Advancing Immunotherapies for HPV-Related Cancers: Exploring Novel Vaccine Strategies and the Influence of Tumor Microenvironment
Abstract
1. Introduction
2. Cancers Related to HPV Infection and Classic Treatment Strategies
3. Immunotherapy and Cancer
4. Study of the Tumor Microenvironment (TME) for the Development of Immunotherapies
5. Cytokines and Immune Cells Found in the TME of HPV-Related Cancers
5.1. Cytokines
5.2. Lymphocytes
5.3. Neutrophils
5.4. Natural Killer Cells
5.5. Macrophages
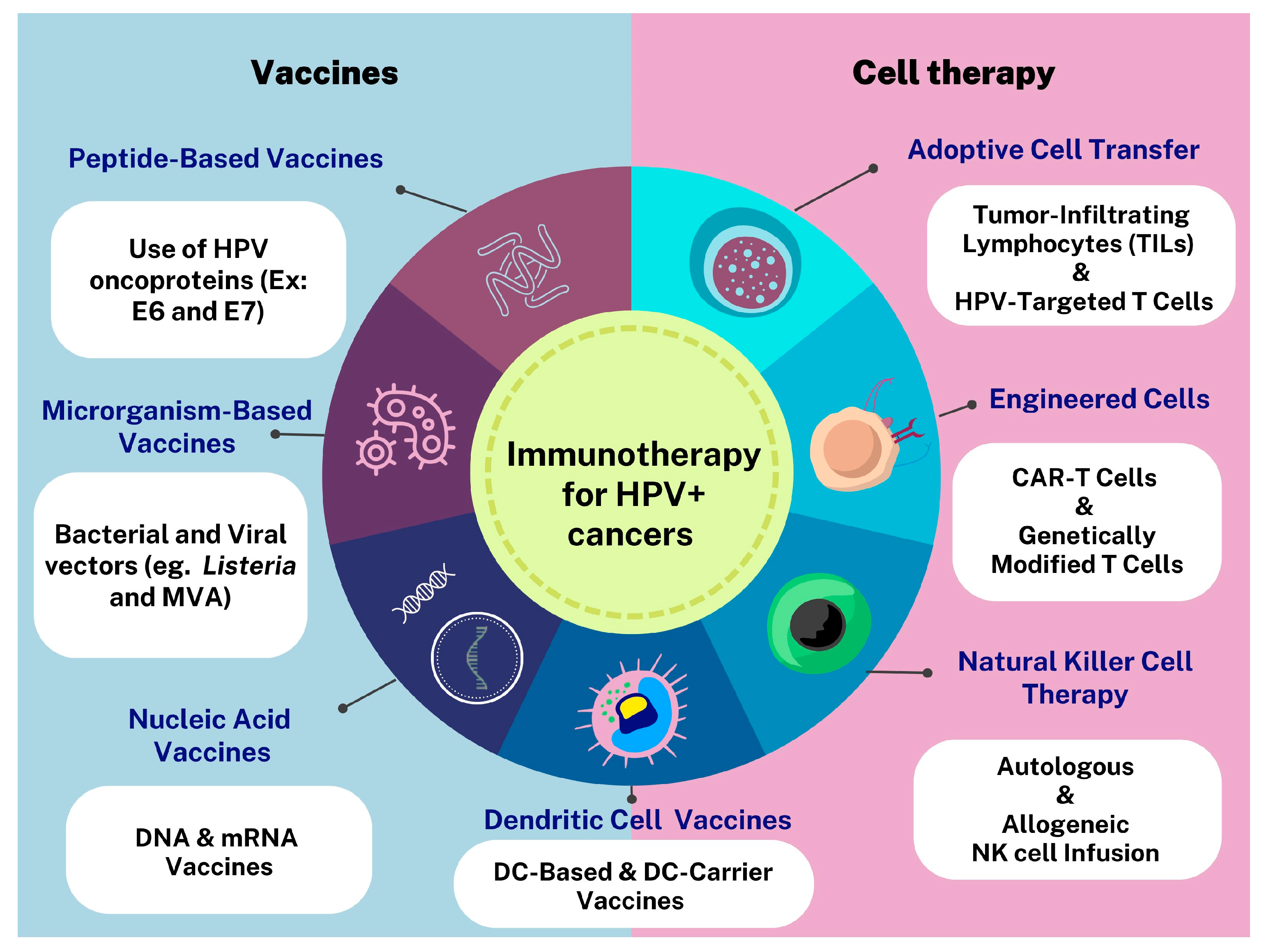
6. Immunotherapies Targeting HPV-Related Cancers
7. Cell Therapy for HPV+ Cancers
8. Antigen-Presenting Cells as Vaccine Targets for the Treatment of HPV-Related Cancers
8.1. Modulation of Tumor-Associated Macrophages as an Immunotherapy Strategy
8.2. Dendritic Cell Vaccines
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Araldi, R.P.; Sant’Ana, T.A.; Módolo, D.G.; de Melo, T.C.; Spadacci-Morena, D.D.; de Cassia Stocco, R.; Cerutti, J.M.; de Souza, E.B. The Human Papillomavirus (HPV)-Related Cancer Biology: An Overview. Biomed. Pharmacother. 2018, 106, 1537–1556. [Google Scholar] [CrossRef] [PubMed]
- Anna Szymonowicz, K.; Chen, J. Biological and Clinical Aspects of HPV-Related Cancers. Cancer Biol. Med. 2020, 17, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Ramberg, I.; Vieira, F.G.; Toft, P.B.; Von Buchwald, C.; Funding, M.; Nielsen, F.C.; Heegaard, S. Genomic Alterations in Human Papillomavirus–Positive and –Negative Conjunctival Squamous Cell Carcinomas. Investig. Opthalmol. Vis. Sci. 2021, 62, 11. [Google Scholar] [CrossRef] [PubMed]
- Illah, O.; Olaitan, A. Updates on HPV Vaccination. Diagnostics 2023, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- St. Laurent, J.; Luckett, R.; Feldman, S. HPV Vaccination and the Effects on Rates of HPV-Related Cancers. Curr. Probl. Cancer 2018, 42, 493–506. [Google Scholar] [CrossRef]
- Hancock, G.; Hellner, K.; Dorrell, L. Therapeutic HPV Vaccines. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 59–72. [Google Scholar] [CrossRef]
- Ruffin, A.T.; Li, H.; Vujanovic, L.; Zandberg, D.P.; Ferris, R.L.; Bruno, T.C. Improving Head and Neck Cancer Therapies by Immunomodulation of the Tumour Microenvironment. Nat. Rev. Cancer 2023, 23, 173–188. [Google Scholar] [CrossRef]
- Yuan, Y.; Cai, X.; Shen, F.; Ma, F. HPV Post-Infection Microenvironment and Cervical Cancer. Cancer Lett. 2021, 497, 243–254. [Google Scholar] [CrossRef]
- Smalley Rumfield, C.; Roller, N.; Pellom, S.T.; Schlom, J.; Jochems, C. Therapeutic Vaccines for HPV-Associated Malignancies. ImmunoTargets Ther. 2020, 9, 167–200. [Google Scholar] [CrossRef]
- Hu, Z.; Ott, P.A.; Wu, C.J. Towards Personalized, Tumour-Specific, Therapeutic Vaccines for Cancer. Nat. Rev. Immunol. 2018, 18, 168–182. [Google Scholar] [CrossRef]
