ProLonged Liposomal Delivery of TLR7/8 Agonist for Enhanced Cancer Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of ProLNG-001 (Liposomes Containing ProLNG-S) and Blank Liposome
2.2. Physicochemical Properties of ProLNG-001
2.3. Activation of Bone Marrow-Derived Dendritic Cells (BMDCs)
2.4. Cytotoxic T-Lymphocyte Response in Human Peripheral Blood Mononuclear Cells (hPBMCs)
2.5. Measurement of IgG Titer in Mouse Plasma
2.6. Analysis of Delayed Cytokine Response in Mouse Plasma
2.7. Hematoxylin and Eosin Staining
2.8. Assessment of the Anti-Cancer Therapeutic Effect of ProLNG-001
2.9. Statistical Analysis
3. Results
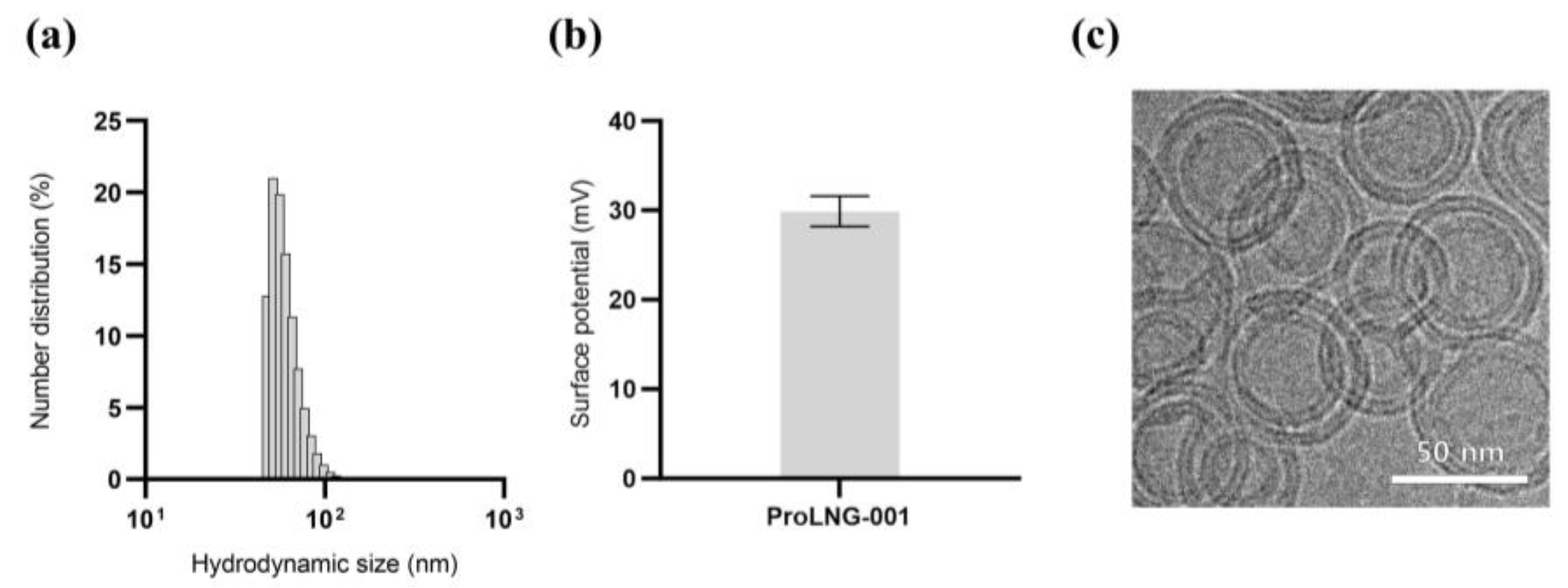
3.1. Synthesis and Characterization of ProLNG-001
3.2. Cytokine Secretion and Activation Marker Expression of Dendritic Cells Induced by ProLNG-001 in Mouse BMDCs
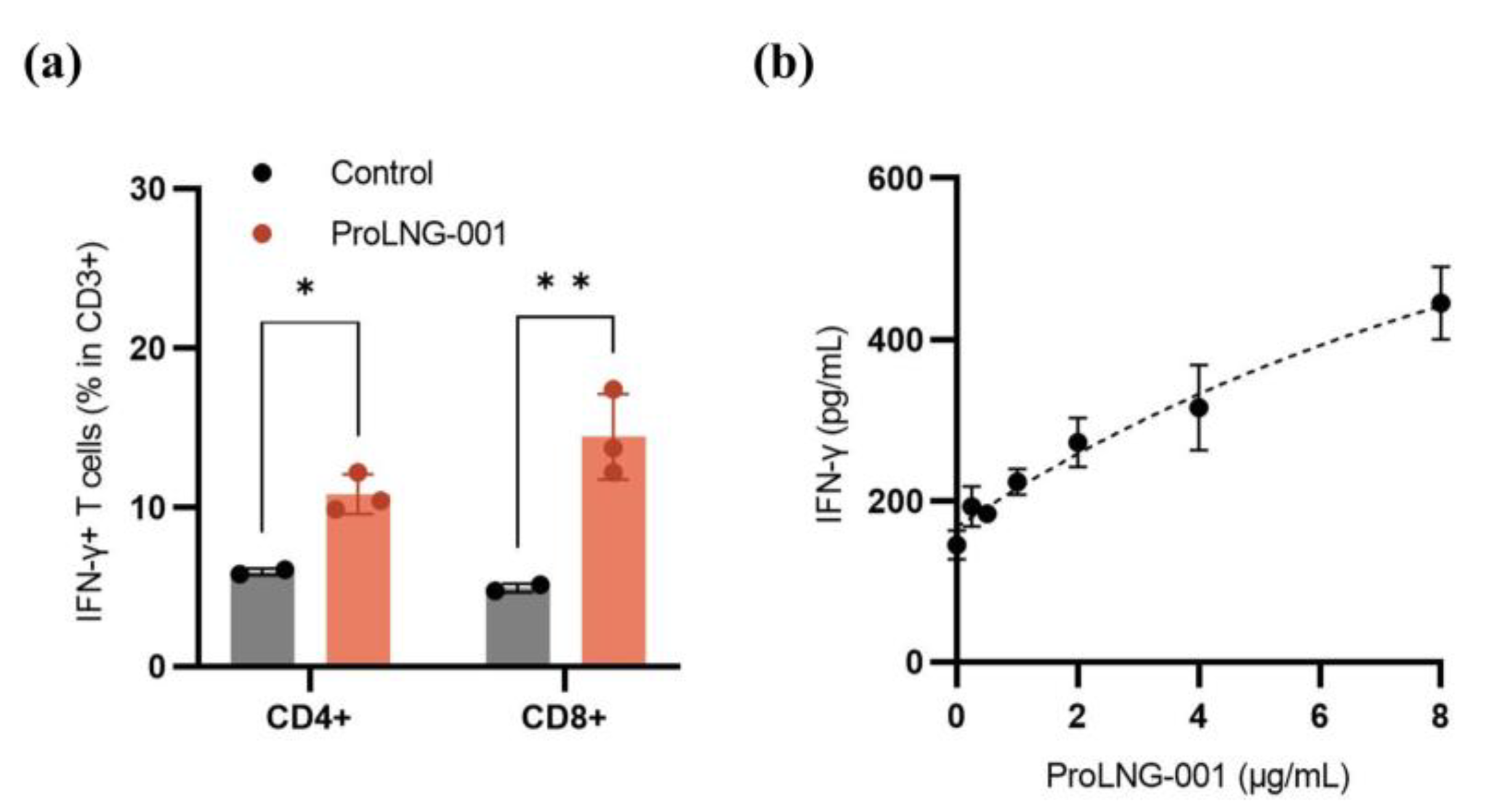
3.3. IFN-γ Secreting T Cells in Human Peripheral Blood Mononuclear Cells (hPBMCs)
3.4. Delayed Cytokine Release in Mouse Plasma Induced by ProLNG-001
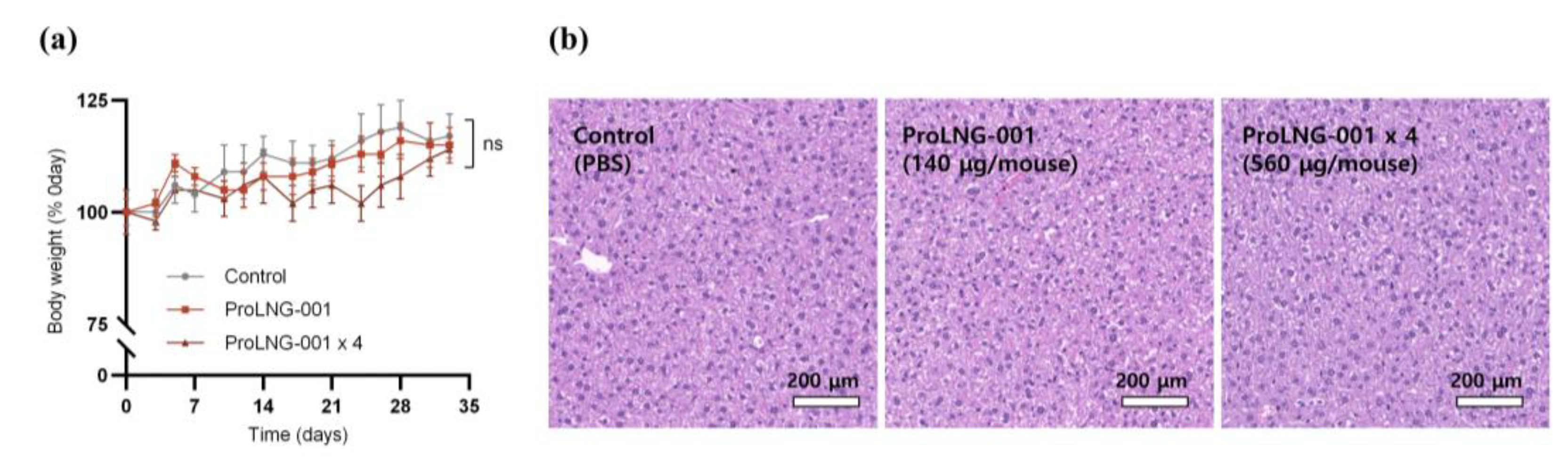
3.5. Anti-Tumor Effects and Recruitment of Activated Immune Cells by ProLNG-001
3.6. Enhanced Anti-Tumor Effects of ProLNG-001 Compared to Commercial Adjuvants
