Association of the Magnitude of Anti-SARS-CoV-2 Vaccine Side Effects with Sex, Allergy History, Chronic Diseases, Medication Intake, and SARS-CoV-2 Infection
Abstract
1. Introduction
2. Methods
2.1. Study Design and Settings
2.2. The Questionnaire
2.3. Study Participants and Sample Size
2.4. Classification of the Side Effect Categories
2.5. Data Analysis
3. Results
3.1. Characteristics of the Participants
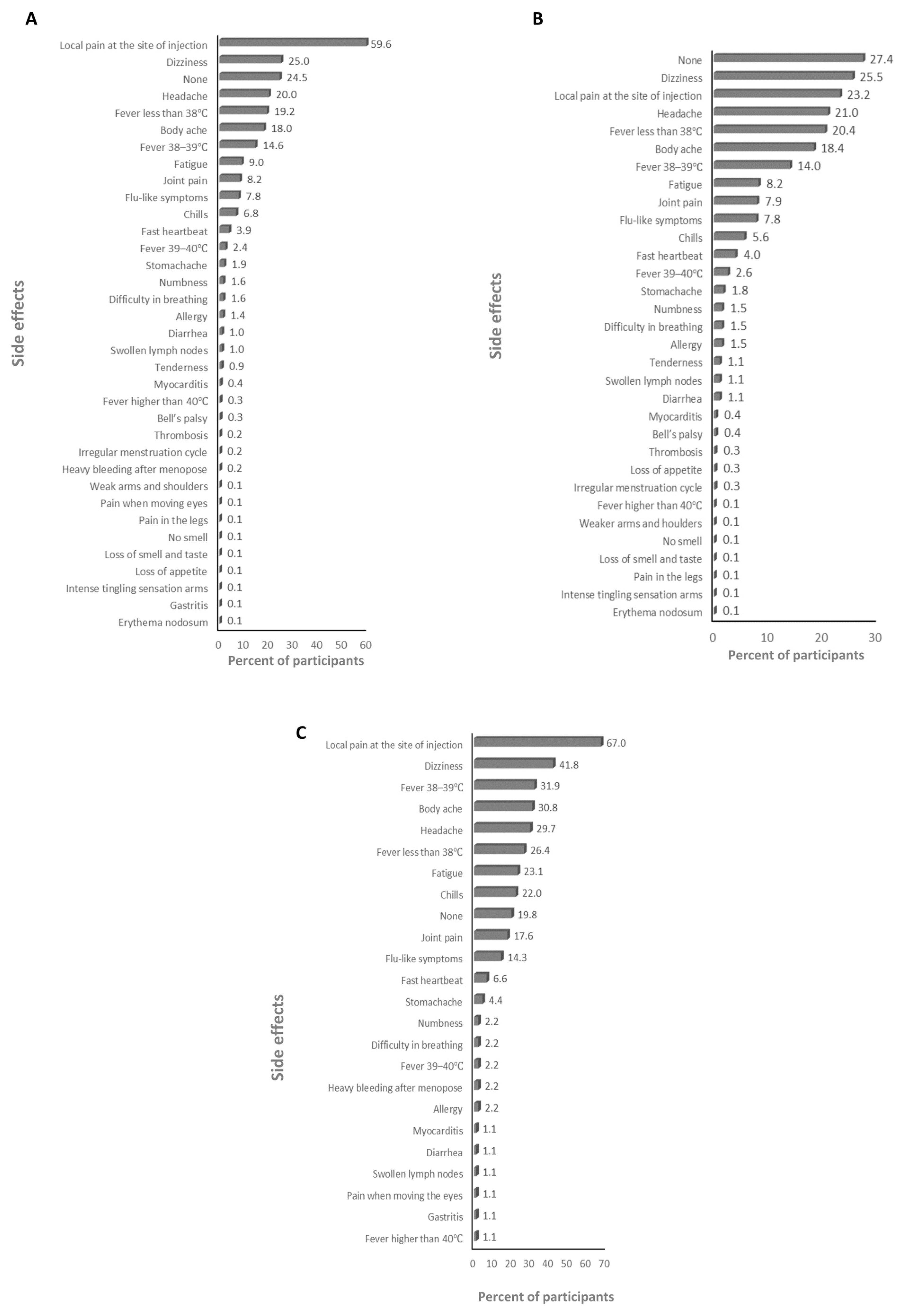
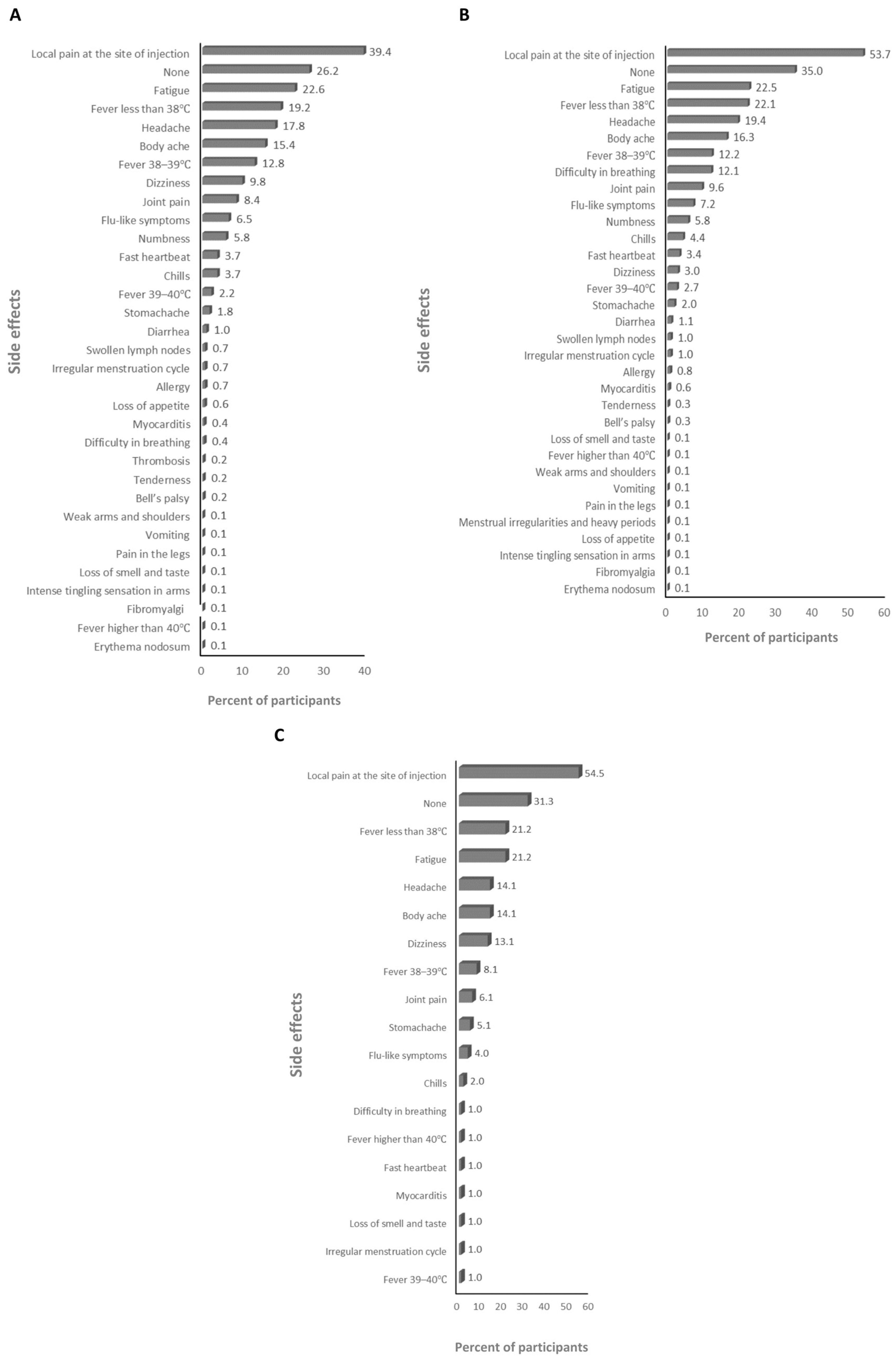
3.2. The Side Effects (SEs) of the Vaccines
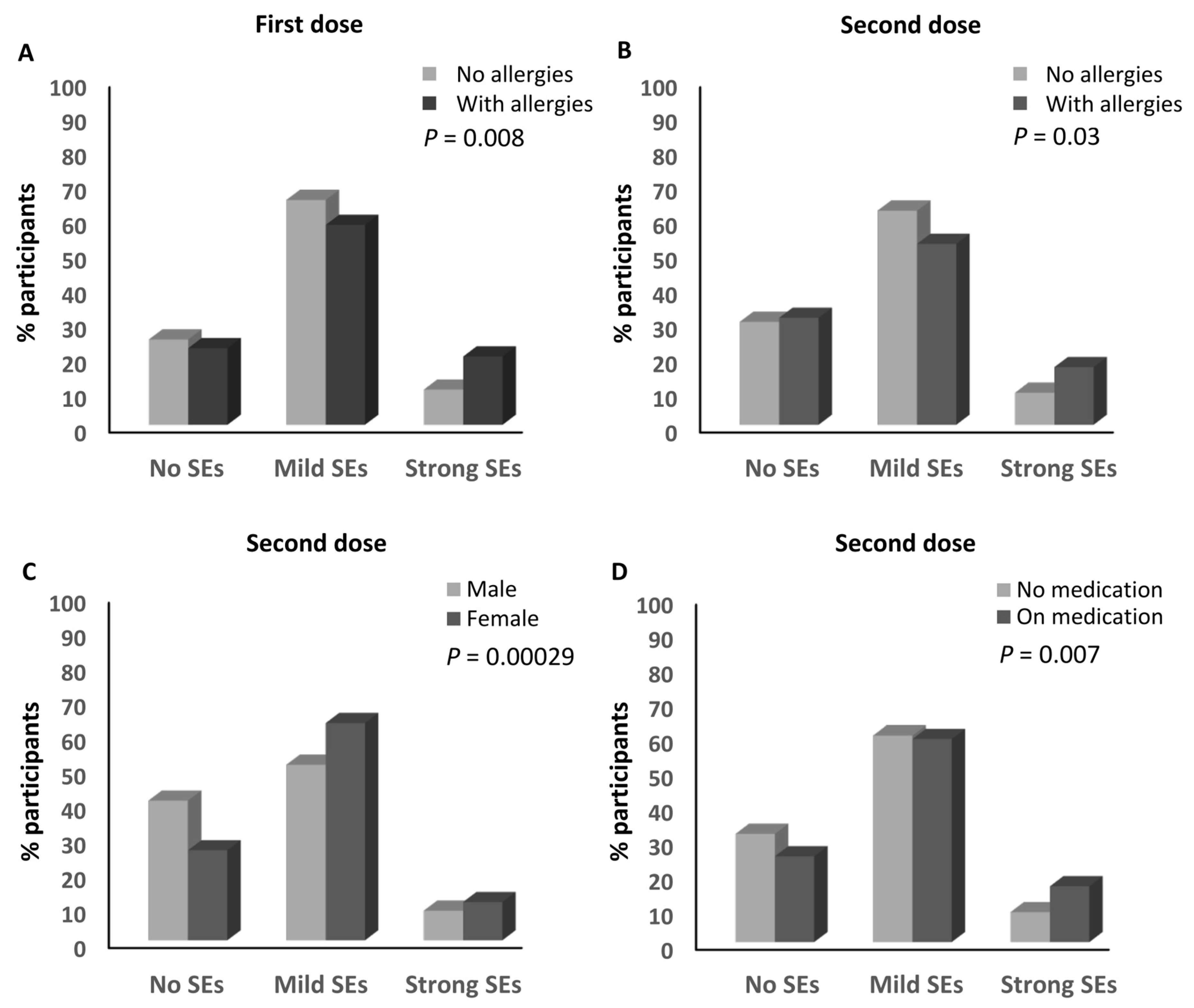
3.3. Parameters Associated with the Magnitude of the Side Effects of the Anti-SARS-CoV-2 Vaccines
3.4. Parameters Associated with the Magnitude of Side Effects for the BNT162b2 Vaccine
3.5. Parameters Associated with the Magnitude of Side Effects of the ChAdOx1-S Vaccine
3.6. Association between Having a History of Allergies and Developing Allergies upon Vaccination
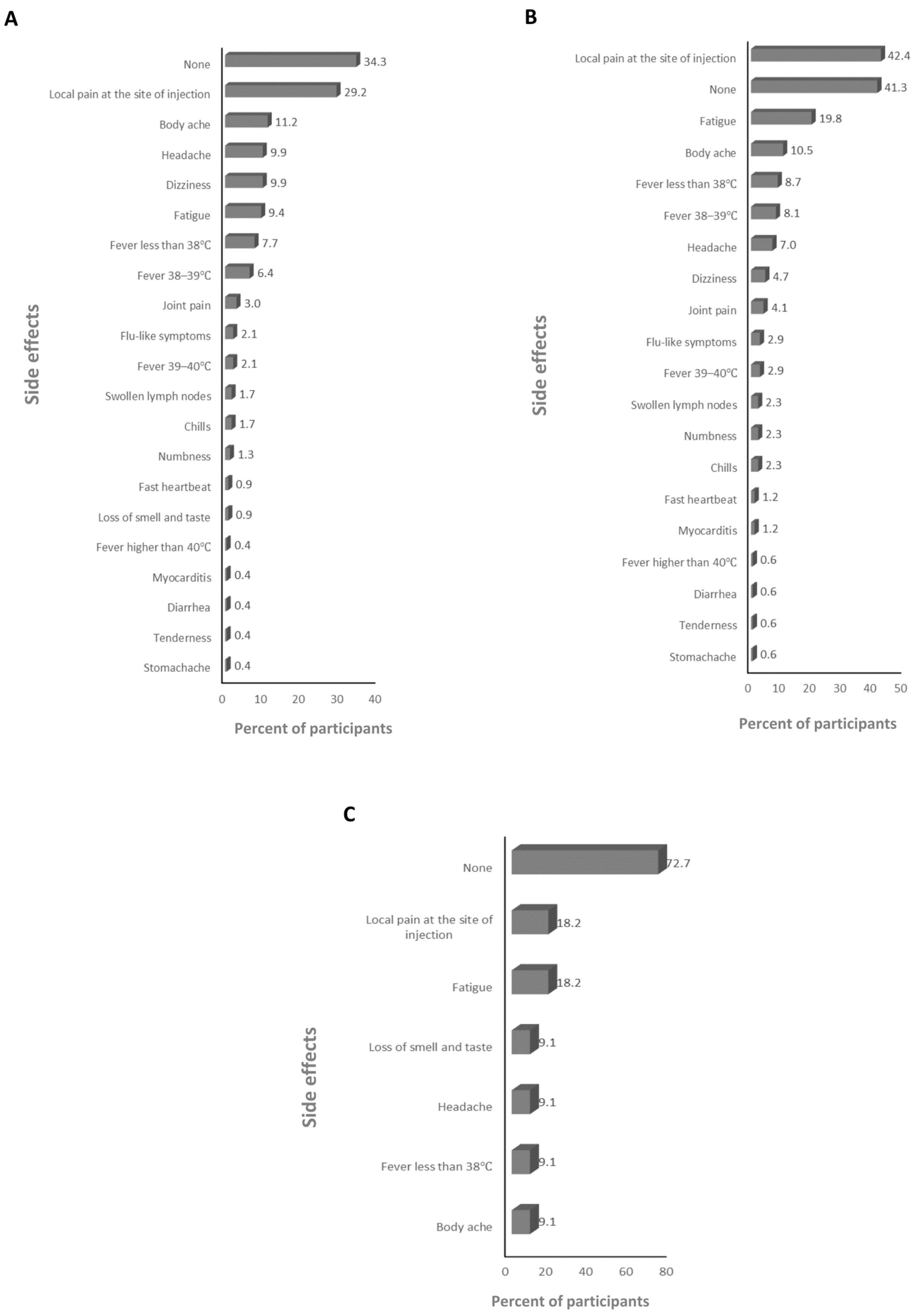
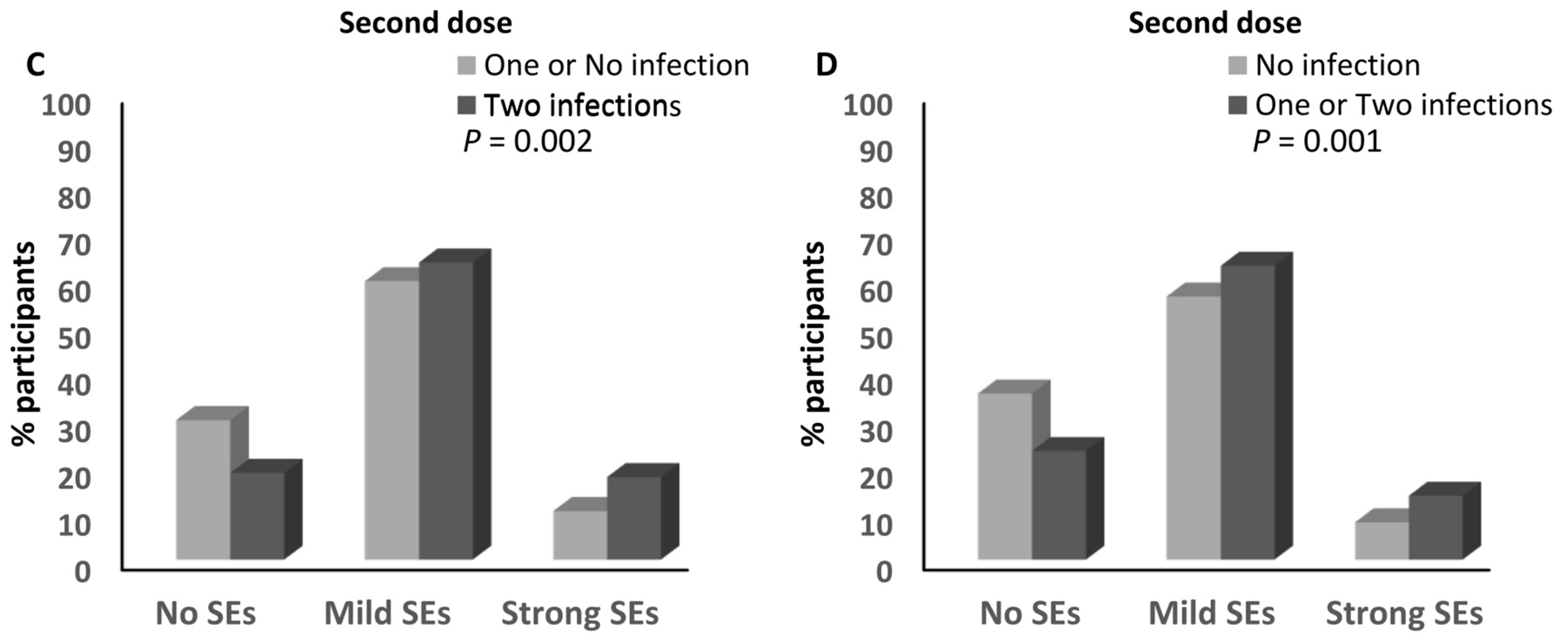
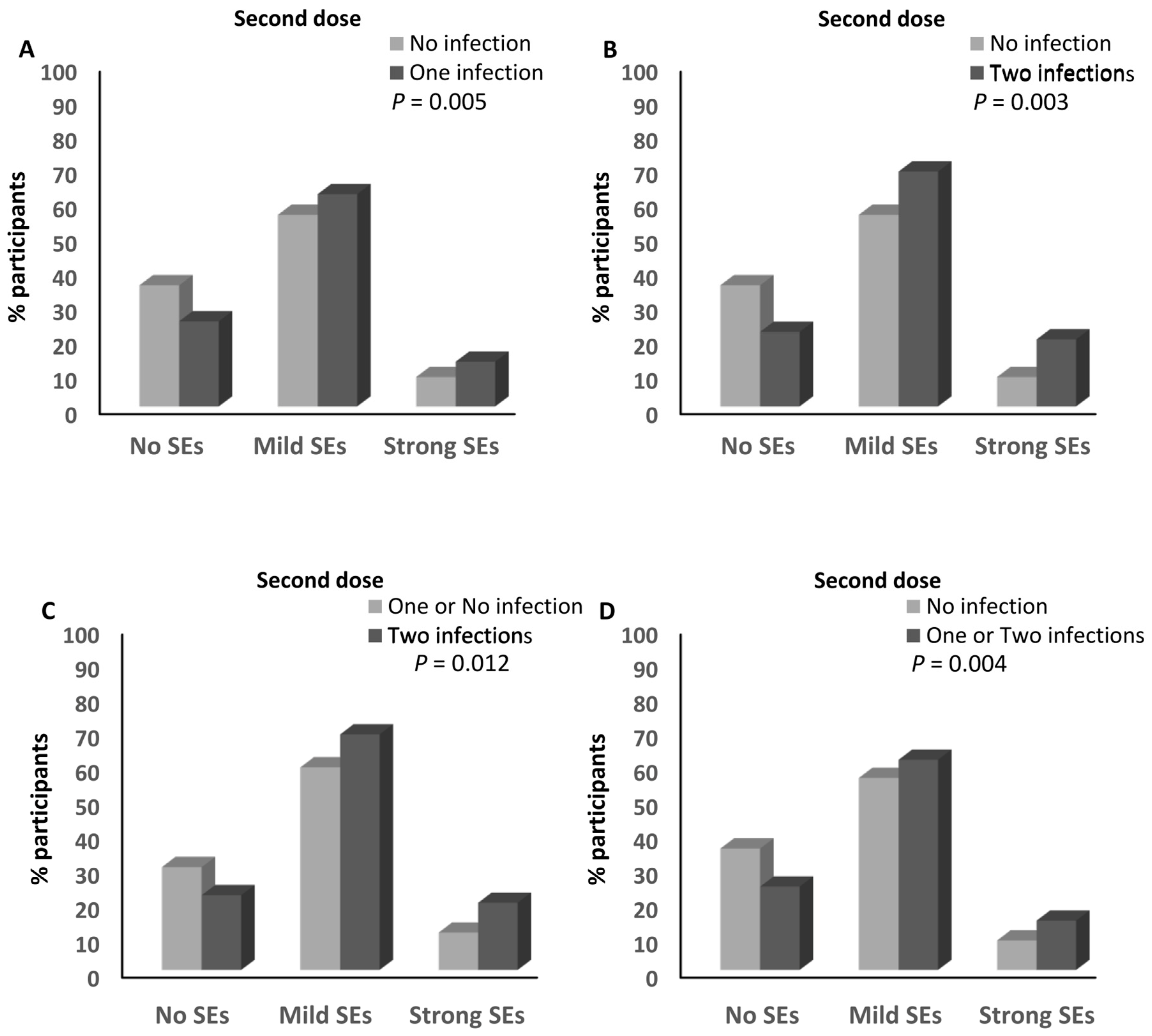
3.7. Infection with SARS-CoV-2 and the Magnitude of Side Effects after the Anti-SARS-CoV-2 Vaccination
3.8. Infection with SARS-CoV-2 and the Magnitude of Side Effects after the BNT162b2 Vaccine
3.9. Infection with SARS-CoV-2 and the Magnitude of Side Effects after the ChAdOx1-S Vaccine
4. Discussion
5. Conclusions
6. Study Strengths
7. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, N.; Zhang, C.; Dong, J.; Sun, L.; Chen, X.; Xie, Z.; Xu, B.; An, S.; Zhang, T.; Yang, F. Molecular evolution of human coronavirus-NL63, -229E, -HKU1 and -OC43 in hospitalized children in China. Front. Microbiol. 2022, 13, 1023847. [Google Scholar] [CrossRef] [PubMed]