- Lee, M.Y.; Allen, C.T. Immunotherapy for HPV Malignancies. Semin. Radiat. Oncol. 2021, 31, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Shamseddine, A.A.; Burman, B.; Lee, N.Y.; Zamarin, D.; Riaz, N. Tumor Immunity and Immunotherapy for HPV-Related Cancers. Cancer Discov. 2021, 11, 1896–1912. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.A.; Laimins, L.A. Human Papillomavirus Oncoproteins: Pathways to Transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Orosco, R.K.; Kedarisetty, S.; Hecht, A.S.; Chang, D.C.; Coffey, C.S.; Weissbrod, P.A. Predictors of High-Risk and Low-Risk Oral HPV Infection in the United States: Predictors of Oral HPV Infection. Laryngoscope 2016, 126, 1365–1372. [Google Scholar] [CrossRef]
- Egawa, N.; Doorbar, J. The Low-Risk Papillomaviruses. Virus Res. 2017, 231, 119–127. [Google Scholar] [CrossRef]
- Della Fera, A.N.; Warburton, A.; Coursey, T.L.; Khurana, S.; McBride, A.A. Persistent Human Papillomavirus Infection. Viruses 2021, 13, 321. [Google Scholar] [CrossRef]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; De Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic Human Papillomavirus Infection. Nat. Rev. Dis. Primer 2016, 2, 16086. [Google Scholar] [CrossRef]
- Castellsagué, X. Natural History and Epidemiology of HPV Infection and Cervical Cancer. Gynecol. Oncol. 2008, 110, S4–S7. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of Incidence and Mortality of Cervical Cancer in 2018: A Worldwide Analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Alemany, L.; Saunier, M.; Tinoco, L.; Quirós, B.; Alvarado-Cabrero, I.; Alejo, M.; Joura, E.A.; Maldonado, P.; Klaustermeier, J.; Salmerón, J.; et al. Large Contribution of Human Papillomavirus in Vaginal Neoplastic Lesions: A Worldwide Study in 597 Samples. Eur. J. Cancer 2014, 50, 2846–2854. [Google Scholar] [CrossRef]
- Assarzadegan, N.; Brooks, E.; Voltaggio, L. HPV-Driven Anal Neoplasia: Review and Recent Developments. Pathology 2022, 54, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Olesen, T.B.; Sand, F.L.; Rasmussen, C.L.; Albieri, V.; Toft, B.G.; Norrild, B.; Munk, C.; Kjær, S.K. Prevalence of Human Papillomavirus DNA and P16INK4a in Penile Cancer and Penile Intraepithelial Neoplasia: A Systematic Review and Meta-Analysis. Lancet Oncol. 2019, 20, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Wakeham, K.; Kavanagh, K.; Cuschieri, K.; Millan, D.; Pollock, K.G.; Bell, S.; Burton, K.; Reed, N.S.; Graham, S.V. HPV Status and Favourable Outcome in Vulvar Squamous Cancer: HPV Status and Vulvar Cancer. Int. J. Cancer 2017, 140, 1134–1146. [Google Scholar] [CrossRef]
- Dayyani, F.; Etzel, C.J.; Liu, M.; Ho, C.-H.; Lippman, S.M.; Tsao, A.S. Meta-Analysis of the Impact of Human Papillomavirus (HPV) on Cancer Risk and Overall Survival in Head and Neck Squamous Cell Carcinomas (HNSCC). Head Neck Oncol. 2010, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide Burden of Cancer Attributable to HPV by Site, Country and HPV Type: Worldwide Burden of Cancer Attributable to HPV. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Götz, C.; Bischof, C.; Wolff, K.-D.; Kolk, A. Detection of HPV Infection in Head and Neck Cancers: Promise and Pitfalls in the Last Ten Years: A Meta-Analysis. Mol. Clin. Oncol. 2018, 10, 17–28. [Google Scholar] [CrossRef]