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Want, M.Y.; Bashir, Z.; Najar, R.A. T Cell Based Immunotherapy for Cancer: Approaches and Strategies. Vaccines 2023, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer Immunotherapy Comes of Age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Reynolds, C.R.; Tran, S.; Jain, M.; Narendran, A. Neoantigen Cancer Vaccines: Generation, Optimization, and Therapeutic Targeting Strategies. Vaccines 2022, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Padinharayil, H.; Alappat, R.R.; Joy, L.M.; Anilkumar, K.V.; Wilson, C.M.; George, A.; Valsala Gopalakrishnan, A.; Madhyastha, H.; Ramesh, T.; Sathiyamoorthi, E.; et al. Advances in the Lung Cancer Immunotherapy Approaches. Vaccines 2022, 10, 1963. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Toll-like Receptor Control of the Adaptive Immune Responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef]
- Heil, F.; Ahmad-Nejad, P.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Gellert, T.; Dietrich, H.; Lipford, G.; Takeda, K.; Akira, S.; et al. The Toll-like Receptor 7 (TLR7)-Specific Stimulus Loxoribine Uncovers a Strong Relationship within the TLR7, 8 and 9 Subfamily. Eur. J. Immunol. 2003, 33, 2987–2997. [Google Scholar] [CrossRef]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like Receptors. Annu. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like Receptor Signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Bernasconi, N.L.; Onai, N.; Lanzavecchia, A. A Role for Toll-like Receptors in Acquired Immunity: Up-Regulation of TLR9 by BCR Triggering in Naive B Cells and Constitutive Expression in Memory B Cells. Blood 2003, 101, 4500–4504. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like Receptors: Critical Proteins Linking Innate and Acquired Immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Khong, H.; Dai, Z.; Huang, X.-F.; Wargo, J.A.; Cooper, Z.A.; Vasilakos, J.P.; Hwu, P.; Overwijk, W.W. Effective Innate and Adaptive Antimelanoma Immunity through Localized TLR7/8 Activation. J. Immunol. 2014, 193, 4722–4731. [Google Scholar] [CrossRef] [PubMed]
- Crofts, K.F.; Page, C.L.; Swedik, S.M.; Holbrook, B.C.; Meyers, A.K.; Zhu, X.; Parsonage, D.; Westcott, M.M.; Alexander-Miller, M.A. An Analysis of Linker-Dependent Effects on the APC Activation and In Vivo Immunogenicity of an R848-Conjugated Influenza Vaccine. Vaccines 2023, 11, 1261. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T Cells in Cancer and Cancer Immunotherapy. Br. J. Cancer 2020, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Garcia, K.C.; Scott, C.A.; Brunmark, A.; Carbone, F.R.; Peterson, P.A.; Wilson, I.A.; Teyton, L. CD8 Enhances Formation of Stable T-Cell Receptor/MHC Class I Molecule Complexes. Nature 1996, 384, 577–581. [Google Scholar] [CrossRef]
- Basu, R.; Whitlock, B.M.; Husson, J.; Le Floc’h, A.; Jin, W.; Oyler-Yaniv, A.; Dotiwala, F.; Giannone, G.; Hivroz, C.; Biais, N.; et al. Cytotoxic T Cells Use Mechanical Force to Potentiate Target Cell Killing. Cell 2016, 165, 100–110. [Google Scholar] [CrossRef]
