Access to DTP-Based Combination Vaccines in Asia–Pacific Countries between 2019 and 2022
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Data Source and Collection
2.2.1. Vaccine Consumption (Availability)
2.2.2. Vaccine Expenditure (Affordability)
2.2.3. Vaccine Coverage Rate (Accessibility)
2.2.4. National Health and Economic Performance
2.2.5. Statistical Analysis
3. Results
3.1. Availability of DTP-Based Vaccines
3.2. Affordability of DTP-Based Vaccines
3.3. Accessibility of DTP-Based Vaccines
3.4. Correlation between Access to DTP-Based Vaccines and National Health and Economic Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNICEF. Diphtheria Tetanus and Pertussis Containing Vaccines: Market and Supply Update. 2023. Available online: https://www.unicef.org/supply/media/17606/file/Diphtheria-Tetanus-Pertussis-Vaccine-Containing-Market-and-Supply-Update-June-2023.pdf (accessed on 24 November 2023).
- Di Fabio, J.L.; de Quadros, C. Considerations for combination vaccine development and use in the developing world. Clin. Infect. Dis. 2001, 33 (Suppl. S4), S340–S345. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vaccination Schedule for Diphtheria. The WHO Immunization Data Portal. Available online: https://immunizationdata.who.int/pages/schedule-by-disease/diphtheria.html?ISO_3_CODE=AUS+IND&TARGETPOP_GENERAL= (accessed on 18 December 2023).
- Aguinaga-Ontoso, I.; Guillen-Aguinaga, S.; Guillen-Aguinaga, L.; Alas-Brun, R.; Onambele, L.; Aguinaga-Ontoso, E.; Guillen-Grima, F. COVID-19 Impact on DTP Vaccination Trends in Africa: A Joinpoint Regression Analysis. Vaccines 2023, 11, 1103. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. The Sate of the World’s Children 2023-for Every Child, Vaccination. 2023. Available online: https://www.unicef.org/media/108161/file/SOWC-2023-full-report-English.pdf (accessed on 24 November 2023).
- Buchy, P.; Chen, J.; Zhang, X.-H.; Benninghoff, B.; Lee, C.; Bibera, G.L. A review of rotavirus vaccine use in Asia and the Pacific regions: Challenges and future prospects. Expert Rev. Vaccines 2021, 20, 1499–1514. [Google Scholar] [CrossRef] [PubMed]
- Zeitouny, S.; Suda, K.J.; Mitsantisuk, K.; Law, M.R.; Tadrous, M. Mapping global trends in vaccine sales before and during the first wave of the COVID-19 pandemic: A cross-sectional time-series analysis. BMJ Glob. Heal. 2021, 6, e006874. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Li, X.; Pratt, N.L.; Hillen, J.B.; Stanford, T.; Ward, M.; Roughead, E.E.; Lai, E.C.-C.; Shin, J.-Y.; Cheng, F.W.; et al. Monoclonal antibodies and Fc-fusion protein biologic medicines: A multinational cross-sectional investigation of accessibility and affordability in Asia Pacific regions between 2010 and 2020. Lancet Reg. Heal. West. Pac. 2022, 26, 100506. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Milkowska-Shibata, M.; Tseng, K.K.; Sharland, M.; Gandra, S.; Pulcini, C.; Laxminarayan, R. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: An analysis of pharmaceutical sales data. Lancet Infect. Dis. 2021, 21, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Hsia, Y.; Sharland, M.; Jackson, C.; Wong, I.C.K.; Magrini, N.; A Bielicki, J. Consumption of oral antibiotic formulations for young children according to the WHO Access, Watch, Reserve (AWaRe) antibiotic groups: An analysis of sales data from 70 middle-income and high-income countries. Lancet Infect. Dis. 2018, 19, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Gandra, S.; Ashok, A.; Caudron, Q.; Grenfell, B.T.; A Levin, S.; Laxminarayan, R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014, 14, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.; Monasch, R.; Lautenbach, B.; Gacic-Dobo, M.; Neill, M.; Karimov, R.; Wolfson, L.; Jones, G.; Birmingham, M. WHO and UNICEF estimates of national infant immunization coverage: Methods and processes. Bull. World Heal. Organ. 2009, 87, 535–541. [Google Scholar] [CrossRef] [PubMed]
- World Bank. World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 18 December 2023).