- WHO. Statement for Healthcare Professionals: How COVID-19 Vaccines Are Regulated for Safety and Effectiveness (Revised March 2022); WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Looi, M.K. COVID-19: Hospital admissions rise in England amid fears of new variant and waning immunity. BMJ 2023, 382, 1833. [Google Scholar] [CrossRef] [PubMed]
- Mascellino, M.T.; Di Timoteo, F.; De Angelis, M.; Oliva, A. Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety. Infect. Drug Resist. 2021, 14, 3459–3476. [Google Scholar] [CrossRef] [PubMed]
- Dickerman, B.A.; Gerlovin, H.; Madenci, A.L.; Kurgansky, K.E.; Ferolito, B.R.; Figueroa Muniz, M.J.; Gagnon, D.R.; Gaziano, J.M.; Cho, K.; Casas, J.P.; et al. Comparative Effectiveness of BNT162b2 and mRNA-1273 Vaccines in U.S. Veterans. N. Engl. J. Med. 2022, 386, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, S.; Ghanavati, R.; Shirani, M.; Ghahramanpour, H.; Sholeh, M.; Shariati, A.; Sadeghifard, N.; Heidary, M. Viral vector and nucleic acid vaccines against COVID-19: A narrative review. Front. Microbiol. 2022, 13, 984536. [Google Scholar] [CrossRef] [PubMed]
- Grana, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef] [PubMed]
- Chapin-Bardales, J.; Gee, J.; Myers, T. Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines. JAMA 2021, 325, 2201–2202. [Google Scholar] [CrossRef]
- Mahallawi, W.H.; Mumena, W.A. Reactogenicity and Immunogenicity of the Pfizer and AstraZeneca COVID-19 Vaccines. Front. Immunol. 2021, 12, 794642. [Google Scholar] [CrossRef]