- Sabatini, M.E.; Chiocca, S. Human Papillomavirus as a Driver of Head and Neck Cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef]
- Spence, T.; Bruce, J.; Yip, K.; Liu, F.-F. HPV Associated Head and Neck Cancer. Cancers 2016, 8, 75. [Google Scholar] [CrossRef]
- Tanaka, T.I.; Alawi, F. Human Papillomavirus and Oropharyngeal Cancer. Dent. Clin. N. Am. 2018, 62, 111–120. [Google Scholar] [CrossRef]
- Cristalli, G.; Venuti, A.; Giudici, F.; Paolini, F.; Ferreli, F.; Mercante, G.; Spriano, G.; Boscolo Nata, F. HPV Infection in Middle Ear Squamous Cell Carcinoma: Prevalence, Genotyping and Prognostic Impact. J. Clin. Med. 2021, 10, 738. [Google Scholar] [CrossRef]
- Jin, Y.T.; Tsai, S.T.; Li, C.; Chang, K.C.; Yan, J.J.; Chao, W.Y.; Eng, H.L.; Chou, T.Y.; Wu, T.C.; Su, I.J. Prevalence of Human Papillomavirus in Middle Ear Carcinoma Associated with Chronic Otitis Media. Am. J. Pathol. 1997, 150, 1327–1333. [Google Scholar] [PubMed]
- Paolini, F.; Bonomo, C.; Terrenato, I.; Pennetti, A.; Covello, R.; Cristalli, G.; Venuti, A. Beta Human Papillomaviruses in Middle Ear Squamous Cell Carcinoma. Oral Oncol. 2019, 90, 134–135. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.H.A.; Do Amaral, C.M.; De França São Marcos, B.; Nascimento, K.C.G.; De Miranda Rios, A.C.; Quixabeira, D.C.A.; Muniz, M.T.C.; Silva Neto, J.D.C.; De Freitas, A.C. Presence and Activity of HPV in Primary Lung Cancer. J. Cancer Res. Clin. Oncol. 2018, 144, 2367–2376. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Lin, C.; Tsai, S.C.-S.; Lin, F.C.-F. Human Papillomavirus Is Associated With Adenocarcinoma of Lung: A Population-Based Cohort Study. Front. Med. 2022, 9, 932196. [Google Scholar] [CrossRef]
- Marcos, B.F.S.; De Oliveira, T.H.A.; Do Amaral, C.M.M.; Muniz, M.T.C.; Freitas, A.C. Correlation between HPV PCNA, P16, and P21 Expression in Lung Cancer Patients. Cell. Microbiol. 2022, 2022, 9144334. [Google Scholar] [CrossRef]
- Ragin, C.; Obikoya-Malomo, M.; Kim, S.; Chen, Z.; Flores-Obando, R.; Gibbs, D.; Koriyama, C.; Aguayo, F.; Koshiol, J.; Caporaso, N.E.; et al. HPV-Associated Lung Cancers: An International Pooled Analysis. Carcinogenesis 2014, 35, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Lee, W.-J.; Fang, C.-L.; Hsu, H.-L.; Chen, B.-J.; Liu, H.-E. Human Papillomavirus Oncoproteins Confer Sensitivity to Cisplatin by Interfering with Epidermal Growth Factor Receptor Nuclear Trafficking Related to More Favorable Clinical Survival Outcomes in Non-Small Cell Lung Cancer. Cancers 2022, 14, 5333. [Google Scholar] [CrossRef]
- Lawson, J.S.; Glenn, W.K.; Salyakina, D.; Clay, R.; Delprado, W.; Cheerala, B.; Tran, D.D.; Ngan, C.C.; Miyauchi, S.; Karim, M.; et al. Human Papilloma Virus Identification in Breast Cancer Patients with Previous Cervical Neoplasia. Front. Oncol. 2016, 5, 298. [Google Scholar] [CrossRef]