- Lydon, P.; Schreiber, B.; Gasca, A.; Dumolard, L.; Urfer, D.; Senouci, K. Vaccine stockouts around the world: Are essential vaccines always available when needed? Vaccine 2017, 35, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- GBD 2020, Release 1, Vaccine Coverage Collaborators. Measuring routine childhood vaccination coverage in 204 countries and territories, 1980–2019: A systematic analysis for the Global Burden of Disease Study 2020, Release 1. Lancet 2021, 398, 503–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Haidari, L.A. The importance of vaccine supply chains to everyone in the vaccine world. Vaccine 2017, 35 Pt A, 4475–4479. [Google Scholar] [CrossRef]
- Muhoza, P.; Danovaro-Holliday, M.C.; Diallo, M.S.; Murphy, P.; Sodha, S.V.; Requejo, J.H.; Wallace, A.S. Routine Vaccination Coverage—Worldwide, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.D.; Miner, D.S.; Lee, A.A.; Asay, A.E.; Nielson, B.U.; Rose, A.M.; Hinton, K.; Poole, B.D. Factors Affecting Vaccine Attitudes Influenced by the COVID-19 Pandemic. Vaccines 2023, 11, 516. [Google Scholar] [CrossRef] [PubMed]
- Ota, M.O.C.; Badur, S.; Romano-Mazzotti, L.; Friedland, L.R. Impact of COVID-19 pandemic on routine immunization. Ann. Med. 2021, 53, 2286–2297. [Google Scholar] [CrossRef] [PubMed]
- Piché-Renaud, P.-P.; Ji, C.; Farrar, D.S.; Friedman, J.N.; Science, M.; Kitai, I.; Burey, S.; Feldman, M.; Morris, S.K. Impact of the COVID-19 pandemic on the provision of routine childhood immunizations in Ontario, Canada. Vaccine 2021, 39, 4373–4382. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Schreiber, B.; Lee, B.Y. Immunization supply chains: Why they matter and how they are changing. Vaccine 2017, 35, 2103–2104. [Google Scholar] [CrossRef] [PubMed]
