Influenza Vaccine Effectiveness against Hospitalization, Season 2021/22: A Test-Negative Design Study in Barcelona
Abstract
1. Introduction
2. Methods
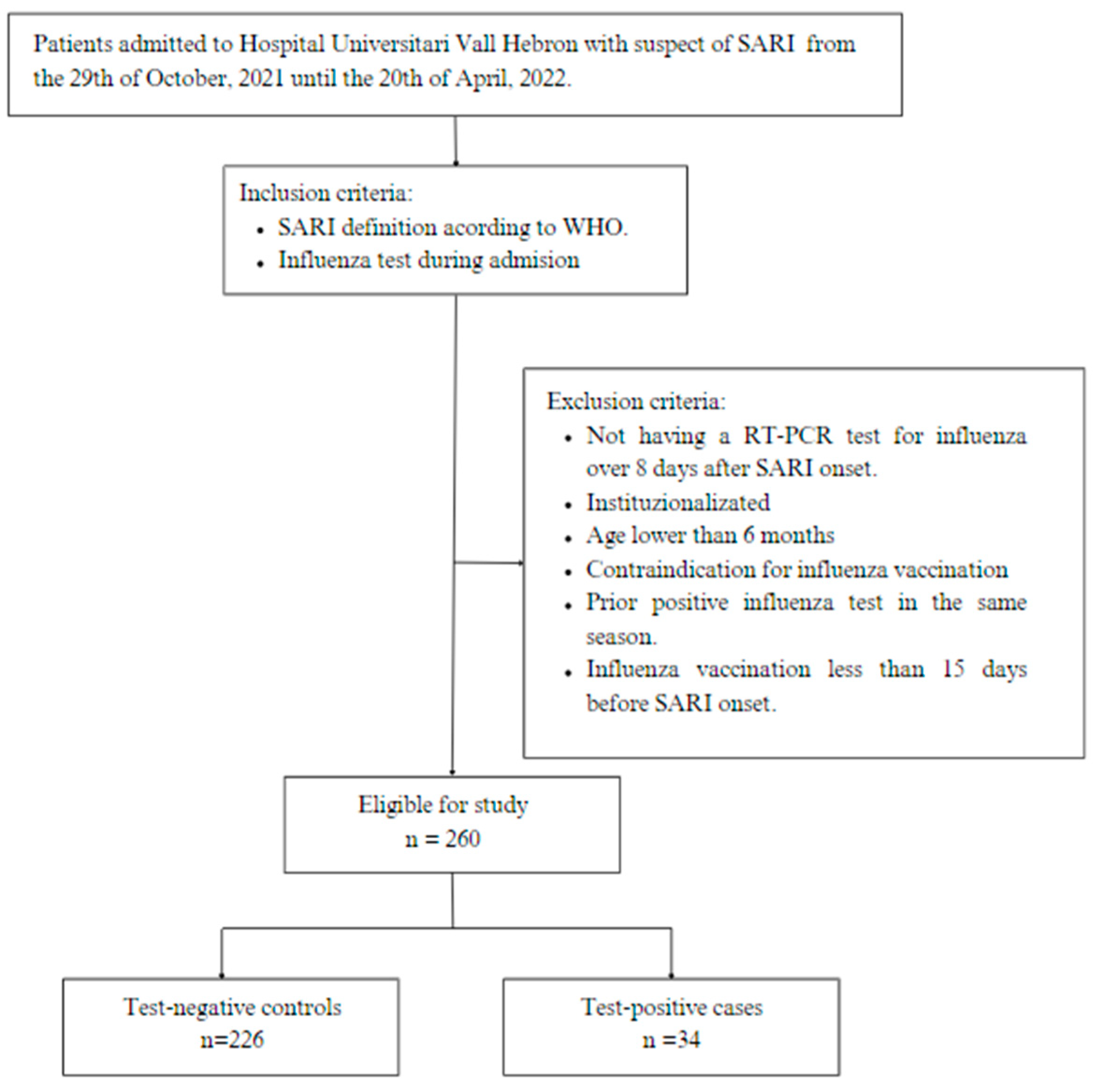
2.1. Study Design and Population
2.2. Sample Size
2.3. Data and Sources of Information
3. Ethical Statement
4. Statistical Analysis
5. Results
Vaccine Effectiveness
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicoll, A.; Ciancio, B.C.; Chavarrias, V.L.; Mølbak, K.; Pebody, R.; Pedzinski, B.; Penttinen, P.; van der Sande, M.; Snacken, R.; Van Kerkhove, M.D. Influenza-related deaths—Available methods for estimating numbers and detecting patterns for seasonal and pandemic influenza in Europe. Eurosurveillance 2012, 17, 20162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rondy, M.; El Omeiri, N.; Thompson, M.G.; Levêque, A.; Moren, A.; Sullivan, S.G. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: A systematic review and meta-analysis of test-negative design case-control studies. J. Infect. 2017, 75, 381–394. [Google Scholar] [CrossRef] [PubMed]
- World Health Organitzation. Recommended Composition of Influenza Virus Vaccines for Use in the 2021–2022 Northern Hemisphere Influenza Season. 2021. Available online: https://cdn.who.int/media/docs/default-source/influenza/202102_recommendation.pdf?sfvrsn=8639f6be_3&download=true (accessed on 23 November 2022).