- Durgeau, A.; Virk, Y.; Corgnac, S.; Mami-Chouaib, F. Recent Advances in Targeting CD8 T-Cell Immunity for More Effective Cancer Immunotherapy. Front. Immunol. 2018, 9, 1. [Google Scholar] [CrossRef]
- Davis, S.J.; Ikemizu, S.; Evans, E.J.; Fugger, L.; Bakker, T.R.; van der Merwe, P.A. The Nature of Molecular Recognition by T Cells. Nat. Immunol. 2003, 4, 217–224. [Google Scholar] [CrossRef]
- Smith, A.A.A.; Gale, E.C.; Roth, G.A.; Maikawa, C.L.; Correa, S.; Yu, A.C.; Appel, E.A. Nanoparticles Presenting Potent TLR7/8 Agonists Enhance Anti-PD-L1 Immunotherapy in Cancer Treatment. Biomacromolecules 2020, 21, 3704–3712. [Google Scholar] [CrossRef]
- VAC. Available online: https://vac.niaid.nih.gov/view?id=34 (accessed on 7 July 2023).
- Jin, S.M.; Yoo, Y.J.; Shin, H.S.; Kim, S.; Lee, S.N.; Lee, C.H.; Kim, H.; Kim, J.-E.; Bae, Y.-S.; Hong, J.; et al. A Nanoadjuvant That Dynamically Coordinates Innate Immune Stimuli Activation Enhances Cancer Immunotherapy and Reduces Immune Cell Exhaustion. Nat. Nanotechnol. 2023, 18, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Helper Activity of NK Cells during the Dendritic Cell-Mediated Induction of Melanoma-Specific Cytotoxic T Cells-PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3057371/ (accessed on 21 July 2023).