- Ngan, C.; Lawson, J.S.; Clay, R.; Delprado, W.; Whitaker, N.J.; Glenn, W.K. Early Human Papilloma Virus (HPV) Oncogenic Influences in Breast Cancer. Breast Cancer Basic Clin. Res. 2015, 9, 93–97. [Google Scholar] [CrossRef]
- Khodabandehlou, N.; Mostafaei, S.; Etemadi, A.; Ghasemi, A.; Payandeh, M.; Hadifar, S.; Norooznezhad, A.H.; Kazemnejad, A.; Moghoofei, M. Human Papilloma Virus and Breast Cancer: The Role of Inflammation and Viral Expressed Proteins. BMC Cancer 2019, 19, 61. [Google Scholar] [CrossRef]
- Dunne, E.F.; Park, I.U. HPV and HPV-Associated Diseases. Infect. Dis. Clin. N. Am. 2013, 27, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Tumban, E. A Current Update on Human Papillomavirus-Associated Head and Neck Cancers. Viruses 2019, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Felix, C.; Wang, P.-C.; Hsu, S.; Basehart, V.; Garst, J.; Beron, P.; Wong, D.; Rosove, M.H.; Rao, S.; et al. Reduced-Dose Radiotherapy for Human Papillomavirus-Associated Squamous-Cell Carcinoma of the Oropharynx: A Single-Arm, Phase 2 Study. Lancet Oncol. 2017, 18, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, H.; Amen, F.; Tao, Y.; Deutsch, E.; Levy, A. Increased Radiosensitivity of HPV-Positive Head and Neck Cancers: Molecular Basis and Therapeutic Perspectives. Cancer Treat. Rev. 2015, 41, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Vengaloor Thomas, T.; Packianathan, S.; Bhanat, E.; Albert, A.; Abraham, A.; Gordy, X.; Kanakamedala, M.; Mehta, D.; Vijayakumar, S. Oligometastatic Head and Neck Cancer: Comprehensive Review. Head Neck 2020, 42, 2194–2201. [Google Scholar] [CrossRef]
- Azizjalali, M.; Ghaffarpour, G.; Mousavifard, B. CO2 Laser Therapy versus Cryotherapy in Treatment of Genital Warts; a Randomized Controlled Trial (RCT). Iran. J. Microbiol. 2012, 4, 187–190. [Google Scholar]
- Basu, P.; Taghavi, K.; Hu, S.-Y.; Mogri, S.; Joshi, S. Management of Cervical Premalignant Lesions. Curr. Probl. Cancer 2018, 42, 129–136. [Google Scholar] [CrossRef]
- Medeiros-Fonseca, B.; Cubilla, A.; Brito, H.; Martins, T.; Medeiros, R.; Oliveira, P.; Gil Da Costa, R.M. Experimental Models for Studying HPV-Positive and HPV-Negative Penile Cancer: New Tools for An Old Disease. Cancers 2021, 13, 460. [Google Scholar] [CrossRef]
- Massad, L.S.; Einstein, M.H.; Huh, W.K.; Katki, H.A.; Kinney, W.K.; Schiffman, M.; Solomon, D.; Wentzensen, N.; Lawson, H.W. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. Obstet. Gynecol. 2013, 121, 829–846. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, Z.; Wei, T.; Liu, Q. Persistent HPV Infection after Conization of Cervical Intraepithelial Neoplasia—A Systematic Review and Meta-Analysis. BMC Women’s Health 2023, 23, 216. [Google Scholar] [CrossRef]
- Duggan, B.D.; Felix, J.C.; Muderspach, L.I.; Gebhardt, J.A.; Groshen, S.; Morrow, C.P.; Roman, L.D. Cold-Knife Conization versus Conization by the Loop Electrosurgical Excision Procedure: A Randomized, Prospective Study. Am. J. Obstet. Gynecol. 1999, 180, 276–282. [Google Scholar] [CrossRef]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical Cancer Therapies: Current Challenges and Future Perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef] [PubMed]