- NHS. COVID-19 Vaccines Side Effects and Safety; NHS: London, UK, 2023. [Google Scholar]
- CDC. Selected Adverse Events Reported after COVID-19 Vaccination; CDC: Atlanta, GA, USA, 2023. [Google Scholar]
- Pomara, C.; Sessa, F.; Ciaccio, M.; Dieli, F.; Esposito, M.; Garozzo, S.F.; Giarratano, A.; Prati, D.; Rappa, F.; Salerno, M.; et al. Post-mortem findings in vaccine-induced thrombotic thombocytopenia. Haematologica 2021, 106, 2291–2293. [Google Scholar] [CrossRef]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Atif, A.R.; Asghar, M.S.; Nimri, N.; Saleem, M.; Bandyopadhyay, D.; Krittanawong, C.; et al. Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun. Inflamm. Dis. 2023, 11, e807. [Google Scholar] [CrossRef]
- Butt, A.A.; Guerrero, M.D.; Canlas, E.B.; Al-Dwairi, H.; Alimam, A.; Mohamad, A.R.; Ali, M.T.; Asaad, N.A.; Alkeldi, A.; Mohammad, M.F.S.; et al. Evaluation of mortality attributable to SARS-CoV-2 vaccine administration using national level data from Qatar. Nat. Commun. 2023, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Nafilyan, V.; Bermingham, C.R.; Ward, I.L.; Morgan, J.; Zaccardi, F.; Khunti, K.; Stanborough, J.; Banerjee, A.; Doidge, J.C. Risk of death following COVID-19 vaccination or positive SARS-CoV-2 test in young people in England. Nat. Commun. 2023, 14, 1541. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Yokoyama, A.; Shichida, A.; Sasuga, K.; Maekawa, T.; Moriyama, T. Impact of Sex and Age on mRNA COVID-19 Vaccine-Related Side Effects in Japan. Microbiol. Spectr. 2022, 10, e0130922. [Google Scholar] [CrossRef] [PubMed]