- World Health Organization Regional Office for Europe and European Centre for Disease Prevention and Control. Influenza Virus Characterization: Summary Report, Europe, September 2022. Copenhagen and Stockholm. 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/influenza-virus-characterization-summary-europe-september-2022 (accessed on 10 October 2022).
- Instituto de Salud Carlos III. Informe Anual SiVIRA de Vigilancia de Gripe, COVID-19 y VRS. Temporada 2021–22. Madrid. 2022. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/GRIPE/INFORMES%20ANUALES/Informe%20SiVIRA%20de%20Vigilancia%20de%20gripe%2c%20COVID-19%20y%20VRS_temporada%202021-22_v14112022.pdf (accessed on 30 November 2022).
- Atlanta: How Flu Vaccine Effectiveness and Efficacy Are Measured. 2021. Available online: https://www.cdc.gov/flu/vaccines-work/effectivenessqa.htm (accessed on 23 November 2022).
- European Centre for Disease Prevention and Control. Systematic Review of the Efficacy, Effectiveness and Safety of Newer and Enhanced Seasonal Influenza Vaccines for the Prevention of Laboratory-Confirmed Influenza in Individuals Aged 18 Years and Over. October 2020 Stockholm. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/seasonal-influenza-vaccines-systematic-review-efficacy.pdf (accessed on 18 September 2022).
- Valenciano, M.; Ciancio, B.C.; I-MOVE Study Team. I-MOVE: A European network to measure the effectiveness of influenza vaccines. Eurosurveillance 2012, 17, 20281. [Google Scholar] [CrossRef] [PubMed]
- Sistema de Vigilancia de la Gripe y otros Virus Respiratorios en España. Madrid. Vigilancia de Gripe y otros Virus Respiratorios en España. Sistemas y Fuentes de Información. Temporada 2020–2021. Available online: https://vgripe.isciii.es/documentos/20202021/home/Sistemas%20y%20fuentes%20de%20informacion%20del%20SVGE_2020-21_vf.pdf (accessed on 18 September 2022).
- Development of Robust and Innovative Vaccine Effectiveness. D7.1 Core Protocol for Type/Brandspecific Influenza Vaccine Effectiveness Studies (Test-Negative Design Studies). 2018. Available online: https://www.drive-eu.org/wp-content/uploads/2018/05/ANNEX1_DRIVE_D7.1_Core-protocol-for-test-negative-design-studies_0.9.pdf (accessed on 5 May 2023).
- Development of Robust and Innovative Vaccine Effectiveness. D7.1.3 Core Protocol for Type/Brandspecific Influenza Vaccine Effectiveness Studies—Test-Negative Design. 2021. Available online: https://www.drive-eu.org/wp-content/uploads/2021/09/DRIVE_D7.1.3_TND-generic-protocol-2021-22_clean_final.pdf (accessed on 5 May 2023).
- World Health Organization. Global Influenza Programme. WHO Surveillance Case Definitions for ILI and SARI—Updated January 2014. Available online: https://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/case-definitions-for-ili-and-sari (accessed on 30 November 2022).
- World Health Organization. Sample Size Calculator for Evaluation of COVID-19 Vaccine Effectiveness. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/sim.4780071208 (accessed on 2 June 2021).
- Comité Asesor de Vacunas. Asociacion Española de Pediatria. Madrid. Coberturas de la Vacunación Antigripal en la Temporada 2021–22. 2022. Available online: https://vacunasaep.org/profesionales/noticias/gripe-coberturas-vacunacion-temporada-2021-22 (accessed on 18 September 2022).
