Assessing the Impact of COVID-19 Vaccination on Preterm Birth: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria and Definitions
2.3. Data Collection Process
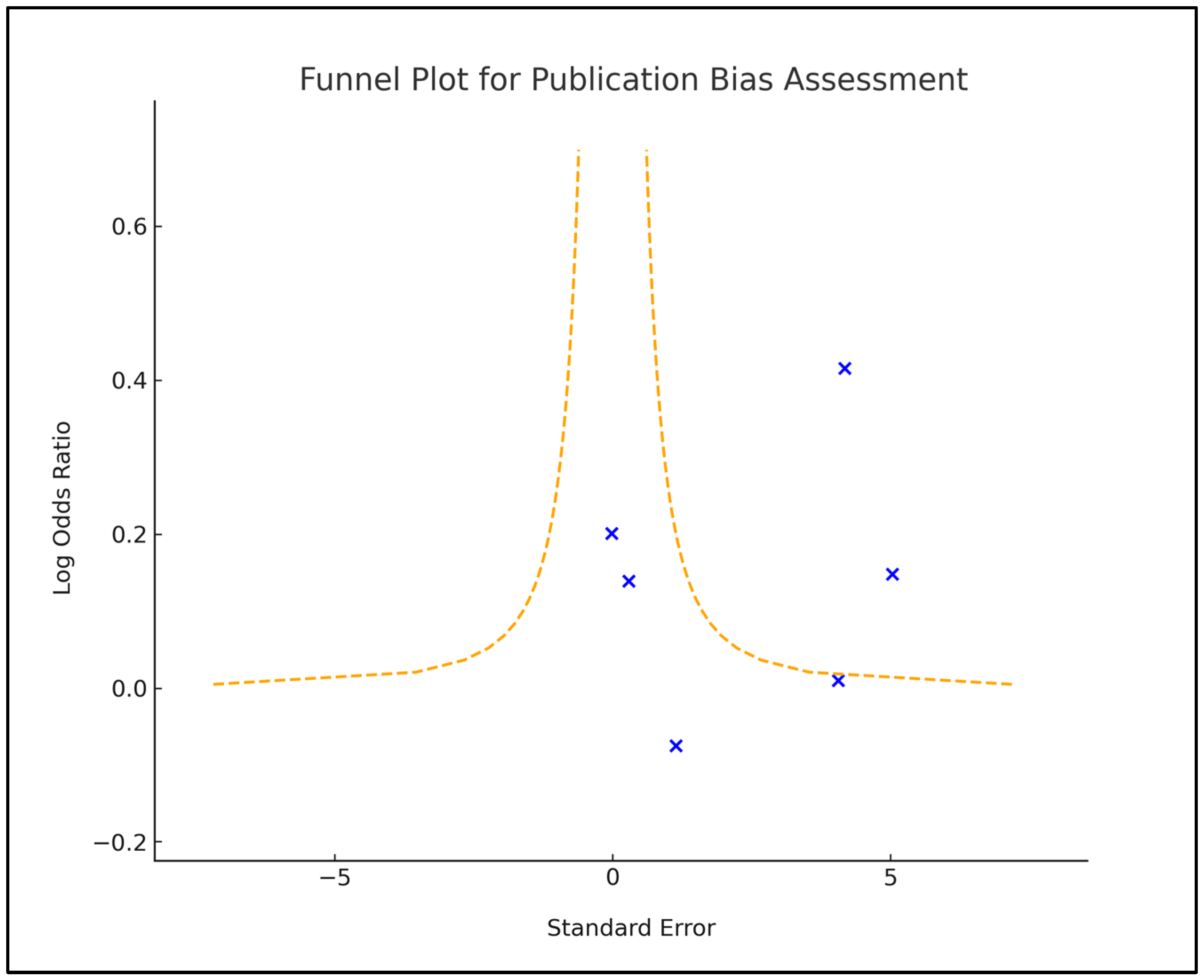
2.4. Risk of Bias and Quality Assessment
2.5. Statistical Analysis Methods
3. Results
3.1. Study Characteristics
3.2. Characteristics of Patients
3.3. COVID-19 Vaccination Characteristics
3.4. Analysis of Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miyah, Y.; Benjelloun, M.; Lairini, S.; Lahrichi, A. COVID-19 Impact on Public Health, Environment, Human Psychology, Global Socioeconomy, and Education. Sci. World J. 2022, 2022, 5578284. [Google Scholar] [CrossRef] [PubMed]
- Feehan, J.; Apostolopoulos, V. Is COVID-19 the worst pandemic? Maturitas 2021, 149, 56–58. [Google Scholar] [CrossRef]
- Nana, M.; Nelson-Piercy, C. COVID-19 in pregnancy. Clin. Med. 2021, 21, e446–e450. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Bappy, M.H.; Desai, S.; Chowdhury, S.; Patel, V.; Chowdhury, M.S.; Fonseca, A.; Sekzer, C.; Zahid, S.; Patousis, A.; et al. COVID-19 and Pregnancy. Discoveries 2022, 10, e147. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, A.; Greco, P.; Loverro, G.; Lopalco, P.L.; Pansini, V.; Selvaggi, L. Maternal complications after caesarean section in HIV infected women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 90, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zeng, J.; Liu, X.; Sun, G.; Gao, Y.; Liao, J.; Yu, J.; Luo, X.; Qi, H. Changes in physiology and immune system during pregnancy and coronavirus infection: A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 255, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Overton, E.E.; Goffman, D.; Friedman, A.M. The Epidemiology of COVID-19 in Pregnancy. Clin. Obstet. Gynecol. 2022, 65, 110–122. [Google Scholar] [CrossRef]
- Altendahl, M.; Mok, T.; Jang, C.; Yeo, S.; Quach, A.; Afshar, Y. Severe COVID-19 in pregnancy has a distinct serum profile, including greater complement activation and dysregulation of serum lipids. PLoS ONE 2022, 17, e0276766. [Google Scholar] [CrossRef]
- Yao, X.D.; Zhu, L.J.; Yin, J.; Wen, J. Impacts of COVID-19 pandemic on preterm birth: A systematic review and meta-analysis. Public Health 2022, 213, 127–134. [Google Scholar] [CrossRef]
