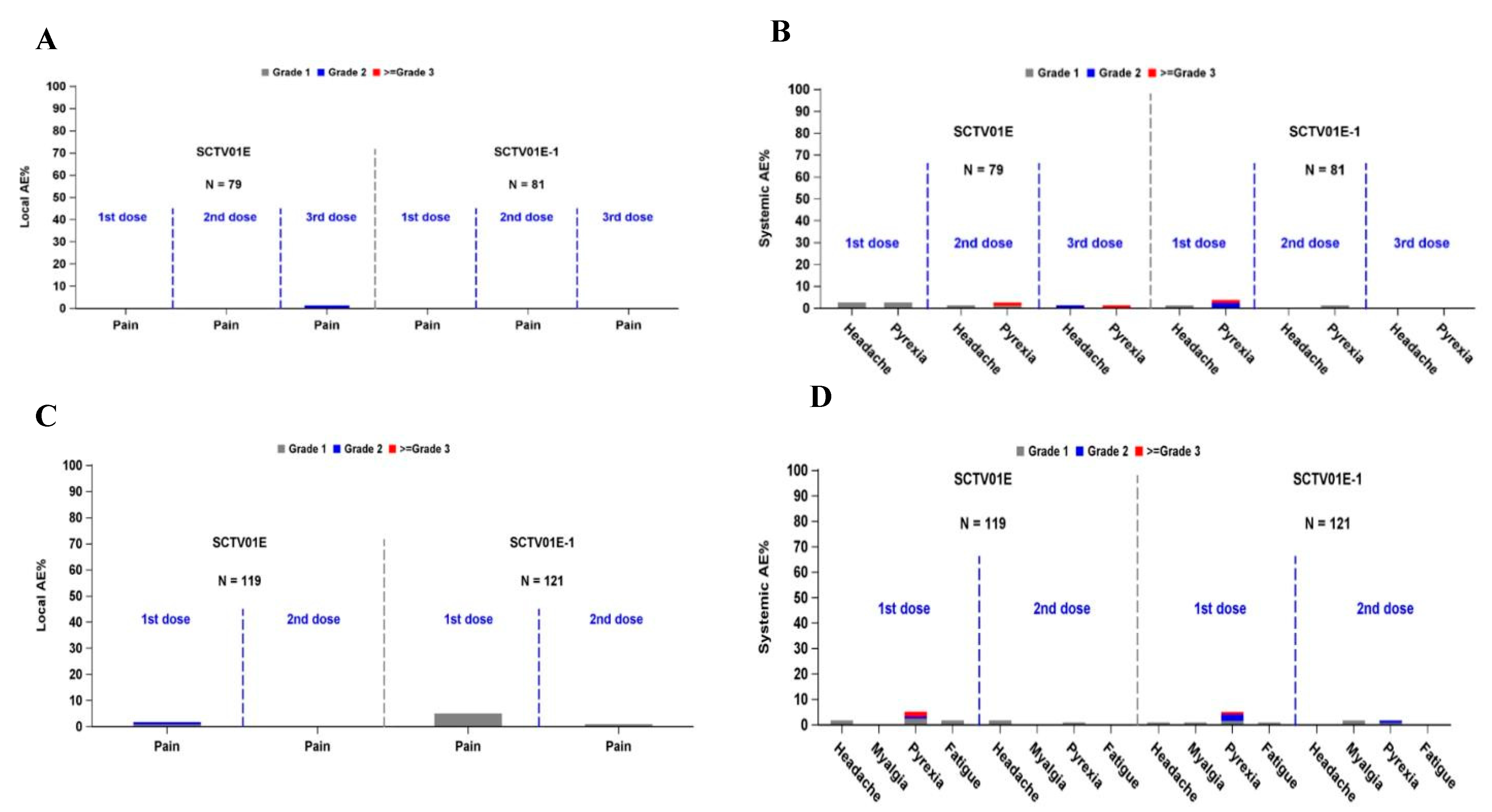
3.3. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Tegally, H.; Moir, M.; Everatt, J.; Giovanetti, M.; Scheepers, C.; Wilkinson, E.; Subramoney, K.; Makatini, Z.; Moyo, S.; Amoako, D.G.; et al. Emergence of SARS-CoV-2 omicron lineages BA. 4 and BA. 5 in South Africa. Nat. Med. 2022, 28, 1785–1790. [Google Scholar] [CrossRef]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Gribben, C.; Bishop, J.; Hanlon, P.; Caldwell, D.; Wood, R.; Reid, M.; McMenamin, J.; Goldberg, D.; Stockton, D.; et al. Effect of vaccination on transmission of SARS-CoV-2. N. Engl. J. Med. 2021, 385, 1718–1720. [Google Scholar] [CrossRef]
- Ai, J.; Wang, X.; He, X.; Zhao, X.; Zhang, Y.; Jiang, Y.; Li, M.; Cui, Y.; Chen, Y.; Qiao, R.; et al. Antibody evasion of SARS-CoV-2 Omicron BA. 1, BA. 1.1, BA. 2, and BA. 3 sub-lineages. Cell Host Microbe 2022, 30, 1077–1083.e4. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022, 608, 593–602. [Google Scholar] [CrossRef]
- Hachmann, N.P.; Miller, J.; Collier, A.R.; Ventura, J.D.; Yu, J.; Rowe, M.; Bondzie, E.A.; Powers, O.; Surve, N.; Hall, K.; et al. Neutralization escape by SARS-CoV-2 Omicron subvariants BA. 2.12. 1, BA. 4, and BA. 5. N. Engl. J. Med. 2022, 387, 86–88. [Google Scholar] [CrossRef] [PubMed]
- WHO Director-General’s Opening Remarks at the Media Briefing—5 May 2023. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing (accessed on 5 May 2023).