- World Health Organization. Evaluation of Influenza Vaccine Effectiveness: A Guide to the Design and Interpretation of Observational Studies. Geneva. Cataloguing-In-Publication (CIP) Data. 2017. Available online: https://apps.who.int/iris/handle/10665/255203 (accessed on 4 May 2023).
- Stuurman, A.L.; Levi, M.; Beutels, P.; Bricout, H.; Descamps, A.; Dos Santos, G.; McGovern, I.; Mira-Iglesias, A.; Nauta, J.; Torcel-Pagnon, L.; et al. Investigating confounding in network-based test-negative design influenza vaccine effectiveness studies—Experience from the DRIVE project. Influenza Other Respir. Viruses 2023, 17, e13087. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 2 June 2023).
- Development of Robust and Innovative Vaccine Effectiveness. D7.9 Brand-Specific Influenza Vaccine Effectiveness in Europe Season 2021/22 REPORT. 2022. Available online: https://www.drive-eu.org/wp-content/uploads/2022/07/DRIVE_D7.9-IVE-Results-Report_Season-2021-22_FINAL.pdf (accessed on 4 June 2023).
- European Centre for Disease Prevention and Control. Influenza Virus Characterisation. Summary Europe, March 2022. ECDC: Stockholm, Sweden. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Influenza-characterisation-report-march-2022.pdf (accessed on 6 June 2023).
- Consejo Interritorial. Sintema Nacional de Salud. Recomendaciones de Vacunación Frente la Gripe. Temporada 2021–2022. 2021. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/programasDeVacunacion/gripe/docs/Recomendaciones_vacunacion_gripe2021_2022.pdf (accessed on 2 June 2023).
- Emborg, H.-D.; Vestergaard, L.S.; Botnen, A.B.; Nielsen, J.; Krause, T.G.; Trebbien, R. A late sharp increase in influenza detections and low interim vaccine effectiveness against the circulating A(H3N2) strain, Denmark, 2021/22 influenza season up to 25 March 2022. Eurosurveillance 2022, 27, 2200278. [Google Scholar] [CrossRef] [PubMed]
- Kronbichler, A.; Kresse, D.; Yoon, S.; Lee, K.H.; Effenberger, M.; Shin, J.I. Asymptomatic patients as a source of COVID-19 infections: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 98, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chuang, E.S.; Sabaiduc, S.; Olsha, R.; Kaweski, S.E.; Zelyas, N.; Gubbay, J.B.; Jassem, A.N.; Charest, H.; De Serres, G.; et al. Influenza vaccine effectiveness against A(H3N2) during the delayed 2021/22 epidemic in Canada. Eurosurveillance 2022, 27, 2200720. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.R.; Kim, S.S.; Kondor, R.J.; Smith, C.; Budd, A.P.; Tartof, S.Y.; Florea, A.; Talbot, H.K.; Grijalva, C.G.; Wernli, K.J.; et al. Interim Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness—United States, February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 365–370. Available online: https://www.cdc.gov/mmwr/volumes/71/wr/pdfs/mm7110a1-H.pdf (accessed on 12 June 2023). [CrossRef] [PubMed]
- Martínez-Baz, I.; Casado, I.; Miqueleiz, A.; Navascués, A.; Pozo, F.; Trobajo-Sanmartín, C.; Albéniz, E.; Elía, F.; Burgui, C.; Fernández-Huerta, M.; et al. Effectiveness of influenza vaccination in preventing influenza in primary care, Navarre, Spain, 2021/22. Eurosurveillance 2022, 27, 2200488. [Google Scholar] [CrossRef] [PubMed]