- Alhumaidan, L.S.; Alhabardi, N.; Aldharman, S.S.; Alfuhaid, A.A.; Alrasheed, M.A.; Almotairi, R.S.; Alhassun, J.A.; Alrohait, G.A.; Almutairi, R.F.; Alsuwailem, F.S.; et al. The Impact of COVID-19 on Preterm Birth Among Pregnant Women in Al-Qassim, Saudi Arabia. Cureus 2023, 15, e40682. [Google Scholar] [CrossRef]
- Vimercati, A.; De Nola, R.; Trerotoli, P.; Metta, M.E.; Cazzato, G.; Resta, L.; Malvasi, A.; Lepera, A.; Ricci, I.; Capozza, M.; et al. COVID-19 Infection in Pregnancy: Obstetrical Risk Factors and Neonatal Outcomes-A Monocentric, Single-Cohort Study. Vaccines 2022, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Karasek, D.; Baer, R.J.; McLemore, M.R.; Bell, A.J.; Blebu, B.E.; Casey, J.A.; Coleman-Phox, K.; Costello, J.M.; Felder, J.N.; Flowers, E.; et al. The association of COVID-19 infection in pregnancy with preterm birth: A retrospective cohort study in California. Lancet Reg. Health Am. 2021, 2, 100027. [Google Scholar] [CrossRef] [PubMed]
- Ciapponi, A.; Berrueta, M.; PK Parker, E.; Bardach, A.; Mazzoni, A.; Anderson, S.A.; Argento, F.J.; Ballivian, J.; Bok, K.; Comandé, D.; et al. Safety of COVID-19 vaccines during pregnancy: A systematic review and meta-analysis. Vaccine 2023, 41, 3688–3700. [Google Scholar] [CrossRef] [PubMed]
- Badell, M.L.; Dude, C.M.; Rasmussen, S.A.; Jamieson, D.J. COVID-19 vaccination in pregnancy. BMJ 2022, 378, e069741. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.H.; Kim, H.I.; Lee, K.S.; Heo, J.S.; Kim, H.Y.; Cho, G.J.; Hong, S.C.; Oh, M.J.; Kim, H.J. COVID-19 and vaccination during pregnancy: A systematic analysis using Korea National Health Insurance claims data. Obstet. Gynecol. Sci. 2022, 65, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.; Asiri, M.; Alsuwaidan, S.; Sufyani, R.; AlSalem, S.; Alghamdi, J. COVID-19 Vaccine During Pregnancy and Perinatal Outcomes. Cureus 2023, 15, e33240. [Google Scholar] [CrossRef]
- Süt, H.; Aynaoğlu Yıldız, G.; Şeker, E.; Ümit, C.; Koçar, M.; Koç, A. Maternal and perinatal outcomes of COVID-19 vaccination during pregnancy. J. Turk. Gynecol. Assoc. 2023, 24, 120–124. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Schiavo, J.H. PROSPERO: An International Register of Systematic Review Protocols. Med. Ref. Serv. Q. 2019, 38, 171–180. [Google Scholar] [CrossRef]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Beharier, O.; Plitman Mayo, R.; Raz, T.; Nahum Sacks, K.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Investig. 2021, 131, e150319, Erratum in J. Clin. Investig. 2021, 131, e154834. [Google Scholar] [CrossRef]