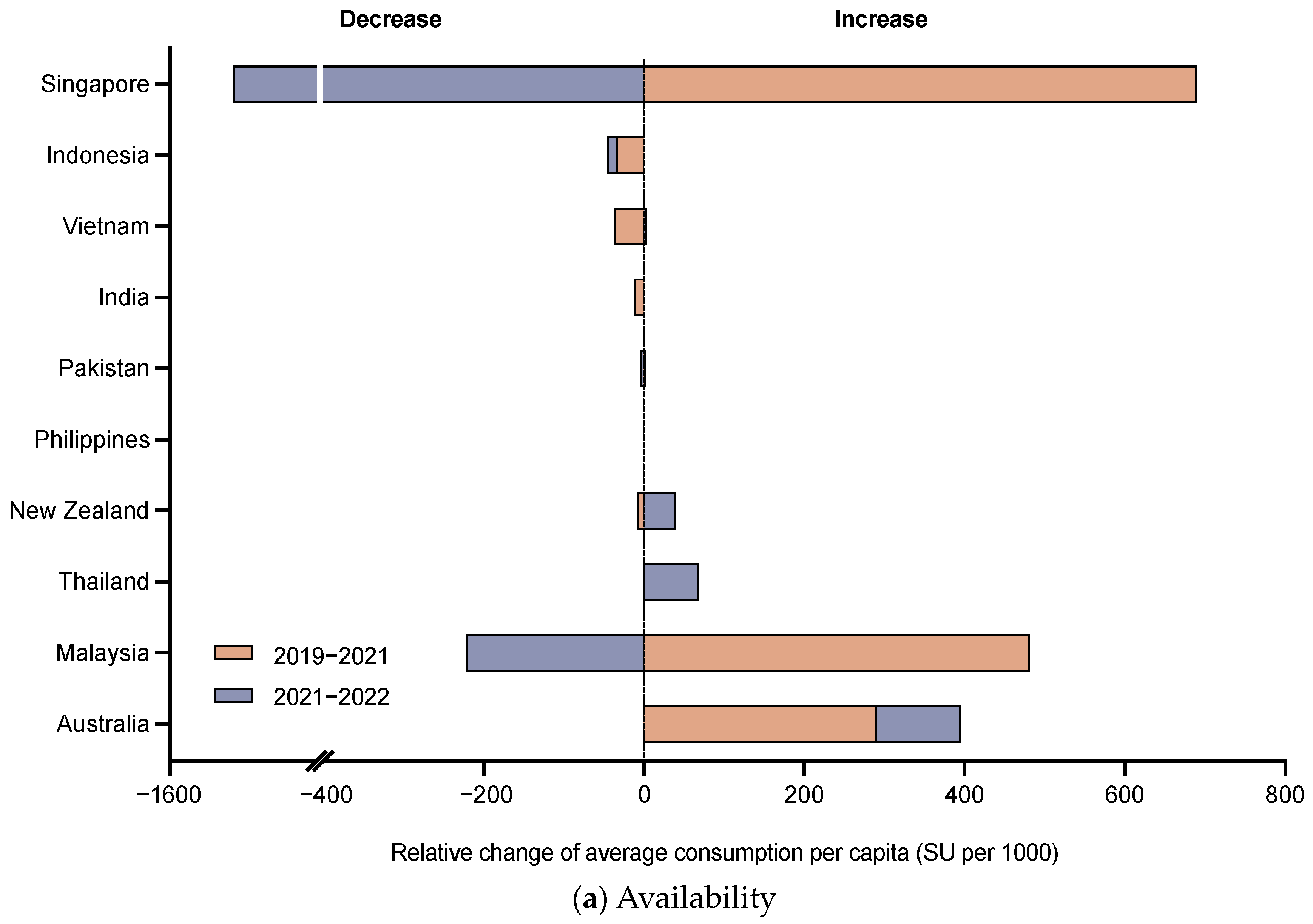
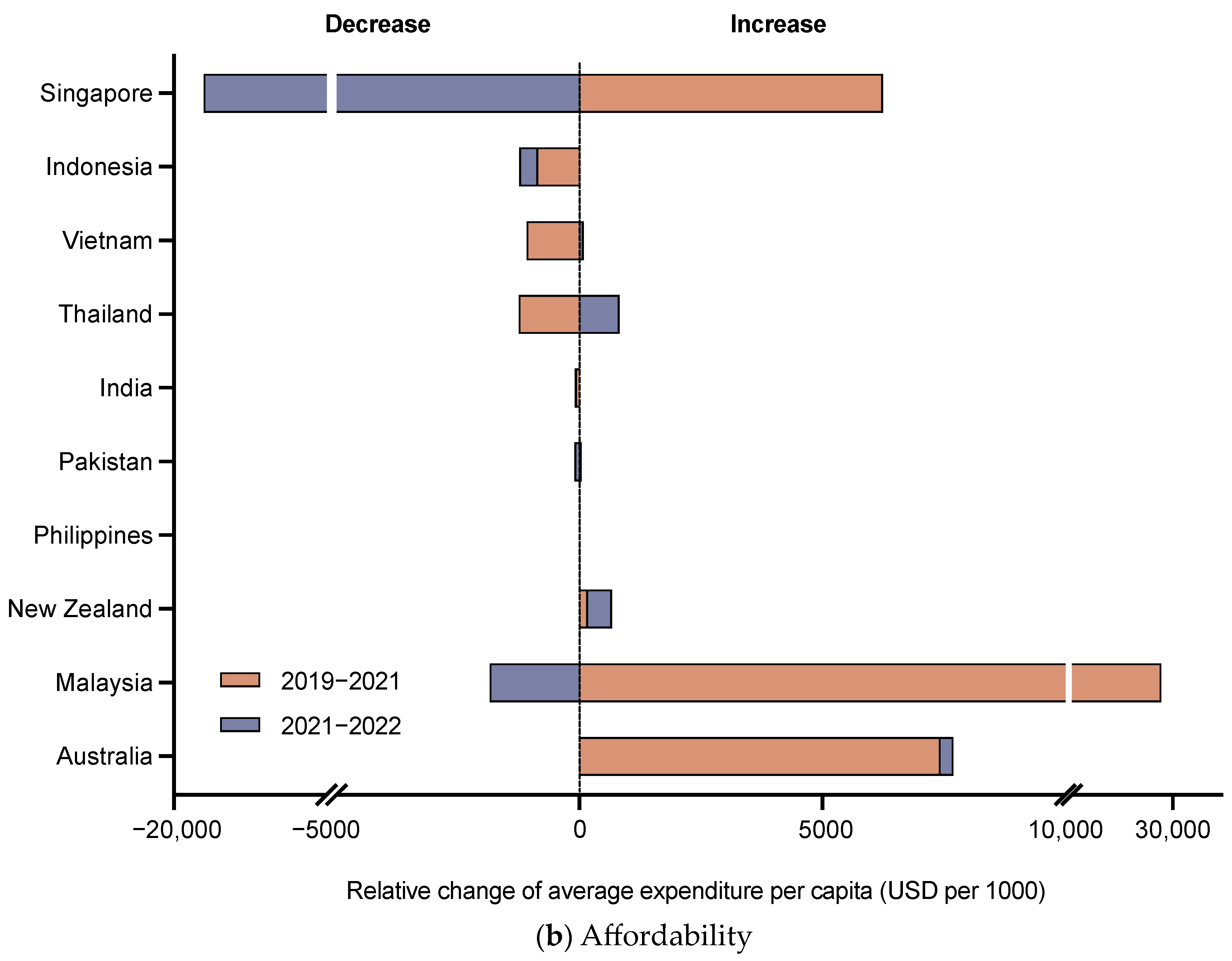

| Characteristic | Australia | India | Indonesia | Malaysia | New Zealand | Pakistan | Philippines | Singapore | Thailand | Vietnam |
|---|---|---|---|---|---|---|---|---|---|---|
| Income level | HIC | LMIC | UMIC | UMIC | HIC | LMIC | LMIC | HIC | UMIC | LMIC |
| Population under 1 year old in 2022 (per 1000) | 298.5 | 23,113.5 | 4496.4 | 511.3 | 63.6 | 6374.7 | 2485.0 | 42.2 | 644.3 | 1462.6 |
| GDP per capita in 2022 (USD per capita) | 64,813.9 | 2391.9 | 4798.1 | 12,465.6 | 47,226.1 | 1650.3 | 3623.6 | 82,807.6 | 7069.6 | 4086.5 |
| Current health expenditure in 2020 (% of GDP) | 10.6 | 3.4 | 3.0 | 4.1 | 10.0 | 3.0 | 5.1 | 6.1 | 4.4 | 4.7 |
| DTP vaccine is included in NIP * | DTaP | DTwP | DTwP | DTaP | DTaP | DTwP | DTwP | DTaP | DTwP | DTwP |
| The incidence rate of diphtheria among children under 1 year old in 2019 (per 100,000) | 0.0008 | 0.1341 | 0.6005 | 0.0371 | 0.0004 | 0.7591 | 0.5813 | 0.0011 | 0.1007 | 0.1374 |
| The incidence rate of tetanus among children under 1 year old in 2019 (per 100,000) | 0.0008 | 43.3039 | 34.7453 | 0.2974 | 0.0006 | 72.5749 | 11.0156 | 0.0006 | 0.2184 | 3.4995 |
| The incidence rate of pertussis among children under 1 year old in 2019 (per 100,000) | 2871 | 6107 | 8425 | 4788 | 3207 | 8091 | 8072 | 3667 | 5183 | 7691 |
| Country | Average Consumption per Capita (SU per 1000) * | Average Expenditure per Capita (USD per 1000) | Average DTP 1 Coverage Rate (%) | Average DTP 3 Coverage Rate (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | IQR | Mean | IQR | Mean | IQR | Mean | IQR | |