- Scher, A.I.; Berjohn, C.M.; Byrne, C.; Colombo, R.E.; Colombo, C.J.; Edwards, M.S.; Ewers, E.C.; Ganesan, A.; Jones, M.; Larson, D.T.; et al. An Analysis of SARS-CoV-2 Vaccine Reactogenicity: Variation by Type, Dose, and History, Severity, and Recency of Prior SARS-CoV-2 Infection. Open Forum Infect. Dis. 2022, 9, ofac314. [Google Scholar] [CrossRef] [PubMed]
- He, Y.F.; Ouyang, J.; Hu, X.D.; Wu, N.; Jiang, Z.G.; Bian, N.; Wang, J. Correlation between COVID-19 vaccination and diabetes mellitus: A systematic review. World J. Diabetes 2023, 14, 892–918. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Khan, M.T.; Zaman, S.; Mujtaba, S.; Batool, A.; Ghanghro, Z.; Anwar, A.; Hashmi, A.A. Side Effects of COVID-19 Vaccines Among Diabetic Subjects and Healthy Individuals. Cureus 2023, 15, e36005. [Google Scholar] [CrossRef]
- Ebrahimian, S.; Amini, S.; Aghoun, Z. Association between COVID-19 vaccine side effects and history of nutritional supplement intake and body mass index (BMI): A retrospective study. Nutr. Food Sci. 2023, 53, 608–617. [Google Scholar] [CrossRef]
- Gualtieri, P.; Trombetta, D.; Smeriglio, A.; Frank, G.; Alibrandi, A.; Leggeri, G.; Marchetti, M.; Zingale, I.; Fanelli, S.; Stocchi, A.; et al. Effectiveness of Nutritional Supplements for Attenuating the Side Effects of SARS-CoV-2 Vaccines. Nutrients 2023, 15, 1807. [Google Scholar] [CrossRef]
- de Heredia, F.P.; Gomez-Martinez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef]
- Geerlings, S.E.; Hoepelman, A.I. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef]
- Oman, N. Oman Population. National Center for statistics and Information, Muscat, Oman. 2022. Available online: https://www.ncsi.gov.om/Pages/AllIndicators.aspx (accessed on 2 January 2024).