- Vaccines and Related Biological Products Advisory Committee June 15, 2023 Meeting Announcement. Available online: https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-june-15-2023-meeting-announcement (accessed on 15 June 2023).
- Weekly Epidemiological Update on COVID-19—9 November 2022. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19 (accessed on 9 November 2022).
- Wang, R.; Huang, H.; Yu, C.; Sun, C.; Ma, J.; Kong, D.; Lin, Y.; Zhao, D.; Zhou, S.; Lu, J.; et al. A spike-trimer protein-based tetravalent COVID-19 vaccine elicits enhanced breadth of neutralization against SARS-CoV-2 Omicron subvariants and other variants. Sci. China Life Sci. 2022, 66, 1818–1830. [Google Scholar] [CrossRef]
- Hannawi, S.; Saf Eldin, L.; Abuquta, A.; Alamadi, A.; Mahmoud, S.A.; Hassan, A.; Xu, S.; Li, J.; Liu, D.; Baidoo, A.A.; et al. Safety and immunogenicity of a tetravalent and bivalent SARS-CoV-2 protein booster vaccine in men. Nat. Commun. 2023, 14, 4043. [Google Scholar] [CrossRef]
- Hannawi, S.; Yan, L.; Eldin, L.S.; Abuquta, A.; Alamadi, A.; Mahmoud, S.A.; Hassan, A.; Zhang, M.; Gao, C.; Chen, Y.; et al. Safety and immunogenicity of multivalent SARS-CoV-2 protein vaccines: A randomized phase 3 trial. EClinicalMedicine 2023, 64, 102195. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, K.; Han, J.; Hu, Z.; Zhang, T.; Wang, Y.; Shi, R.; Li, Y.; Song, Q.; Du, H.; et al. Safety and immunogenicity of a bivalent SARS-CoV-2 recombinant protein vaccine, SCTV01C in unvaccinated adults: A randomized, double-blinded, placebo-controlled, phase I clinical trial. J. Infect. 2023, 86, 154–225. [Google Scholar] [CrossRef] [PubMed]
- Bergeri, I.; Whelan, M.G.; Ware, H.; Subissi, L.; Nardone, A.; Lewis, H.C.; Li, Z.; Ma, X.; Valenciano, M.; Cheng, B.; et al. Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: A systematic review and meta-analysis of standardized population-based studies. PLoS Med. 2022, 19, e1004107. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Link-Gelles, R.; Patel, M.M. Long-term Protection Associated With COVID-19 Vaccination and Prior Infection. JAMA 2022, 328, 1402–1404. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Liang, Z.; Wang, N.; Liu, S.; Li, T.; Yu, Y.; Cui, Q.; Wu, X.; Nie, J.; et al. Cross-reactivity of eight SARS-CoV-2 variants rationally predicts immunogenicity clustering in sarbecoviruses. Signal Transduct. Target. Ther. 2022, 7, 256. [Google Scholar] [CrossRef] [PubMed]
- Lyke, K.E.; Atmar, R.L.; Islas, C.D.; Posavad, C.M.; Szydlo, D.; Chourdhury, R.P.; Deming, M.E.; Eaton, A.; Jackson, L.A.; Branche, A.R.; et al. Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep. Med. 2022, 3, 100679. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bowen, A.; Tam, A.R.; Valdez, R.; Stoneman, E.; Mellis, I.A.; Gordon, A.; Liu, L.; Ho, D.D. SARS-CoV-2 neutralising antibodies after bivalent versus monovalent booster. Lancet Infect. Dis. 2023, 23, 527–528. [Google Scholar] [CrossRef]
- Srivastava, K.; Carreño, J.M.; Gleason, C.; Monahan, B.; Singh, G.; Abbad, A.; Tcheou, J.; Raskin, A.; Kleiner, G.; van Bakel, H. Kinetics and durability of humoral responses to SARS-CoV-2 infection and vaccination. medRxiv 2023. [Google Scholar] [CrossRef]