| Australia | 922.8 | 751.1–1097.9 | 19,105.1 | 15,321.8–22,485.5 | 96 | 92.0–98.0 | 93.5 | 92.0–95.0 |
| India | 138.7 | 133.4–144.8 | 2509.7 | 2473.4–2555.2 | 90.3 | 88.5–92.5 | 86.1 | 82.5–89.5 |
| Indonesia | 58.6 | 40.8–78.5 | 1850.6 | 1344.6–2379.9 | 88.5 | 87.5–90.0 | 83.2 | 81.0–86.0 |
| Malaysia | 4079.4 | 3633.7–4450.3 | 58,632.7 | 42,371.3–73,938.2 | 98.8 | 98.5–99.0 | 97.4 | 96.5–98.5 |
| New Zealand | 172.8 | 154.6–195.8 | 1877.3 | 1567.7–2261.4 | 93.8 | 93.0–95.0 | 92.3 | 92.0–93.0 |
| Pakistan | 9.9 | 7.7–11.7 | 161.1 | 118.1–200.0 | 82.6 | 78.0–88.5 | 72.8 | 64.5–81.5 |
| Philippines | 0.1 | 0.1–0.2 | 4.2 | 3.1–5.1 | 81.7 | 74.0–91.0 | 77.9 | 71.5–86.0 |
| Singapore | 7664.3 | 7139.5–8284.1 | 138,398.5 | 127,622.4–148,839.5 | 98.2 | 98.0–99.0 | 96.8 | 96.0–97.0 |
| Thailand | 659.4 | 642.0–693.1 | 17,711.4 | 17,194.8–18,306.1 | 99 | 99 | 98.2 | 97.0–99.0 |
| Vietnam | 132.2 | 98.0–180.6 | 4126.3 | 3095.3–5625.5 | 92.9 | 89.5–97.5 | 89.1 | 86.0–95.5 |
| Indicators | Average Consumption per Capita | Average Expenditure per Capita | Average DTP 1 Coverage Rate | Average DTP 3 Coverage Rate | ||||
|---|---|---|---|---|---|---|---|---|
| Overall | Relative Change | Overall | Relative Change | Overall | Relative Change | Overall | Relative Change | |
| GDP per capita | ||||||||
| Coefficient | 0.802 ** | 0.103 | 0.676 ** | 0.009 | 0.641 ** | −0.093 | 0.673 ** | −0.076 |
| p value | 0.000 | 0.588 | 0.000 | 0.964 | 0.000 | 0.624 | 0.000 | 0.689 |
| Incidence burden | ||||||||
| Coefficient | −0.799 ** | −0.302 | −0.615 ** | −0.25 | −0.674 ** | 0.056 | −0.700 ** | 0.069 |
| p value | 0.000 | 0.104 | 0.000 | 0.183 | 0.000 | 0.771 | 0.000 | 0.717 |
| Death burden | ||||||||