- Al Awaidy, S.T.; Khatiwada, M.; Castillo, S.; Al Siyabi, H.; Al Siyabi, A.; Al Mukhaini, S.; Dochez, C. Knowledge, Attitude, and Acceptability of COVID-19 Vaccine in Oman: A Cross-sectional Study. Oman Med. J. 2022, 37, e380. [Google Scholar] [CrossRef] [PubMed]
- Nittner-Marszalska, M.; Rosiek-Biegus, M.; Kopec, A.; Pawlowicz, R.; Kosinska, M.; Lata, A.; Szenborn, L. Pfizer-BioNTech COVID-19 Vaccine Tolerance in Allergic versus Non-Allergic Individuals. Vaccines 2021, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.V.; Nguyen, B.T.; Duong, H.N.V.; Lenh, P.T.; Tran, K.T.; Tran, H.M.; Nguyen, T.C.; Nguyen, D.P.; Ta, M.N.; Trieu, N.N.M.; et al. Side effects following first dose of COVID-19 vaccination in Ho Chi Minh City, Vietnam. Hum. Vaccin. Immunother. 2023, 19, 2176066. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.N.; Alotaibi, M.I.; Alqahtani, A.S.; Al Aboud, D.; Abdel-Moneim, A.S. BNT162b2 and ChAdOx1 SARS-CoV-2 Post-vaccination Side-Effects Among Saudi Vaccinees. Front. Med. 2021, 8, 760047. [Google Scholar] [CrossRef]
- Green, M.S.; Peer, V.; Magid, A.; Hagani, N.; Anis, E.; Nitzan, D. Gender Differences in Adverse Events Following the Pfizer-BioNTech COVID-19 Vaccine. Vaccines 2022, 10, 233. [Google Scholar] [CrossRef]
- Ossato, A.; Tessari, R.; Trabucchi, C.; Zuppini, T.; Realdon, N.; Marchesini, F. Comparison of medium-term adverse reactions induced by the first and second dose of mRNA BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine: A post-marketing Italian study conducted between 1 January and 28 February 2021. Eur. J. Hosp. Pharm. 2021, 30, e15. [Google Scholar] [CrossRef]
- Bettinger, J.A.; Irvine, M.A.; Shulha, H.P.; Valiquette, L.; Muller, M.P.; Vanderkooi, O.G.; Kellner, J.D.; Top, K.A.; Sadarangani, M.; McGeer, A.; et al. Adverse Events Following Immunization With mRNA and Viral Vector Vaccines in Individuals with Previous Severe Acute Respiratory Syndrome Coronavirus 2 Infection from the Canadian National Vaccine Safety Network. Clin. Infect. Dis. 2023, 76, 1088–1102. [Google Scholar] [CrossRef]
- Potluri, T.; Fink, A.L.; Sylvia, K.E.; Dhakal, S.; Vermillion, M.S.; Vom Steeg, L.; Deshpande, S.; Narasimhan, H.; Klein, S.L. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. npj Vaccines 2019, 4, 29. [Google Scholar] [CrossRef]
- Halsey, N.A.; Griffioen, M.; Dreskin, S.C.; Dekker, C.L.; Wood, R.; Sharma, D.; Jones, J.F.; LaRussa, P.S.; Garner, J.; Berger, M.; et al. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: Reports to VAERS. Vaccine 2013, 31, 6107–6112. [Google Scholar] [CrossRef]
- Voskuhl, R.R. The effect of sex on multiple sclerosis risk and disease progression. Mult. Scler. 2020, 26, 554–560. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Spiering, A.E.; de Vries, T.J. Why Females Do Better: The X Chromosomal TLR7 Gene-Dose Effect in COVID-19. Front. Immunol. 2021, 12, 756262. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.; Mills, K.H.G. Sex differences regulate immune responses in experimental autoimmune encephalomyelitis and multiple sclerosis. Eur. J. Immunol. 2022, 52, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Haq, H.N.; Khan, H.; Chaudhry, H.; Nimmala, S.; Demidovich, J.; Papudesi, B.N.; Potluri, S.D. Pfizer-BioNTech (BNT162b2), Moderna (mRNA-1273) COVID-19 mRNA vaccines and hypersensitivity reactions. J. Natl. Med. Assoc. 2022, 114, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Hatmal, M.M.; Al-Hatamleh, M.A.I.; Olaimat, A.N.; Mohamud, R.; Fawaz, M.; Kateeb, E.T.; Alkhairy, O.K.; Tayyem, R.; Lounis, M.; Al-Raeei, M.; et al. Reported Adverse Effects and Attitudes among Arab Populations Following COVID-19 Vaccination: A Large-Scale Multinational Study Implementing Machine Learning Tools in Predicting Post-Vaccination Adverse Effects Based on Predisposing Factors. Vaccines 2022, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Kanizsai, A.; Zavori, L.; Molnar, T.; Tokes-Fuzesi, M.; Szalai, Z.; Berecz, J.; Varnai, R.; Peterfi, Z.; Schwarcz, A.; Csecsei, P. Adverse Reactions after Booster SARS-CoV-2 Vaccination Have Less Impact on Antibody Response than after Basic Vaccination Scheme. Vaccines 2023, 11, 182. [Google Scholar] [CrossRef]