- Citu, I.M.; Citu, C.; Gorun, F.; Sas, I.; Tomescu, L.; Neamtu, R.; Motoc, A.; Gorun, O.M.; Burlea, B.; Bratosin, F.; et al. Immunogenicity Following Administration of BNT162b2 and Ad26.COV2.S COVID-19 Vaccines in the Pregnant Population during the Third Trimester. Viruses 2022, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.; Rosenbloom, J.I.; Gutman-Ido, E.; Lessans, N.; Cahen-Peretz, A.; Chill, H.H. Safety of SARS-CoV-2 vaccination during pregnancy- obstetric outcomes from a large cohort study. BMC Pregnancy Childbirth 2022, 22, 166. [Google Scholar] [CrossRef]
- Goldshtein, I.; Steinberg, D.M.; Kuint, J.; Chodick, G.; Segal, Y.; Shapiro Ben David, S.; Ben-Tov, A. Association of BNT162b2 COVID-19 Vaccination During Pregnancy with Neonatal and Early Infant Outcomes. JAMA Pediatr. 2022, 176, 470–477. [Google Scholar] [CrossRef]
- Rottenstreich, M.; Sela, H.Y.; Rotem, R.; Kadish, E.; Wiener-Well, Y.; Grisaru-Granovsky, S. COVID-19 vaccination during the third trimester of pregnancy: Rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG 2022, 129, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Theiler, R.N.; Wick, M.; Mehta, R.; Weaver, A.L.; Virk, A.; Swift, M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am. J. Obstet. Gynecol. MFM 2021, 3, 100467. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; Vale, M.S.D.; Cardona-Perez, J.A.; et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: The INTERCOVID multinational cohort study. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Skirrow, H.; Barnett, S.; Bell, S.; Riaposova, L.; Mounier-Jack, S.; Kampmann, B.; Holder, B. Women’s views on accepting COVID-19 vaccination during and after pregnancy, and for their babies: A multi-methods study in the UK. BMC Pregnancy Childbirth 2022, 22, 33. [Google Scholar] [CrossRef]
- Munoz, F.M. Can We Protect Pregnant Women and Young Infants from COVID-19 through Maternal Immunization? JAMA Pediatr. 2021, 175, 561–562. [Google Scholar] [CrossRef]
- Flannery, D.D.; Gouma, S.; Dhudasia, M.B.; Mukhopadhyay, S.; Pfeifer, M.R.; Woodford, E.C.; Triebwasser, J.E.; Gerber, J.S.; Morris, J.S.; Weirick, M.E.; et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr. 2021, 175, 594–600. [Google Scholar] [CrossRef]