- Cheetham, N.J.; Kibble, M.; Wong, A.; Silverwood, R.J.; Knuppel, A.; Williams, D.M.; Hamilton, O.K.; Lee, P.H.; Bridger Staatz, C.; Di Gessa, G. Antibody levels following vaccination against SARS-CoV-2: Associations with post-vaccination infection and risk factors in two UK longitudinal studies. Elife 2023, 12, e80428. [Google Scholar] [CrossRef]
- Franzese, M.; Coppola, L.; Silva, R.; Santini, S.A.; Cinquanta, L.; Ottomano, C.; Salvatore, M.; Incoronato, M. SARS-CoV-2 antibody responses before and after a third dose of the BNT162b2 vaccine in Italian healthcare workers aged≤ 60 years: One year of surveillance. Front. Immunol. 2022, 13, 947187. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N. Engl. J. Med. 2022, 387, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Al-Rifai, R.H.; Acuna, J.; Al Hossany, F.I.; Aden, B.; Al Memari, S.A.; Al Mazrouei, S.K.; Ahmed, L.A. Epidemiological characterization of symptomatic and asymptomatic COVID-19 cases and positivity in subsequent RT-PCR tests in the United Arab Emirates. PLoS ONE 2021, 16, e0246903. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Gui, X.; Xiong, Y. Comparison of Clinical Characteristics of Patients with Asymptomatic vs Symptomatic Coronavirus Disease 2019 in Wuhan, China. JAMA Netw. Open 2020, 3, e2010182. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Collier, A.-R.Y.; Yu, J.; McMahan, K.; Liu, J.; Chandrashekar, A.; Maron, J.S.; Atyeo, C.; Martinez, D.R.; Ansel, J.L.; Aguayo, R.; et al. Differential kinetics of immune responses elicited by COVID-19 vaccines. N. Engl. J. Med. 2021, 385, 2010–2012. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, S.; Ma, W.; Li, X.; Wei, K.; Xie, F.; Zhao, C.; Zhao, X.; Li, C.; Qiao, R.; et al. Enhanced neutralization of SARS-CoV-2 XBB sub-lineages and BA. 2.86 by a tetravalent COVID-19 vaccine booster. bioRxiv 2023. [Google Scholar] [CrossRef]
- Formeister, E.J.; Wu, M.J.; Chari, D.A.; Meek, R.; Rauch, S.D.; Remenschneider, A.K.; Quesnel, A.M.; de Venecia, R.; Lee, D.J.; Chien, W.; et al. Assessment of sudden sensorineural hearing loss after COVID-19 vaccination. Arch. Otolaryngol. Neck Surg. 2022, 148, 307–315. [Google Scholar] [CrossRef]
| SCTV01E (N = 79) n (%) | SCTV01E-1 (N = 81) n (%) | Total (N = 160) n (%) | |
|---|---|---|---|
| Age (years) | |||
| n | 79 | 81 | 160 |
| Mean (SD) | 27.9 (7.36) | 29.1 (8.32) | 28.5 (7.86) |
| Median | 26.0 | 26.0 | 26.0 |
| Min, Max | 18, 48 | 18, 57 | 18, 57 |
| Age Group, n (%) | |||
| 18–54 | 79 (100.0) | 80 (98.8) | 159 (99.4) |
| ≥55 | 0 | 1 (1.2) | 1 (0.6) |
| Gender, n (%) | |||
| Female | 1 (1.3) | 0 | 1 (0.6) |
| Male | 78 (98.7) | 81 (100.0) | 159 (99.4) |
| Race, n (%) | |||
| American Indian or Alaska Native | 0 | 1 (1.2) | 1 (0.6) |
| Asian | 74 (93.7) | 74 (91.4) | 148 (92.5) |
| Black or African American | 2 (2.5) | 2 (2.5) | 4 (2.5) |
| White | 2 (2.5) | 1 (1.2) | 3 (1.9) |
| Not Reported | 1 (1.3) | 2 (2.5) | 3 (1.9) |
| Unknown | 0 | 1 (1.2) | 1 (0.6) |