- Cheng, A.; Hsieh, M.J.; Chang, S.Y.; Ieong, S.M.; Cheng, C.Y.; Sheng, W.H.; Chang, S.C. Correlation of adverse effects and antibody responses following homologous and heterologous COVID19 prime-boost vaccinations. J. Formos. Med. Assoc. 2023, 122, 384–392. [Google Scholar] [CrossRef]
- Coppeta, L.; Ferrari, C.; Somma, G.; Mazza, A.; D’Ancona, U.; Marcuccilli, F.; Grelli, S.; Aurilio, M.T.; Pietroiusti, A.; Magrini, A.; et al. Reduced Titers of Circulating Anti-SARS-CoV-2 Antibodies and Risk of COVID-19 Infection in Healthcare Workers during the Nine Months after Immunization with the BNT162b2 mRNA Vaccine. Vaccines 2022, 10, 141. [Google Scholar] [CrossRef]
- Coggins, S.A.; Laing, E.D.; Olsen, C.H.; Goguet, E.; Moser, M.; Jackson-Thompson, B.M.; Samuels, E.C.; Pollett, S.D.; Tribble, D.R.; Davies, J.; et al. Adverse Effects and Antibody Titers in Response to the BNT162b2 mRNA COVID-19 Vaccine in a Prospective Study of Healthcare Workers. Open Forum Infect. Dis. 2022, 9, ofab575. [Google Scholar] [CrossRef]
- Francois Watkins, L.K.; Mitruka, K.; Dorough, L.; Bressler, S.S.; Kugeler, K.J.; Sadigh, K.S.; Birhane, M.G.; Nolen, L.D.; Fischer, M. Characteristics of Reported Deaths Among Fully Vaccinated Persons with Coronavirus Disease 2019-United States, January-April 2021. Clin. Infect. Dis. 2022, 75, e645–e652. [Google Scholar] [CrossRef]
- Esposito, M.; Cocimano, G.; Vanaria, F.; Sessa, F.; Salerno, M. Death from COVID-19 in a Fully Vaccinated Subject: A Complete Autopsy Report. Vaccines 2023, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Valiati, N.C.M.; Noronha, T.G.; Porto, V.B.G.; Pacheco, A.G.; Freitas, L.P.; Coelho, F.C.; Gomes, M.; Bastos, L.S.; Cruz, O.G.; et al. The effectiveness of COVID-19 vaccines against severe cases and deaths in Brazil from 2021 to 2022: A registry-based study. Lancet Reg. Health Am. 2023, 20, 100465. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Bautista, P.F.; Grajales Muniz, C.; Cabrera Gaytan, D.A.; Rojas Mendoza, T.; Vallejos Paras, A.; Santacruz Tinoco, C.E.; Alvarado Yaah, J.E.; Anguiano Hernandez, Y.M.; Sandoval Gutierrez, N.; Jaimes Betancourt, L. Impact of vaccination on infection or death from COVID-19 in individuals with laboratory-confirmed cases: Case-control study. PLoS ONE 2023, 18, e0265698. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Side Effect Magnitude; n (%) | Odds Ratio | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| None | Mild | Strong | None/ Mild | None/ Strong | Mild/ Strong | |||
| First dose | ||||||||
| Sex | Male | 53 (23.7%) | 148 (66.1%) | 23 (10.3%) | 1.08 | 0.87 | 0.81 | 0.680 |
| Female | 151 (24.6%) | 389 (63.3%) | 75 (12.2%) | |||||
| Allergies | No | 176 (24.7%) | 464 (65.1%) | 73 (10.2%) | 1.01 | 0.46 | 0.46 | 0.008 |
| Yes | 28 (22.2%) | 73 (57.9%) | 25 (19.8%) | |||||
| Chronic disease | No | 182 (24.6%) | 471 (63.7%) | 86 (11.6%) | 0.86 | 0.87 | 1.00 | 0.850 |
| Yes | 22 (22%) | 66 (66%) | 12 (12%) | |||||
| Medications | No | 159 (24.4%) | 418 (64.1%) | 75 (11.5%) | 0.99 | 0.92 | 0.93 | 0.960 |
| Yes | 45 (24.1%) | 119 (63.6%) | 23 (12.3%) | |||||
| Autoimmune disease | No | 200 (24.2%) | 530 (64.1%) | 97 (11.7%) | 1.51 | 1.94 | 1.28 | 0.750 |
| Yes | 4 (33.3%) | 7 (58.3%) | 1 (8.3%) | |||||
| Age | 18–50 | 193 (24.1%) | 513 (64.1%) | 94 (11.8%) | 1.22 | 1.34 | 1.10 | 0.830 |
| >50 | 11 (28.2%) | 24 (61.5%) | 4 (10.3%) | |||||
| Second dose | ||||||||
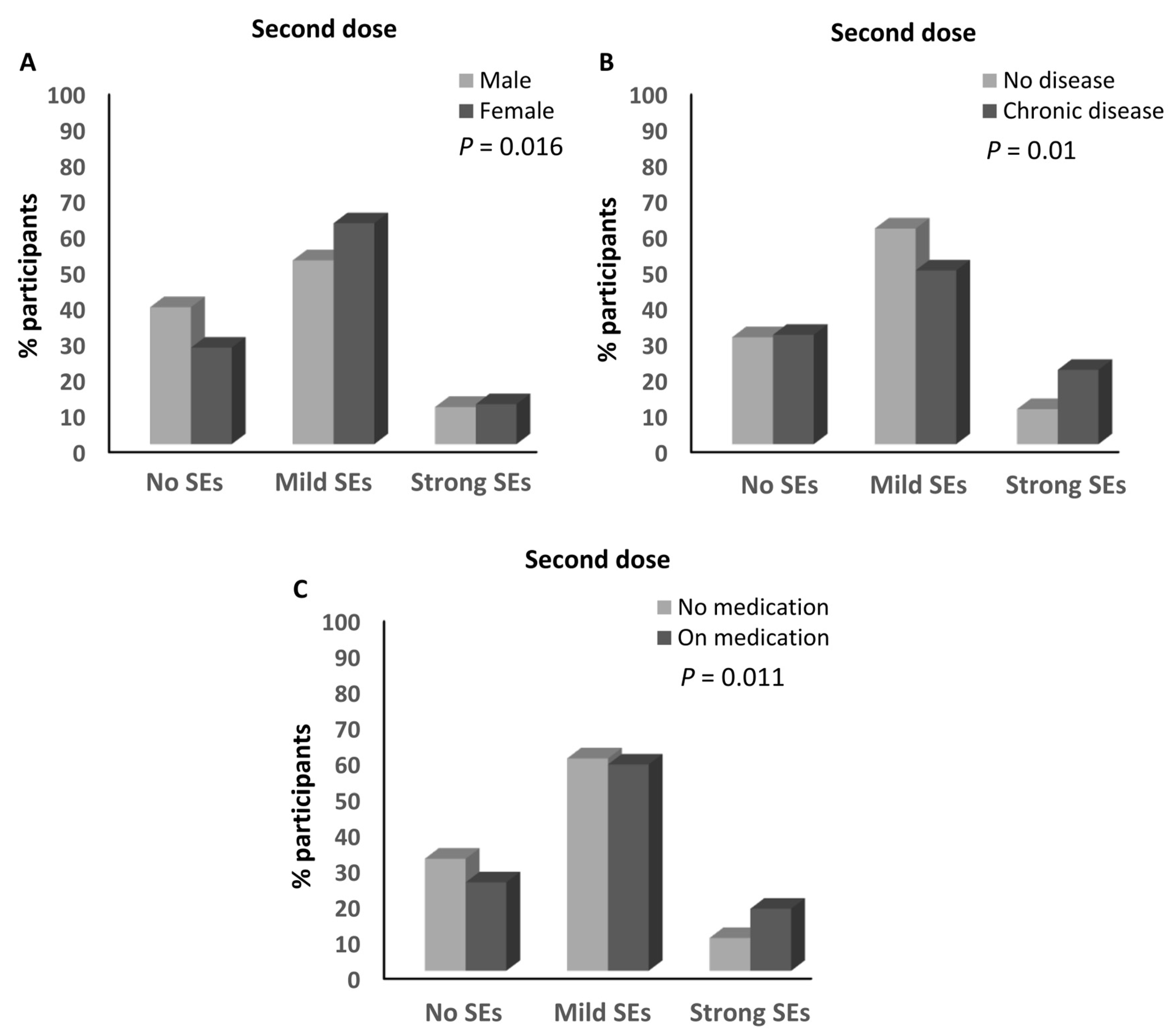
| Sex | Male | 90 (40.5%) | 113 (50.9%) | 19 (8.6%) | 0.52 | 0.50 | 0.96 | 0.000299 |