- Atyeo, C.; Pullen, K.M.; Bordt, E.A.; Fischinger, S.; Burke, J.; Michell, A.; Slein, M.D.; Loos, C.; Shook, L.L.; Boatin, A.A.; et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell 2021, 184, 628–642. [Google Scholar] [CrossRef] [PubMed]
- Edlow, A.G.; Li, J.Z.; Collier, A.-R.Y.; Atyeo, C.; James, K.E.; Boatin, A.A.; Gray, K.J.; Bordt, E.A.; Shook, L.L.; Yonker, L.M.; et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw. Open 2020, 3, e2030455. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. COVID-19 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 225, 303.e1–303.e17. [Google Scholar]
- Citu, C.; Neamtu, R.; Sorop, V.B.; Horhat, D.I.; Gorun, F.; Tudorache, E.; Gorun, O.M.; Boarta, A.; Tuta-Sas, I.; Citu, I.M. Assessing SARS-CoV-2 Vertical Transmission and Neonatal Complications. J. Clin. Med. 2021, 10, 5253. [Google Scholar] [CrossRef]
- Karimi, H.; Mansouri, V.; Rezaei, N. Vertical transmission and maternal passive immunity post-SARS-CoV-2. Future Virol. 2023, 18, 895–912. [Google Scholar] [CrossRef] [PubMed]
- Magnus, M.C.; Örtqvist, A.K.; Dahlqwist, E.; Ljung, R.; Skår, F.; Oakley, L.; Macsali, F.; Pasternak, B.; Gjessing, H.K.; Håberg, S.E.; et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA 2022, 327, 1469. [Google Scholar] [CrossRef]

| Study & Author | Country | Publication Year | Study Design | Study Quality |
|---|---|---|---|---|
| 1 [21] Beharier et al. | Israel | 2021 | Prospective cohort | Medium |
| 2 [22] Citu et al. | Romania | 2022 | Prospective cohort | Low |
| 3 [23] Dick et al. | USA | 2022 | Retrospective cohort | Medium |
| 4 [24] Goldshtein et al. | Israel | 2022 | Prospective cohort | High |
| 5 [25] Rottenstreich et al. | Israel | 2022 | Retrospective cohort | Medium |
| 6 [26] Theiler et al. | USA | 2021 | Retrospective cohort | Medium |
| Study Number | Number of Patients | Age (Mean/Median) | Comorbidities | Comparison Group | Patient Characteristics |
|---|---|---|---|---|---|
| 1 [21] Beharier et al. | 1094 (94 unvaccinated with previous COVID-19, and 895 unvaccinated without prior SARS-CoV-2 infection) | 28.8 years (COVID-19 group) vs. 31.7 years (no COVID-19 group) | DM 5.4% (COVID-19 group) vs. 8.7% (no COVID-19 group) | 213 mother-child pairs (65 PCR positive, 86 vaccinated, 62 unvaccinated noninfected) | Societal groups: ~75% Jewish, ~20% Arab, ~5% other |