- Albarrán, V.; Villamayor, M.L.; Pozas, J.; Chamorro, J.; Rosero, D.I.; San Román, M.; Guerrero, P.; Pérez De Aguado, P.; Calvo, J.C.; García De Quevedo, C.; et al. Current Landscape of Immunotherapy for Advanced Sarcoma. Cancers 2023, 15, 2287. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Yahya, E.B.; Mohamed Ibrahim Mohamed, M.; Rashid, S.; Iqbal, M.O.; Kontek, R.; Abdulsamad, M.A.; Allaq, A.A. Recent Advances in Molecular Mechanisms of Cancer Immunotherapy. Cancers 2023, 15, 2721. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. IL-2: The First Effective Immunotherapy for Human Cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef]
- Akhatova, A.; Chan, C.K.; Azizan, A.; Aimagambetova, G. The Efficacy of Therapeutic DNA Vaccines Expressing the Human Papillomavirus E6 and E7 Oncoproteins for Treatment of Cervical Cancer: Systematic Review. Vaccines 2021, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Bakker, N.A.M.; Rotman, J.; van Beurden, M.; Zijlmans, H.J.M.; van Ruiten, M.; Samuels, S.; Nuijen, B.; Beijnen, J.; De Visser, K.; Haanen, J.; et al. HPV-16 E6/E7 DNA Tattoo Vaccination Using Genetically Optimized Vaccines Elicit Clinical and Immunological Responses in Patients with Usual Vulvar Intraepithelial Neoplasia (UVIN): A Phase I/II Clinical Trial. J. Immunother. Cancer 2021, 9, e002547. [Google Scholar] [CrossRef]
- Choi, Y.J.; Hur, S.Y.; Kim, T.-J.; Hong, S.R.; Lee, J.K.; Cho, C.-H.; Park, K.S.; Woo, J.W.; Sung, Y.C.; Suh, Y.S.; et al. A Phase II, Prospective, Randomized, Multicenter, Open-Label Study of GX-188E, an HPV DNA Vaccine, in Patients with Cervical Intraepithelial Neoplasia 3. Clin. Cancer Res. 2020, 26, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, Efficacy, and Immunogenicity of VGX-3100, a Therapeutic Synthetic DNA Vaccine Targeting Human Papillomavirus 16 and 18 E6 and E7 Proteins for Cervical Intraepithelial Neoplasia 2/3: A Randomised, Double-Blind, Placebo-Controlled Phase 2b Trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef]
- Shiri Aghbash, P.; Hemmat, N.; Baradaran, B.; Mokhtarzadeh, A.; Poortahmasebi, V.; Ahangar Oskuee, M.; Bannazadeh Baghi, H. The Effect of Wnt/β-Catenin Signaling on PD-1/PDL-1 Axis in HPV-Related Cervical Cancer. Oncol. Res. 2022, 30, 99–116. [Google Scholar] [CrossRef]
- Doran, S.L.; Stevanović, S.; Adhikary, S.; Gartner, J.J.; Jia, L.; Kwong, M.L.M.; Faquin, W.C.; Hewitt, S.M.; Sherry, R.M.; Yang, J.C.; et al. T-Cell Receptor Gene Therapy for Human Papillomavirus–Associated Epithelial Cancers: A First-in-Human, Phase I/II Study. J. Clin. Oncol. 2019, 37, 2759–2768. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.B.; Norberg, S.M.; Sinkoe, A.L.; Adhikary, S.; Meyer, T.J.; Lack, J.B.; Warner, A.C.; Schweitzer, C.; Doran, S.L.; Korrapati, S.; et al. TCR-Engineered T Cells Targeting E7 for Patients with Metastatic HPV-Associated Epithelial Cancers. Nat. Med. 2021, 27, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Abbott, M.; Ustoyev, Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923. [Google Scholar] [CrossRef] [PubMed]