| Female | 158 (26.1%) | 381 (62.9%) | 67 (11.1%) | |||||
| Allergies | No | 209 (29.8%) | 428 (61%) | 65 (9.3%) | 1.21 | 0.58 | 0.48 | 0.03 |
| Yes | 39 (31%) | 66 (52.4%) | 21 (16.7%) | |||||
| Chronic disease | No | 219 (30%) | 442 (60.5%) | 69 (9.5% | 1.13 | 0.54 | 0.48 | 0.05 |
| Yes | 29 (29.6%) | 52 (53.1%) | 17 (17.3%) | |||||
| Medications | No | 202 (31.4%) | 385 (59.9%) | 56 (8.7%) | 0.80 | 0.43 | 0.53 | 0.01 |
| Yes | 46 (24.9) | 109 (58.9%) | 30 (16.2) | |||||
| Autoimmune disease | No | 247 (30.3%) | 486 (59.6%) | 83 (10.2%) | 0.25 | 0.11 | 0.46 | 0.11 |
| Yes | 1 (8.3%) | 8 (66.7%) | 3 (25%) | |||||
| Age | 18–50 | 234 (29.7%) | 475 (60.2%) | 80 (10.1%) | 1.50 | 0.80 | 0.53 | 0.32 |
| >50 | 14 (35.9%) | 19 (48.9%) | 6 (15.4%) | |||||
| Third dose | ||||||||
| Sex | Male | 18 (40%) | 24 (53.3%) | 3 (6.7%) | 0.53 | 0.93 | 1.78 | 0.224 |
| Female | 28 (26.9%) | 71 (68.3%) | 5 (4.8%) | |||||
| Allergies | No | 42 (33.9%) | 74 (59.7%) | 8 (6.5%) | 0.34 | - | - | 0.06 |
| Yes | 4 (16%) | 21 (84%) | 0 (0%) | |||||
| Chronic disease | No | 38 (32.8%) | 74 (63.8%) | 4 (3.4%) | 0.74 | 0.21 | 0.28 | 0.16 |
| Yes | 8 (24.2%) | 21 (63.6%) | 4 (12.1%) | |||||
| Medications | No | 31 (34.1%) | 58 (63.73%) | 2 (2.2%) | 0.76 | 0.16 | 0.21 | 0.79 |
| Yes | 15 (25.9%) | 37 (63.8%) | 6 (10.3%) | |||||
| Autoimmune disease | No | 45 (31.25%) | 91 (63.2%) | 8 (5.6%) | 0.51 | - | - | 0.62 |
| Yes | 1 (20%) | 4 (80%) | 0 (0%) | |||||
| Age | 18–50 | 40 (31.5%) | 81 (63.8%) | 6 (4.7%) | 0.87 | 0.45 | 0.52 | 0.71 |
| >50 | 6 (27.3%) | 14 (63.6%) | 2 (9.1%) | |||||
| Parameters | Side Effect Magnitude; n (%) | Odds Ratio | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| None | Mild | Strong | None/ Mild | None/ Strong | Mild/ Strong | |||
| First dose | ||||||||
| Sex | Male | 40 (21.6%) | 125 (67.6%) | 20 (10.8%) | 1.30 | 1.10 | 0.84 | 0.42 |
| Female | 134 (25.9%) | 322 (62.3%) | 61 (11.8%) | |||||
| Allergies | No | 151 (25.3%) | 385 (64.4%) | 62 (10.4%) | 0.95 | 0.50 | 0.53 | 0.07 |
| Yes | 23 (22.1%) | 62 (59.6%) | 19 (18.3%) | |||||
| Chronic disease | No | 156 (24.8%) | 399 (63.5%) | 73 (11.6%) | 0.96 | 1.05 | 1.10 | 0.97 |
| Yes | 18 (24.3%) | 48 (64.9%) | 8 (10.8%) | |||||
| Medications | No | 135 (24.5%) | 351 (63.7%) | 65 (11.8%) | 1.06 | 1.17 | 1.11 | 0.89 |
| Yes | 39 (25.8%) | 96 (63.6%) | 16 (11.5%) | |||||
| Autoimmune disease | No | 170 (24.6%) | 441 (63.8%) | 80 (11.6%) | 1.73 | 1.88 | 1.09 | 0.67 |
| Yes | 4 (36.4%) | 6 (54.5%) | 1 (9.1%) | |||||
| Age | 18–50 | 166 (24.5%) | 432 (63.7%) | 80 (11.8%) | 1.39 | 3.86 | 2.78 | 0.39 |
| >50 | 8 (33.3%) | 15 (62.5%) | 1 (4.2%) | |||||
| Second dose | ||||||||
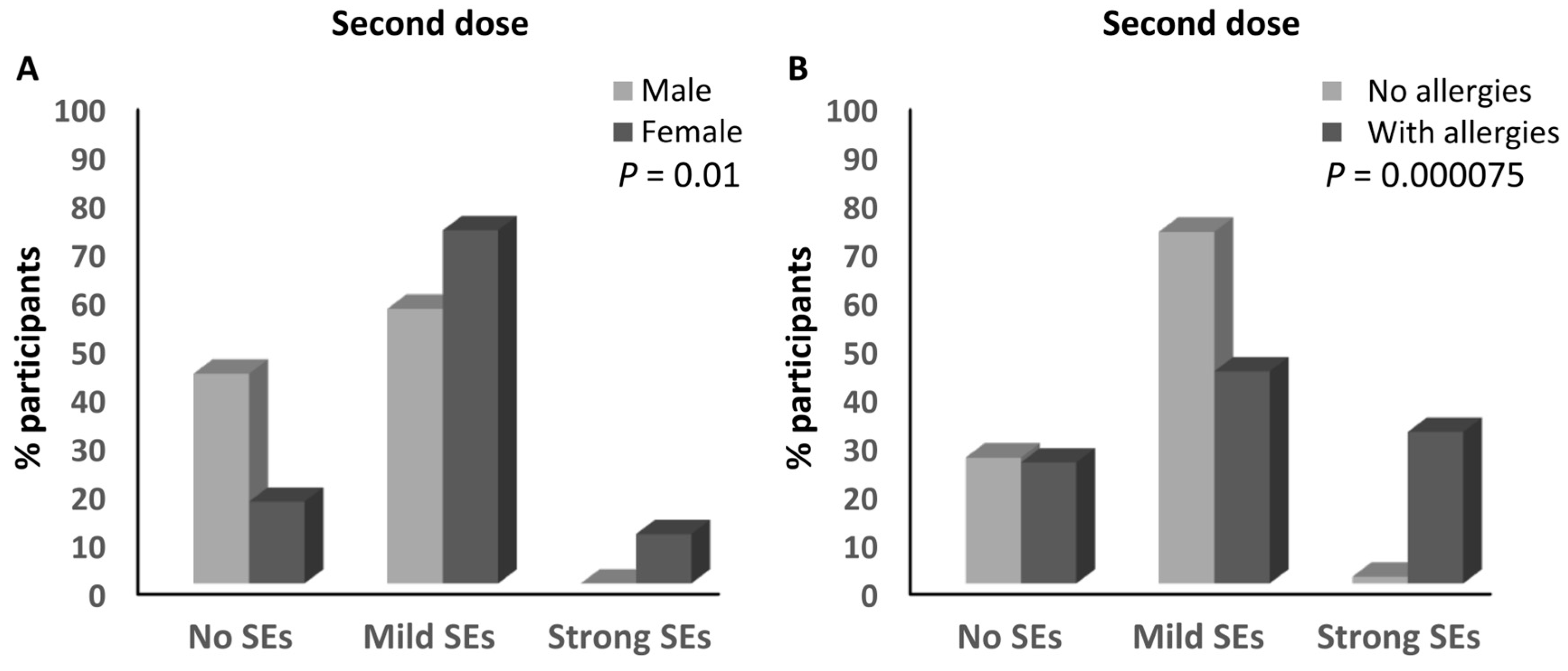
| Sex | Male | 70 (38.3%) | 94 (51.4%) | 19 (10.4%) | 0.59 | 0.66 | 1.12 | 0.016 |
| Female | 138 (27%) | 316 (61.8%) | 57 (11.2%) | |||||
| Allergies | No | 178 (30.2%) | 352 (59.7%) | 60 (10.2%) | 1.02 | 0.63 | 0.62 | 0.29 |
| Yes | 30 (28.8%) | 58 (55.8%) | 16 (15.4%) | |||||
| Chronic disease | No | 186 (29.9%) | 375 (60.3%) | 61 (9.8%) | 1.27 | 0.48 | 0.38 | 0.01 |
| Yes | 22 (30.6%) | 35 (48.6%) | 15 (20.8%) | |||||
| Medications | No | 171 (31.4%) | 324 (59.4%) | 50 (9.2%) | 0.82 | 0.42 | 0.51 | 0.01 |
| Yes | 37 (24.8%) | 86 (57.7%) | 26 (17.4%) | |||||
| Autoimmune disease | No | 207 (30.3%) | 403 (59%) | 73 (10.7%) | 0.28 | 0.12 | 0.42 | 0.11 |
| Yes | 1 (9.1%) | 7 (63.6%) | 3 (27.33%) | |||||
| Age | 18–50 | 201 (30%) | 398 (59.4%) | 71 (10.6%) | 1.16 | 0.49 | 0.43 | 0.28 |
| >50 | 7 (29.2%) | 12 (50%) | 5 (20.8%) | |||||
| Third dose | ||||||||
| Sex | Male | 16 (40%) | 21 (52.5%) | 3 (7.5%) | 0.48 | 1.50 | 3.14 | 0.11 |
| Female | 24 (25.8%) | 66 (70.9%) | 3 (3.2%) | |||||
| Allergies | No | 37 (33%) | 69 (61.6%) | 6 (5.4%) | 0.31 | - | - | 0.05 |
| Yes | 3 (14.3%) | 18 (85.7%) | 0 (0%) | |||||