| 2 [22] Citu et al. | 835 (Vaccinated: 227, Unvaccinated: 608) | Vaccinated: 29.8 years, Unvaccinated: 31.2 years | Obesity (Vaccinated: 19.6%, Unvaccinated: 22.4%) | 173 seronegative vs. 54 seropositive | Rural origin: 28.9% vaccinated vs. 62.1% vaccinated, Multiparous vs. primiparous: 41.6% vaccinated vs. 59.4% vaccinated), History of abortion (Vaccinated: 17.9%) |
| 3 [23] Dick et al. | 5618 (2305 vaccinated vs. 3313 unvaccinated) | 30 years (matched in both groups) | Hypertensive Disorder of Pregnancy: 25 (1.1%); Gestational DM: 222 (9.6%) | Unvaccinated group, Pregnancy trimester groups | Primiparous 1 (0.3%); Nulliparous: 611 (26.5%); Smoking: 79 (3.4%) |
| 4 [24] Goldshtein et al. | 24,288 (Pre-IPTW Post-IPTWa unvaccinated—7591; Post-IPTW unvaccinated—7452; vaccinated 738) | Average age: 31.61 years | Obesity: 1768 (10.6%); Infertility: 304 (1.8%); Cancer: 168 (1.0%); Hypertension: 159 (1.0%); CKD: 118 (0.7%); Diabetes: 145 (0.9%); Cardiovascular Disease: 8 (<0.1%) | Unvaccinated group, IPTW groups | Nulliparous: 32.7% Jewish (Secular 63.9%, Arab 10.9%); Socioeconomic higher in vaccinated patients; Smoking: 798 (4.8%) |
| 5 [25] Rottenstreich et al. | 1775 (1063 unvaccinated vs. 712 vaccinated) | Vaccinated: 30.6 years; Unvaccinated: 29.5 years | Hypertensive Disorders: Vaccinated 1.4%, Unvaccinated 1.8%; Diabetes: Vaccinated 6.3%, Unvaccinated 4.2%; Obesity (BMI ≥ 30 kg/m2): Vaccinated 14.2%, Unvaccinated 13.2% | Unvaccinated group | Previous miscarriages: vaccinated 33.7%, unvaccinated 27.8%; previous caesarean delivery: vaccinated 16.4%, unvaccinated 12.9%; fertility treatments: vaccinated 4.6%, unvaccinated 2.3% |
| 6 [26] Theiler et al. | 2002 (1862 vaccinated vs. 140 unvaccinated) | Vaccinated: 31.8 years; Unvaccinated: 30.1 years | Pregestational diabetes mellitus: 1.4%; Pregestational hypertension: 4.3%; Asthma: 10.7% | Unvaccinated group | Education > 16 years: 46.6% in vaccinated patients; Smoking: 0% in vaccinated; Infertility treatment: 4.3% in vaccinated; Gravidity: 1 (40%) in vaccinated; Pre-pregnancy BMI: <25 in 56.5% of vaccinated patients |
| Study Number | Vaccine Type * | Number of Doses | Time of Vaccination | Immune Response | Newborn Features |
|---|---|---|---|---|---|
| 1 [21] Beharier et al. | BNT162b2 | 2 doses | Median 34.5 weeks GA | Strong maternal IgG response, crossing placenta; lower IgG transfer ratio for third-trimester infections; no significant differences in maternal-neonatal serological correlations between SARS-CoV-2 infected and vaccinated groups | NR |