- Weigelin, B.; den Boer, A.T.; Wagena, E.; Broen, K.; Dolstra, H.; de Boer, R.J.; Figdor, C.G.; Textor, J.; Friedl, P. Cytotoxic T Cells Are Able to Efficiently Eliminate Cancer Cells by Additive Cytotoxicity. Nat. Commun. 2021, 12, 5217. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, K.S.; Kaschek, L.; Knörck, A.; Cappello, S.; Lünsmann, N.; Küchler, N.; Hoxha, C.; Schäfer, G.; Iden, S.; Bogeski, I.; et al. Interdependence of Sequential Cytotoxic T Lymphocyte and Natural Killer Cell Cytotoxicity against Melanoma Cells. J. Physiol. 2022, 600, 5027–5054. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Ugolini, S.; Blaise, D.; Chabannon, C.; Brossay, L. Targeting Natural Killer Cells and Natural Killer T Cells in Cancer. Nat. Rev. Immunol. 2012, 12, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Lonsdorf, A.S.; Hwang, S.T. Immunotherapy for Advanced Melanoma. J. Investig. Dermatol. 2008, 128, 2596–2605. [Google Scholar] [CrossRef][Green Version]
- Madaan, A.; Verma, R.; Singh, A.T.; Jain, S.K.; Jaggi, M. A Stepwise Procedure for Isolation of Murine Bone Marrow and Generation of Dendritic Cells. J. Biol. Methods 2014, 1, e1. [Google Scholar] [CrossRef]
- Pesce, B.; Ribeiro, C.H.; Larrondo, M.; Ramos, V.; Soto, L.; Catalán, D.; Aguillón, J.C. TNF-α Affects Signature Cytokines of Th1 and Th17 T Cell Subsets through Differential Actions on TNFR1 and TNFR2. Int. J. Mol. Sci. 2022, 23, 9306. [Google Scholar] [CrossRef]
- Hernandez-Pando, R.; Rook, G.A.W. The Role of TNF-alpha in T-Cell-Mediated Inflammation Depends on the Thl/Th2 Cytokine Balance. Immunology 1994, 82, 591–595. [Google Scholar]
- Everds, N.E. Evaluation of Clinical Pathology Data:Correlating Changes with Other Study Data. Toxicol. Pathol. 2015, 43, 90–97. [Google Scholar] [CrossRef]
- Boehm, O.; Zur, B.; Koch, A.; Tran, N.; Freyenhagen, R.; Hartmann, M.; Zacharowski, K. Clinical Chemistry Reference Database for Wistar Rats and C57/BL6 Mice. Biol. Chem. 2007, 388, 547–554. [Google Scholar] [CrossRef]
- Ye, H.; He, X.; Feng, X. Developing Neobavaisoflavone Nanoemulsion Suppresses Lung Cancer Progression by Regulating Tumor Microenvironment. Biomed. Pharmacother. 2020, 129, 110369. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.F.; Hussain, S.Z.; Saeed, H.; Javed, I.; Sarwar, H.S.; Nadhman, A.; Huma, Z.E.; Rehman, M.; Jahan, S.; Hussain, I.; et al. Polymeric Nanocapsules Embedded with Ultra-Small Silver Nanoclusters for Synergistic Pharmacology and Improved Oral Delivery of Docetaxel. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Pereira, C.; Silva, V.; Costa, V.M.; Silva, R.; Garcia, J.; Gonçalves-Monteiro, S.; Duarte-Araújo, M.; Santos-Silva, A.; Coimbra, S.; Dinis-Oliveira, R.J.; et al. Histological and Toxicological Evaluation, in Rat, of a P-Glycoprotein Inducer and Activator: 1-(Propan-2-Ylamino)-4-Propoxy-9H-Thioxanthen-9-One (TX5). EXCLI J. 2019, 18, 697. [Google Scholar] [CrossRef] [PubMed]