| BMI (kg/m2) | |||
| n | 79 | 81 | 160 |
| Mean (SD) | 22.94 (3.342) | 23.65 (3.639) | 23.30 (3.503) |
| Median | 22.50 | 23.30 | 22.90 |
| Min, Max | 16.8, 30.5 | 17.1, 32.8 | 16.8, 32.8 |
| Anti-SARS-CoV-2 nucleocapsid antibody test, n (%) | |||
| Positive | 30 (38.0) | 31 (38.3) | 61 (38.1) |
| Negative | 49 (62.0) | 50 (61.7) | 99 (61.9) |
| SCTV01E (N = 119) n (%) | SCTV01E-1 (N = 121) n (%) | Total (N = 240) n (%) | |
|---|---|---|---|
| Age (years) | |||
| n | 119 | 121 | 240 |
| Mean (SD) | 28.2 (6.61) | 28.3 (7.30) | 28.2 (6.95) |
| Median | 26.0 | 26.0 | 26.0 |
| Min, Max | 19, 54 | 18, 59 | 18, 59 |
| Age Group, n (%) | |||
| 18–54 | 119 (100.0) | 120 (99.2) | 239 (99.6) |
| ≥55 | 0 | 1 (0.8) | 1 (0.4) |
| Gender, n (%) | |||
| Female | 1 (0.8) | 1 (0.8) | 2 (0.8) |
| Male | 118 (99.2) | 120 (99.2) | 238 (99.2) |
| Race, n (%) | |||
| Asian | 119 (100.0) | 120 (99.2) | 239 (99.6) |
| Black or African American | 0 | 1 (0.8) | 1 (0.4) |
| BMI (kg/m2) | |||
| n | 119 | 121 | 240 |
| Mean (SD) | 23.75 (4.173) | 23.72 (3.877) | 23.73 (4.018) |
| Median | 23.50 | 23.50 | 23.50 |
| Min, Max | 16.4, 36.0 | 16.2, 37.2 | 16.2, 37.2 |
| Number of previous COVID-19 vaccines, n (%) | |||
| 2 | 107 (89.9) | 109 (90.1) | 216 (90.0) |
| 3 | 12 (10.1) | 12 (9.9) | 24 (10.0) |
| Time since last COVID-19 vaccination (month), n (%) | |||
| 3–5 | 1 (0.8) | 0 | 1 (0.4) |
| 6–24 | 118 (99.2) | 121 (100.0) | 239 (99.6) |
| COVID-19 Vaccine name, n (%) | |||
| Sinopharm Inactivated COVID-19 Vaccine | 88 (73.9) | 81 (66.9) | 169 (70.4) |
| CoronaVac | 31 (26.1) | 40 (33.1) | 71 (29.6) |
| Diagnosed with COVID-19, n (%) | |||
| Yes | 3 (2.5) | 5 (4.1) | 8 (3.3) |
| No | 116 (97.5) | 116 (95.9) | 232 (96.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannawi, S.; Abuquta, A.; Eldin, L.S.; Hassan, A.; Alamadi, A.; Gao, C.; Baidoo, A.A.H.; Yang, X.; Su, H.; Zhang, J.; et al. Immunogenicity and Safety of Omicron-Containing Multivalent COVID-19 Vaccines in Unvaccinated and Previously Vaccinated Adults. Vaccines 2024, 12, 1109. https://doi.org/10.3390/vaccines12101109
Hannawi S, Abuquta A, Eldin LS, Hassan A, Alamadi A, Gao C, Baidoo AAH, Yang X, Su H, Zhang J, et al. Immunogenicity and Safety of Omicron-Containing Multivalent COVID-19 Vaccines in Unvaccinated and Previously Vaccinated Adults. Vaccines. 2024; 12(10):1109. https://doi.org/10.3390/vaccines12101109
Chicago/Turabian StyleHannawi, Suad, Alaa Abuquta, Linda Saf Eldin, Aala Hassan, Ahmad Alamadi, Cuige Gao, Adam Abdul Hakeem Baidoo, Xinjie Yang, Huo Su, Jinxiu Zhang, and et al. 2024. "Immunogenicity and Safety of Omicron-Containing Multivalent COVID-19 Vaccines in Unvaccinated and Previously Vaccinated Adults" Vaccines 12, no. 10: 1109. https://doi.org/10.3390/vaccines12101109
APA StyleHannawi, S., Abuquta, A., Eldin, L. S., Hassan, A., Alamadi, A., Gao, C., Baidoo, A. A. H., Yang, X., Su, H., Zhang, J., & Xie, L. (2024). Immunogenicity and Safety of Omicron-Containing Multivalent COVID-19 Vaccines in Unvaccinated and Previously Vaccinated Adults. Vaccines, 12(10), 1109. https://doi.org/10.3390/vaccines12101109