| 2 [22] Citu et al. | BNT162b2 and Ad26.COV2.S | 1–2 doses | 3rd trimester (>27 weeks) | Spike antibodies before vaccination 0.41 U/mL (seronegative) vs. 145 U/mL (seropositive), at 4 months 1083 U/mL (seronegative) vs. 10,759 U/mL (seropositive) | GW 3149 g (vaccinated) vs. 3207 g (unvaccinated)APGAR <7 at 5 min 6.3% (vaccinated) vs. 6.6% (unvaccinated) |
| 3 [23] Dick et al. | BNT162b2 and mRNA-1273 | NR | 2nd and 3rd trimester | NR | Birthweight: 3280 (2980, 3590) g; APGAR 5 min < 7: 42 (1.8%); Umbilical arterial pH < 7.1: 89 (7.4%) |
| 4 [24] Goldshtein et al. | BNT162b2 | 1–2 doses | 1st, 2nd, and 3rd trimester | NR | 49% Female; 96% Born at ≥37 Weeks’ Gestation; Follow-up: Median 126 Days (76–179) in Exposed, 152 Days (88–209) in Unexposed |
| 5 [25] Rottenstreich et al. | BNT162b2 | 2 doses | 3rd trimester (>27 weeks) | NR | Birthweight: vaccinated 3317.8 g, unvaccinated 3339.5 g; male gender: vaccinated 49.6%, unvaccinated 50.7%; APGAR score ≤ 7 at 1 min: vaccinated 4.2%, unvaccinated 4.6%; APGAR score ≤ 7 at 5 min: vaccinated 2.9%, unvaccinated 2.5%; |
| 6 [26] Theiler et al. | BNT162b2, Ad26.COV2.S, and mRNA-1273 | >1 dose | Median 32 weeks GA | NR | Gestational Age at Delivery: ≥37 0/7 weeks: 90.7%; Low Birthweight (<2500 g): 7.9%; Very Low Birthweight (<1500 g): 2.1%; |
| Study Number | Pregnancy Complications | Neonatal Complications | Preterm Birth Risk (OR/RR/HR) | Other Risks (OR/RR/HR) |
|---|---|---|---|---|
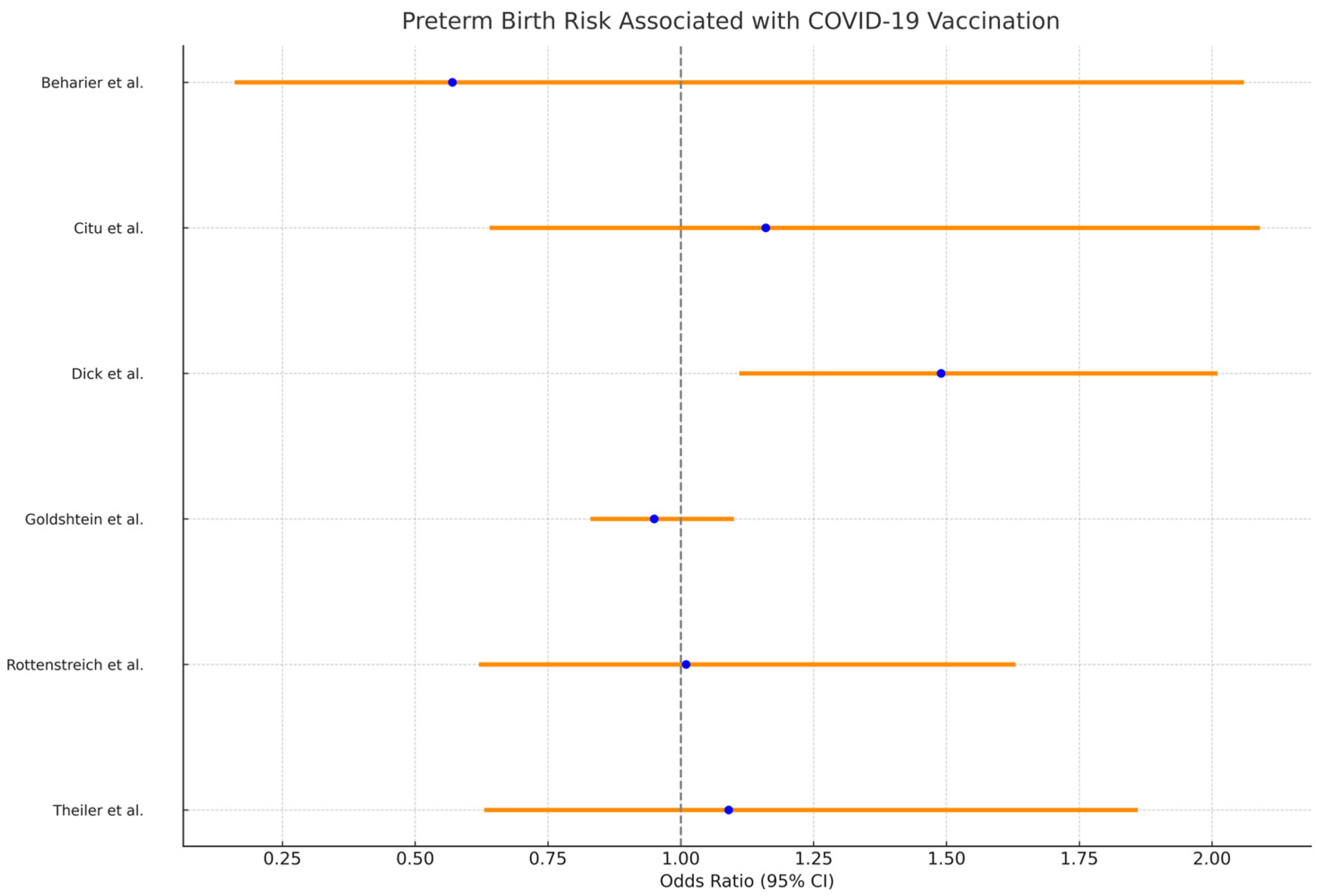
| 1 [21] Beharier et al. | Preterm birth—0.6%Other adverse outcomes—0.2% | No illness post-childbirth | 0.57 (0.16–2.06) | Adverse pregnancy outcomes—2.87 (0.33–25.09) |
| 2 [22] Citu et al. | Preterm birth—2.9% | SGA—0.9% | 1.16 (0.64–2.09) | Adverse neonatal outcomes—0.96 (0.50–1.85); SGA—0.71 (0.30–1.69) |
| 3 [23] Dick et al. | Preterm birth: 127 (5.5%); | SGA: 142 (6.2%); Cesarean Delivery: 358 (15.5%); Postpartum Hemorrhage: 79 (3.4%); Intrauterine fetal demise: 20 (0.87%); Hypertensive disorder of pregnancy: 25 (1.1%); Gestational diabetes: 222 (9.6%) | Preterm birth in 2nd Trimester vaccinated: OR 1.49 (1.11, 2.01); Preterm birth in 3rd Trimester vaccinated: OR 0.49 (0.34, 0.71); | SGA in 2nd trimester vaccinated: OR 0.73 (0.52, 1.03); SGA in 3rd trimester vaccinated: OR 0.85 (0.64, 1.13) |