- Firacative, C.; Gressler, A.E.; Schubert, K.; Schulze, B.; Müller, U.; Brombacher, F.; von Bergen, M.; Alber, G. Identification of T Helper (Th)1- and Th2-Associated Antigens of Cryptococcus Neoformans in a Murine Model of Pulmonary Infection. Sci. Rep. 2018, 8, 2681. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Garcia, A.; Meyer, C.; Tuschl, T.; Merghoub, T.; Wolchok, J.D.; Deng, L. Heat-Inactivated Modified Vaccinia Virus Ankara Boosts Th1 Cellular and Humoral Immunity as a Vaccine Adjuvant. NPJ Vaccines 2022, 7, 120. [Google Scholar] [CrossRef]
- Lee, M.S.J.; Natsume-Kitatani, Y.; Temizoz, B.; Fujita, Y.; Konishi, A.; Matsuda, K.; Igari, Y.; Tsukui, T.; Kobiyama, K.; Kuroda, E.; et al. B Cell-intrinsic MyD88 Signaling Controls IFN-γ-mediated Early IgG2c Class Switching in Mice in Response to a Particulate Adjuvant. Eur. J. Immunol. 2019, 49, 1433–1440. [Google Scholar] [CrossRef]
- Nazeri, S.; Zakeri, S.; Mehrizi, A.A.; Sardari, S.; Djadid, N.D. Measuring of IgG2c Isotype Instead of IgG2a in Immunized C57BL/6 Mice with Plasmodium Vivax TRAP as a Subunit Vaccine Candidate in Order to Correct Interpretation of Th1 versus Th2 Immune Response. Exp. Parasitol. 2020, 216, 107944. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in Tumor Progression and Regression: A Review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Gocher, A.M.; Workman, C.J.; Vignali, D.A.A. Interferon-γ: Teammate or Opponent in the Tumour Microenvironment? Nat. Rev. Immunol. 2022, 22, 158–172. [Google Scholar] [CrossRef]
- Van Horssen, R.; ten Hagen, T.L.M.; Eggermont, A.M.M. TNF-α in Cancer Treatment: Molecular Insights, Antitumor Effects, and Clinical Utility. Oncologist 2006, 11, 397–408. [Google Scholar] [CrossRef]
- Wu, S.Y.; Fu, T.; Jiang, Y.Z.; Shao, Z.M. Natural Killer Cells in Cancer Biology and Therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Cibrián, D.; Sánchez-Madrid, F. CD69: From Activation Marker to Metabolic Gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, Y.; Zhang, P.; Xing, H.; Zhao, S.; Song, Y.; Wan, D.; Yu, J. Targeting Toll-like Receptor 7/8 for Immunotherapy: Recent Advances and Prospectives. Biomark. Res. 2022, 10, 89. [Google Scholar] [CrossRef]
- Krummen, M.; Balkow, S.; Shen, L.; Heinz, S.; Loquai, C.; Probst, H.-C.; Grabbe, S. Release of IL-12 by Dendritic Cells Activated by TLR Ligation Is Dependent on MyD88 Signaling, Whereas TRIF Signaling Is Indispensable for TLR Synergy. J. Leukoc. Biol. 2010, 88, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Rubtsova, K.; Rubtsov, A.V.; Halemano, K.; Li, S.X.; Kappler, J.W.; Santiago, M.L.; Marrack, P. T Cell Production of IFNγ in Response to TLR7/IL-12 Stimulates Optimal B Cell Responses to Viruses. PLoS ONE 2016, 11, e0166322. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Cao, Z.; Li, J.; Long, H.; Wu, Y.; Zhang, Z.; Sun, Y. R848 Is Involved in the Antibacterial Immune Response of Golden Pompano (Trachinotus Ovatus) Through TLR7/8-MyD88-NF-κB-Signaling Pathway. Front. Immunol. 2021, 11, 617522. [Google Scholar] [CrossRef]
- Taniguchi, T.; Takaoka, A. A Weak Signal for Strong Responses: Interferon-Alpha/Beta Revisited. Nat. Rev. Mol. Cell Biol. 2001, 2, 378–386. [Google Scholar] [CrossRef]
- Sirén, J.; Pirhonen, J.; Julkunen, I.; Matikainen, S. IFN-α Regulates TLR-Dependent Gene Expression of IFN-α, IFN-β, IL-28, and IL-29. J. Immunol. 2005, 174, 1932–1937. [Google Scholar] [CrossRef]