| Coefficient | −0.793 ** | −0.189 | −0.647 ** | −0.117 | −0.655 ** | 0.129 | −0.684 ** | 0.097 |
| p value | 0.000 | 0.318 | 0.000 | 0.537 | 0.000 | 0.496 | 0.000 | 0.609 |
| Current health expenditure per capita | ||||||||
| Coefficient | 0.734 ** | 0.297 | 0.572 ** | 0.231 | 0.579 ** | −0.091 | 0.608 ** | −0.084 |
| p value | 0.000 | 0.112 | 0.000 | 0.219 | 0.000 | 0.633 | 0.000 | 0.659 |
| Current health expenditure (% GDP) | ||||||||
| Coefficient | 0.417 ** | 0.393 * | 0.269 | 0.389 * | 0.28 | −0.01 | 0.301 | −0.028 |
| p value | 0.007 | 0.032 | 0.093 | 0.033 | 0.081 | 0.957 | 0.059 | 0.883 |
| UHC service coverage index | ||||||||
| Coefficient | 0.860 ** | 0.242 | 0.739 ** | 0.144 | 0.751 ** | −0.105 | 0.773 ** | −0.058 |
| p value | 0.000 | 0.198 | 0.000 | 0.448 | 0.000 | 0.581 | 0.000 | 0.759 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Sun, Y.; Cui, Y.; Dong, J.; Huang, Y. Access to DTP-Based Combination Vaccines in Asia–Pacific Countries between 2019 and 2022. Vaccines 2024, 12, 33. https://doi.org/10.3390/vaccines12010033
Ma J, Sun Y, Cui Y, Dong J, Huang Y. Access to DTP-Based Combination Vaccines in Asia–Pacific Countries between 2019 and 2022. Vaccines. 2024; 12(1):33. https://doi.org/10.3390/vaccines12010033
Chicago/Turabian StyleMa, Jiyan, Yinuo Sun, Yuxuan Cui, Jingya Dong, and Yangmu Huang. 2024. "Access to DTP-Based Combination Vaccines in Asia–Pacific Countries between 2019 and 2022" Vaccines 12, no. 1: 33. https://doi.org/10.3390/vaccines12010033
APA StyleMa, J., Sun, Y., Cui, Y., Dong, J., & Huang, Y. (2024). Access to DTP-Based Combination Vaccines in Asia–Pacific Countries between 2019 and 2022. Vaccines, 12(1), 33. https://doi.org/10.3390/vaccines12010033