| 4 [24] Goldshtein et al. | Preterm birth: 6.1% Pre-IPTW unvaccinated vs. 7.9% vaccinated; 6.6% Post-IPTW unvaccinated vs. 6.2% vaccinated | Low birth weight: 5.8% Pre-IPTW unvaccinated vs. 7.6% vaccinated; SGA: 6.8% Pre-IPTW unvaccinated vs. 7.5% vaccinated; | 0.95 (0.83–1.10) | SGA: 0.97 (0.87–1.08); Congenital anomalies: 0.69 (0.44–1.04); All-cause neonatal hospitalizations: 0.99 (0.88–1.12); Post-neonatal hospitalizations: 0.95 (0.84–1.07); Infant mortality: 0.84 (0.43–1.72); Major heart malformations: 0.46 (0.24–0.82) |
| 5 [25] Rottenstreich et al. | Preterm birth: 4.4%; elective caesarean delivery: vaccinated 11.5%, unvaccinated 7.6%; vacuum-assisted delivery: vaccinated 3.2%, unvaccinated 6.2%; postpartum hemorrhage: vaccinated 7.3%, unvaccinated 10%; | Intrauterine fetal death: vaccinated 0.7%, unvaccinated 0.5%; NICU admission: vaccinated 4.1%, unvaccinated 4.5% | 1.01 (0.62–1.63) | COVID-19 vaccination not associated with maternal composite adverse outcome: 0.8 (0.61–1.03); composite adverse neonatal outcome: vaccinated 7.9%, unvaccinated 11.4%; significantly reduced risk with vaccination: 0.5 (0.36–0.74) |
| 6 [26] Theiler et al. | Preterm birth: 3.5%;Adverse outcomes: vaccinated 5.0%, unvaccinated 4.9%; eclampsia or preeclampsia: 0.7% in vaccinated; gestational hypertension: 13.6% in vaccinated | C-section 9.9%; Postpartum hemorrhage 0.1%; Stillbirth 2.9%; No significant differences in neonatal outcomes | 1.09 (0.63–1.86) | C-section—1.05 (0.82–1.34); Postpartum hemorrhage—2.66 (0.31–22.61); Stillbirth—1.02 (0.06–18.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uta, M.; Craina, M.; Marc, F.; Enatescu, I. Assessing the Impact of COVID-19 Vaccination on Preterm Birth: A Systematic Review with Meta-Analysis. Vaccines 2024, 12, 102. https://doi.org/10.3390/vaccines12010102
Uta M, Craina M, Marc F, Enatescu I. Assessing the Impact of COVID-19 Vaccination on Preterm Birth: A Systematic Review with Meta-Analysis. Vaccines. 2024; 12(1):102. https://doi.org/10.3390/vaccines12010102
Chicago/Turabian StyleUta, Mihaela, Marius Craina, Felicia Marc, and Ileana Enatescu. 2024. "Assessing the Impact of COVID-19 Vaccination on Preterm Birth: A Systematic Review with Meta-Analysis" Vaccines 12, no. 1: 102. https://doi.org/10.3390/vaccines12010102
APA StyleUta, M., Craina, M., Marc, F., & Enatescu, I. (2024). Assessing the Impact of COVID-19 Vaccination on Preterm Birth: A Systematic Review with Meta-Analysis. Vaccines, 12(1), 102. https://doi.org/10.3390/vaccines12010102