| Chronic disease | No | 34 (32.7%) | 67 (64.4%) | 3 (2.9%) | 0.59 | 0.18 | 0.30 | 0.17 |
| Yes | 6 (20.7%) | 20 (68.9%) | 3 (10.3) | |||||
| Medications | No | 27 (33.3%) | 52 (64.2%) | 2 (2.5%) | 0.72 | 0.24 | 0.34 | 0.27 |
| Yes | 13 (25%) | 35 (67.3%) | 4 (7.7%) | |||||
| Autoimmune disease | No | 39 (30.5%) | 83 (64.8%) | 6 (4.7%) | 0.53 | - | - | 0.67 |
| Yes | 1 (20%) | 4 (80%) | 0 (0%) | |||||
| Age | 18–50 | 5 (27.8%) | 12 (66.7%) | 1 (5.6%) | 1.12 | 1.40 | 1.25 | 0.96 |
| >50 | 35 (30.4%) | 75 (65.2%) | 5 (4.3%) | |||||
| Parameters | Side Effect Magnitude; n (%) | Odds Ratio | p Value | |||||
|---|---|---|---|---|---|---|---|---|
| None | Mild | Strong | None/ Mild | None/ Strong | Mild/ Strong | |||
| First dose | ||||||||
| Sex | Male | 9 (30.0%) | 19 (63.3%) | 2 (6.7%) | 0.51 | 0.25 | 0.48 | 0.24 |
| Female | 10 (16.7%) | 41 (68.3%) | 9 (15%) | |||||
| Allergies | No | 17 (23%) | 50 (67.6%) | 7 (9.5%) | 0.59 | 0.21 | 0.35 | 0.19 |
| Yes | 2 (12.5%) | 10 (62.5%) | 4 (25%) | |||||
| Chronic disease | No | 15 (22.1%) | 44 (64.7%) | 9 (13.2%) | 0.73 | 1.20 | 1.64 | 0.77 |
| Yes | 4 (18.2%) | 16 (72.7%) | 2 (9.1%) | |||||
| Medications | No | 13 (22%) | 40 (67.8%) | 6 (10.2%) | 0.92 | 0.55 | 0.60 | 0.71 |
| Yes | 6 (19.4%) | 20 (64.5%) | 5 (16.1%) | |||||
| Autoimmune disease | No | 19 (21.3%) | 59 (66.3%) | 11 (12.4%) | 0.00 | - | - | 0.78 |
| Yes | 0 | 1 (100%) | 0 | |||||
| Age | 18–50 | 16 (21.1%) | 51 (67.1%) | 9 (11.8%) | 1.06 | 0.84 | 0.79 | 0.96 |
| >50 | 3 (21.4%) | 9 (64.3%) | 2 (14.3%) | |||||
| Second dose | ||||||||
| Sex | Male | 13 (43.3%) | 17 (56.7%) | 0 | 0.30 | - | - | 0.01 |
| Female | 10 (16.9%) | 43 (72.9%) | 6 (10.2%) | |||||
| Allergies | No | 19 (26%) | 53 (72.6%) | 1 (1.4%) | 1.59 | 0.04 | 0.03 | 0.000075 |
| Yes | 4 (25%) | 7 (43.8%) | 5 (31.3%) | |||||
| Chronic disease | No | 16 (23.9%) | 46 (68.7%) | 5 (7.5%) | 1.44 | 2.19 | 1.52 | 0.71 |
| Yes | 7 (31.8%) | 14 (63.6%) | 1 (4.5%) | |||||
| Medications | No | 15 (25.9%) | 40 (69%) | 3 (5.2%) | 1.07 | 0.53 | 0.50 | 0.72 |
| Yes | 8 (25.8%) | 20 (64.5%) | 3 (9.7%) | |||||
| Autoimmune disease | No | 23 (26.1%) | 59 (67%) | 6 (6.8%) | - | - | - | 0.78 |
| Yes | 0 | 1 (100%) | 0 | |||||
| Age | 18–50 | 17 (22.7%) | 53 (70.7%) | 5 (6.7%) | 2.67 | 1.76 | 0.66 | 0.27 |
| >50 | 6 (42.9%) | 7 (50%) | 1 (7.1%) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said, E.A.; Al-Rubkhi, A.; Jaju, S.; Koh, C.Y.; Al-Balushi, M.S.; Al-Naamani, K.; Al-Sinani, S.; Al-Busaidi, J.Z.; Al-Jabri, A.A. Association of the Magnitude of Anti-SARS-CoV-2 Vaccine Side Effects with Sex, Allergy History, Chronic Diseases, Medication Intake, and SARS-CoV-2 Infection. Vaccines 2024, 12, 104. https://doi.org/10.3390/vaccines12010104
Said EA, Al-Rubkhi A, Jaju S, Koh CY, Al-Balushi MS, Al-Naamani K, Al-Sinani S, Al-Busaidi JZ, Al-Jabri AA. Association of the Magnitude of Anti-SARS-CoV-2 Vaccine Side Effects with Sex, Allergy History, Chronic Diseases, Medication Intake, and SARS-CoV-2 Infection. Vaccines. 2024; 12(1):104. https://doi.org/10.3390/vaccines12010104
Chicago/Turabian StyleSaid, Elias A., Afnan Al-Rubkhi, Sanjay Jaju, Crystal Y. Koh, Mohammed S. Al-Balushi, Khalid Al-Naamani, Siham Al-Sinani, Juma Z. Al-Busaidi, and Ali A. Al-Jabri. 2024. "Association of the Magnitude of Anti-SARS-CoV-2 Vaccine Side Effects with Sex, Allergy History, Chronic Diseases, Medication Intake, and SARS-CoV-2 Infection" Vaccines 12, no. 1: 104. https://doi.org/10.3390/vaccines12010104
APA StyleSaid, E. A., Al-Rubkhi, A., Jaju, S., Koh, C. Y., Al-Balushi, M. S., Al-Naamani, K., Al-Sinani, S., Al-Busaidi, J. Z., & Al-Jabri, A. A. (2024). Association of the Magnitude of Anti-SARS-CoV-2 Vaccine Side Effects with Sex, Allergy History, Chronic Diseases, Medication Intake, and SARS-CoV-2 Infection. Vaccines, 12(1), 104. https://doi.org/10.3390/vaccines12010104