- Ghamande, S.; Shaver, C.; Murthy, K.; Raiyani, C.; White, H.D.; Lat, T.; Arroliga, A.C.; Wyatt, D.; Talbot, H.K.; Martin, E.T.; et al. Vaccine effectiveness against acute respiratory illness hospitalizations for influenza-associated pneumonia during the 2015–2016 to 2017–2018 seasons, US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN). Clin. Infect. Dis. 2022, 74, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Mira-Iglesias, A.; López-Labrador, F.X.; Baselga-Moreno, V.; Tortajada-Girbés, M.; Mollar-Maseres, J.; Carballido-Fernández, M.; Schwarz-Chavarri, G.; Puig-Barberà, J.; Díez-Domingo, J.; on behalf of the Valencia Hospital Network for the Study of Influenza and Respiratory Viruses Disease. Influenza vaccine effectiveness against laboratory-confirmed influenza in hospitalised adults aged 60years or older, Valencia Region, Spain, 2017/18 influenza season. Eurosurveillance 2019, 24, 1800461. [Google Scholar] [CrossRef] [PubMed]
- Ainslie, K.E.C.; Haber, M.; Orenstein, W.A. Challenges in estimating influenza vaccine effectiveness. Expert Rev. Vaccines 2019, 18, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.M.; Layton, J.B.; Krueger, W.S.; Kshirsagar, A.V.; McGrath, L.J. Assessing Residual Bias in Estimating Influenza Vaccine Effectiveness: Comparison of High-dose Versus Standard-dose Vaccines. Med. Care 2019, 57, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Braeye, T.; Emborg, H.-D.; Llorente-García, A.; Huerta, C.; Martín-Merino, E.; Duarte-Salles, T.; Danieli, G.; Tramontan, L.; Weibel, D.; McGee, C.; et al. Age-specific vaccination coverage estimates for influenza, human papillomavirus and measles containing vaccines from seven population-based healthcare databases from four EU countries—The ADVANCE project. Vaccine 2020, 38, 3243–3254. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | n = 260 | Vaccinated n = 139 | Unvaccinated n = 121 | p-Value 1,2 | Case n = 34 | Control n = 226 | p-Value 1,3 |
|---|---|---|---|---|---|---|---|
| Age in years: median (IQR) | 69 (60–78) | 74 (66–81) | 62 (50–73) | <0.001 | 59 (37–71) | 70 (61–79) | <0.001 |
| Age group: <18 y | 0/260 (0%) | 0/139 (0%) | 0/121 (0%) | <0.001 | 0/34 (0%) | 0/226 (0%) | 0.021 |
| Age group: 18–64 y | 95/260 (37%) | 27/139 (19%) | 68/121 (56%) | 19/34 (56%) | 76/226 (34%) | ||
| Age group: 65+ y | 165/260 (63%) | 112/139 (81%) | 53/121 (44%) | 15/34 (44%) | 150/226 (66%) | ||
| Sex female | 108/260 (42%) | 60/139 (43%) | 48/121 (40%) | 0.6 | 15/34 (44%) | 93/226 (41%) | 0.7 |
| Any chronic conditions | 238/260 (92%) | 135/139 (97%) | 103/121 (85%) | <0.001 | 28/34 (82%) | 210/226 (93%) | 0.050 |
| Smoking habits | 0.078 | 0.8 | |||||
| Never | 133/232 (57%) | 71/129 (55%) | 62/103 (60%) | 18/34 (53%) | 115/198 (58%) | ||
| Ex-smoker | 78/232 (34%) | 50/129 (39%) | 28/103 (27%) | 14/34 (41%) | 64/198 (32%) | ||
| Occasional | 3/232 (1.3%) | 2/129 (1.6%) | 1/103 (1.0%) | 0/34 (0%) | 3/198 (1.5%) | ||
| Daily | 18/232 (7.8%) | 6/129 (4.7%) | 12/103 (12%) | 2/34 (5.9%) | 16/198 (8.1%) | ||
| Unknown | 28/260 (11%) | 10/139 (7.2%) | 18/121 (15%) | 0/34 (0%) | 28/226 (12%) | ||
| Lung disease | 98/260 (38%) | 61/139 (44%) | 37/121 (31%) | 0.027 | 12/34 (35%) | 86/226 (38%) | 0.8 |
| Renal disease | 57/260 (22%) | 35/139 (25%) | 22/121 (18%) | 0.2 | 9/34 (26%) | 48/226 (21%) | 0.5 |
| Liver disease | 39/260 (15%) | 24/139 (17%) | 15/121 (12%) | 0.3 | 8/34 (24%) | 31/226 (14%) | 0.14 |
| Diabetes | 64/260 (25%) | 39/139 (28%) | 25/121 (21%) | 0.2 | 6/34 (18%) | 58/226 (26%) | 0.3 |