- Bender, A.T.; Tzvetkov, E.; Pereira, A.; Wu, Y.; Kasar, S.; Przetak, M.M.; Vlach, J.; Niewold, T.B.; Jensen, M.A.; Okitsu, S.L. TLR7 and TLR8 Differentially Activate the IRF and NF-κB Pathways in Specific Cell Types to Promote Inflammation. ImmunoHorizons 2020, 4, 93–107. [Google Scholar] [CrossRef]
- Schudel, A.; Francis, D.M.; Thomas, S.N. Material Design for Lymph Node Drug Delivery. Nat. Rev. Mater. 2019, 4, 415–428. [Google Scholar] [CrossRef]
- Saadat, M.; Zahednezhad, F.; Zakeri-Milani, P.; Heidari, H.R.; Shahbazi-Mojarrad, J.; Valizadeh, H. Drug Targeting Strategies Based on Charge Dependent Uptake of Nanoparticles into Cancer Cells. J. Pharm. Pharm. Sci. 2019, 22, 131–364. [Google Scholar] [CrossRef] [PubMed]
- Nersesian, S.; Schwartz, S.L.; Grantham, S.R.; MacLean, L.K.; Lee, S.N.; Pugh-Toole, M.; Boudreau, J.E. NK Cell Infiltration Is Associated with Improved Overall Survival in Solid Cancers: A Systematic Review and Meta-Analysis. Transl. Oncol. 2021, 14, 100930. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Jiménez-Cortegana, C.; Tay, A.H.M.; Wickström, S.; Galluzzi, L.; Lundqvist, A. NK Cells and Solid Tumors: Therapeutic Potential and Persisting Obstacles. Mol. Cancer 2022, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Cózar, B.; Greppi, M.; Carpentier, S.; Narni-Mancinelli, E.; Chiossone, L.; Vivier, E. Tumor-Infiltrating Natural Killer Cells. Cancer Discov. 2021, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.; Curti, B.; Bilen, M.; Brohl, A.; Domingo-Musibay, E.; Borazanci, E.; Fanton, C.; Haglund, C.; Vimal, M.; Muhsin, M.; et al. 368 REVEAL: Phase 1 Dose-escalation Study of NKTR-262, A Novel TLR7/8 agonist, Plus Bempegaldesleukin: Local Innate Immune Activation and Systemic Adaptive Immune Expansion for Treating Solid Tumors. J. Immunother. Cancer 2020, 8 (Suppl. 3), 368. [Google Scholar] [CrossRef]



| No. | Sample Information |
|---|---|
| Group 1 | TBS |
| Group 2 | OVA 10 μg |
| Group 3 | OVA 10 μg + ProLNG-001 140 μg |
| No. | Sample Information |
|---|---|
| Group 1 | TBS |
| Group 2 | OVA 10 μg |
| Group 3 | OVA 10 μg + ProLNG-001 140 μg |
| Group 4 | OVA 10 μg + AS01 5 μg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Park, Y.; Kim, J.; Kim, S.; Choi, K.; Kang, T.; Lee, I.; Lim, Y.T.; Um, S.H.; Kim, C. ProLonged Liposomal Delivery of TLR7/8 Agonist for Enhanced Cancer Vaccine. Vaccines 2023, 11, 1503. https://doi.org/10.3390/vaccines11091503
Kim S, Park Y, Kim J, Kim S, Choi K, Kang T, Lee I, Lim YT, Um SH, Kim C. ProLonged Liposomal Delivery of TLR7/8 Agonist for Enhanced Cancer Vaccine. Vaccines. 2023; 11(9):1503. https://doi.org/10.3390/vaccines11091503
Chicago/Turabian StyleKim, Sehui, Yeji Park, Jeonghun Kim, Sohyun Kim, Kyungmin Choi, Taegyun Kang, Inho Lee, Yong Taik Lim, Soong Ho Um, and Chul Kim. 2023. "ProLonged Liposomal Delivery of TLR7/8 Agonist for Enhanced Cancer Vaccine" Vaccines 11, no. 9: 1503. https://doi.org/10.3390/vaccines11091503
APA StyleKim, S., Park, Y., Kim, J., Kim, S., Choi, K., Kang, T., Lee, I., Lim, Y. T., Um, S. H., & Kim, C. (2023). ProLonged Liposomal Delivery of TLR7/8 Agonist for Enhanced Cancer Vaccine. Vaccines, 11(9), 1503. https://doi.org/10.3390/vaccines11091503