- Carlo, M.I.; Attalla, K.; Mazaheri, Y.; Gupta, S.; Yildirim, O.; Murray, S.J.; Coskey, D.T.; Kotecha, R.; Lee, C.-H.; Feldman, D.R.; et al. Phase II Study of Neoadjuvant Nivolumab in Patients with Locally Advanced Clear Cell Renal Cell Carcinoma Undergoing Nephrectomy. Eur. Urol. 2022, 81, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Rohaan, M.W.; Borch, T.H.; Van Den Berg, J.H.; Met, Ö.; Kessels, R.; Geukes Foppen, M.H.; Stoltenborg Granhøj, J.; Nuijen, B.; Nijenhuis, C.; Jedema, I.; et al. Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2022, 387, 2113–2125. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, J.; Sun, Y.; Lin, Y.; Liu, J.; Zhuo, Y.; Huang, Z.; Huang, S.; Chen, Y.; Chen, L.; et al. Efficacy and Safety of Sintilimab Plus Anlotinib for PD-L1–Positive Recurrent or Metastatic Cervical Cancer: A Multicenter, Single-Arm, Prospective Phase II Trial. J. Clin. Oncol. 2022, 40, 1795–1805. [Google Scholar] [CrossRef]
- Lorenzo-Herrero, S.; López-Soto, A.; Sordo-Bahamonde, C.; Gonzalez-Rodriguez, A.; Vitale, M.; Gonzalez, S. NK Cell-Based Immunotherapy in Cancer Metastasis. Cancers 2018, 11, 29. [Google Scholar] [CrossRef]
- Kim, S.K.; Cho, S.W. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 868695. [Google Scholar] [CrossRef]
- Koury, J.; Lucero, M.; Cato, C.; Chang, L.; Geiger, J.; Henry, D.; Hernandez, J.; Hung, F.; Kaur, P.; Teskey, G.; et al. Immunotherapies: Exploiting the Immune System for Cancer Treatment. J. Immunol. Res. 2018, 2018, 9585614. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Conlon, K.C.; Miljkovic, M.D.; Waldmann, T.A. Cytokines in the Treatment of Cancer. J. Interferon Cytokine Res. 2019, 39, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Vasaturo, A.; Di Blasio, S.; Peeters, D.G.A.; De Koning, C.C.H.; De Vries, J.M.; Figdor, C.G.; Hato, S.V. Clinical Implications of Co-Inhibitory Molecule Expression in the Tumor Microenvironment for DC Vaccination: A Game of Stop and Go. Front. Immunol. 2013, 4, 417. [Google Scholar] [CrossRef] [PubMed]
- Shing, J.Z.; Hu, S.; Herrero, R.; Hildesheim, A.; Porras, C.; Sampson, J.N.; Schussler, J.; Schiller, J.T.; Lowy, D.R.; Sierra, M.S.; et al. Precancerous Cervical Lesions Caused by Non-Vaccine-Preventable HPV Types after Vaccination with the Bivalent AS04-Adjuvanted HPV Vaccine: An Analysis of the Long-Term Follow-up Study from the Randomised Costa Rica HPV Vaccine Trial. Lancet Oncol. 2022, 23, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor-Infiltrating Immune Cells and Their Therapeutic Implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Chan, I.S.; Bhatia, S.; Kaufman, H.L.; Lipson, E.J. Immunotherapy for Merkel Cell Carcinoma: A Turning Point in Patient Care. J. Immunother. Cancer 2018, 6, 23. [Google Scholar] [CrossRef]