| Cardiovascular disease | 112/260 (43%) | 68/139 (49%) | 44/121 (36%) | 0.041 | 13/34 (38%) | 99/226 (44%) | 0.5 |
| Cancer | 76/260 (29%) | 50/139 (36%) | 26/121 (21%) | 0.010 | 9/34 (26%) | 67/226 (30%) | 0.7 |
| Immunodeficiency or OT | 24/260 (9.2%) | 13/139 (9.4%) | 11/121 (9.1%) | 0.9 | 4/34 (12%) | 20/226 (8.8%) | 0.5 |
| COVID-19 infection in the past 12 months | 150/260 (58%) | 77/139 (55%) | 73/121 (60%) | 0.4 | 7/34 (21%) | 143/226 (63%) | <0.001 |
| Death | 25/260 (9.6%) | 14/139 (10%) | 11/121 (9.1%) | 0.8 | 3/34 (8.8%) | 22/226 (9.7%) | 0.9 |
| Characteristic | n = 260 | Vaccinated n = 139 | Unvaccinated 1 n = 121 | p-Value 1,2 | Case n = 34 | Control n = 226 | p-Value 1,3 |
|---|---|---|---|---|---|---|---|
| Previous vaccination (−1 season) | 129/226 (57%) | 115/135 (85%) | 14/91 (15%) | <0.001 | 13/26 (50%) | 116/200 (58%) | 0.4 |
| Unknown | 34/260 (13%) | 4/139 (2.9%) | 30/121 (25%) | 8/34 (24%) | 26/226 (11%) | ||
| Pneumococcal vaccination | 140/259 (54%) | 107/139 (77%) | 33/120 (28%) | <0.001 | 12/33 (36%) | 128/226 (57%) | 0.029 |
| Unknown | 1/260 (0.4%) | 0/139 (0%) | 1/121 (0.8%) | 1/34 (2.9%) | 0/226 (0%) | ||
| 1st COVID-19 vaccine | 235/260 (90%) | 138/139 (99%) | 97/121 (80%) | <0.001 | 31/34 (91%) | 204/226 (90%) | 0.9 |
| 2nd COVID-19 vaccine | 229/260 (88%) | 137/139 (99%) | 92/121 (76%) | <0.001 | 31/34 (91%) | 198/226 (88%) | 0.8 |
| 3rd COVID-19 vaccine | 156/260 (60%) | 111/139 (80%) | 45/121 (37%) | <0.001 | 24/34 (71%) | 132/226 (58%) | 0.2 |
| Vaccinated n = 139 | Unvaccinated n = 121 | Crude Influenza Vaccine Effectiveness (95% CI) | Adjusted Influenza Vaccine Effectiveness * (95% CI) | |
|---|---|---|---|---|
| Case, n = 34 | 15 | 19 | 36% | 26% |
| Control, n = 226 | 124 | 102 | (−34% to 68%) | (−69% to 112%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fornaguera, M.; Parés-Badell, O.; Carbonés-Fargas, Í.; Andrés, C.; Rodrigo-Pendás, J.Á.; Borras-Bermejo, B.; Armadans-Gil, L.; Tejada, G.; Guananga, D.; Vivet-Escalé, M.; et al. Influenza Vaccine Effectiveness against Hospitalization, Season 2021/22: A Test-Negative Design Study in Barcelona. Vaccines 2023, 11, 1450. https://doi.org/10.3390/vaccines11091450
Fornaguera M, Parés-Badell O, Carbonés-Fargas Í, Andrés C, Rodrigo-Pendás JÁ, Borras-Bermejo B, Armadans-Gil L, Tejada G, Guananga D, Vivet-Escalé M, et al. Influenza Vaccine Effectiveness against Hospitalization, Season 2021/22: A Test-Negative Design Study in Barcelona. Vaccines. 2023; 11(9):1450. https://doi.org/10.3390/vaccines11091450
Chicago/Turabian StyleFornaguera, Mar, Oleguer Parés-Badell, Íngrid Carbonés-Fargas, Cristina Andrés, José Ángel Rodrigo-Pendás, Blanca Borras-Bermejo, Lluís Armadans-Gil, Gabriela Tejada, David Guananga, Martí Vivet-Escalé, and et al. 2023. "Influenza Vaccine Effectiveness against Hospitalization, Season 2021/22: A Test-Negative Design Study in Barcelona" Vaccines 11, no. 9: 1450. https://doi.org/10.3390/vaccines11091450
APA StyleFornaguera, M., Parés-Badell, O., Carbonés-Fargas, Í., Andrés, C., Rodrigo-Pendás, J. Á., Borras-Bermejo, B., Armadans-Gil, L., Tejada, G., Guananga, D., Vivet-Escalé, M., Peñalver-Piñol, A., Torrecilla-Martínez, I., del Oso, A., Martínez-Gómez, X., Antón, A., & Otero-Romero, S. (2023). Influenza Vaccine Effectiveness against Hospitalization, Season 2021/22: A Test-Negative Design Study in Barcelona. Vaccines, 11(9), 1450. https://doi.org/10.3390/vaccines11091450