- Gavvovidis, I.; Leisegang, M.; Willimsky, G.; Miller, N.; Nghiem, P.; Blankenstein, T. Targeting Merkel Cell Carcinoma by Engineered T Cells Specific to T-Antigens of Merkel Cell Polyomavirus. Clin. Cancer Res. 2018, 24, 3644–3655. [Google Scholar] [CrossRef]
- Zaggana, E.; Konstantinou, M.P.; Krasagakis, G.H.; De Bree, E.; Kalpakis, K.; Mavroudis, D.; Krasagakis, K. Merkel Cell Carcinoma—Update on Diagnosis, Management and Future Perspectives. Cancers 2022, 15, 103. [Google Scholar] [CrossRef]
- Hosseinkhani, N.; Derakhshani, A.; Kooshkaki, O.; Abdoli Shadbad, M.; Hajiasgharzadeh, K.; Baghbanzadeh, A.; Safarpour, H.; Mokhtarzadeh, A.; Brunetti, O.; Yue, S.; et al. Immune Checkpoints and CAR-T Cells: The Pioneers in Future Cancer Therapies? Int. J. Mol. Sci. 2020, 21, 8305. [Google Scholar] [CrossRef]
- Marofi, F.; Al-Awad, A.S.; Sulaiman Rahman, H.; Markov, A.; Abdelbasset, W.K.; Ivanovna Enina, Y.; Mahmoodi, M.; Hassanzadeh, A.; Yazdanifar, M.; Stanley Chartrand, M.; et al. CAR-NK Cell: A New Paradigm in Tumor Immunotherapy. Front. Oncol. 2021, 11, 673276. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery Technologies for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor Microenvironment Complexity and Therapeutic Implications at a Glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-Associated Stromal Cells as Key Contributors to the Tumor Microenvironment. Breast Cancer Res. 2016, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef]
- Margetis, A.T. Metabolic Targeting of Malignant Tumors: A Need for Systemic Approach. J. Cancer Res. Clin. Oncol. 2023, 149, 2115–2138. [Google Scholar] [CrossRef]
- Ho, P.-C.; Bihuniak, J.D.; Macintyre, A.N.; Staron, M.; Liu, X.; Amezquita, R.; Tsui, Y.-C.; Cui, G.; Micevic, G.; Perales, J.C.; et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-Tumor T Cell Responses. Cell 2015, 162, 1217–1228. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, H.; Wang, X.; Jiang, G.; Liu, H.; Zhang, G.; Wang, H.; Fang, R.; Bu, X.; Cai, S.; et al. TGF-β Induces M2-like Macrophage Polarization via SNAIL-Mediated Suppression of a pro-Inflammatory Phenotype. Oncotarget 2016, 7, 52294–52306. [Google Scholar] [CrossRef]
- Albini, A.; Bruno, A.; Noonan, D.M.; Mortara, L. Contribution to Tumor Angiogenesis From Innate Immune Cells Within the Tumor Microenvironment: Implications for Immunotherapy. Front. Immunol. 2018, 9, 527. [Google Scholar] [CrossRef]
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer Immunotherapy: The Beginning of the End of Cancer? BMC Med. 2016, 14, 73. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic Viruses: A New Class of Immunotherapy Drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef] [PubMed]
- Nouri Rouz, F.; Shirkhoda, M.; Memari, F.; Dana, H.; Mahmoodi, C.G.; Mahmoodzad, H.; Samarghand, N.; Gharagozlo, E.; Mohammadi, M.H.; Maleki, A.R.; et al. Immunotherapy a New Hope for Cancer Treatment: A Review. Pak. J. Biol. Sci. 2018, 21, 135–150. [Google Scholar] [CrossRef]
- Twumasi-Boateng, K.; Pettigrew, J.L.; Kwok, Y.Y.E.; Bell, J.C.; Nelson, B.H. Oncolytic Viruses as Engineering Platforms for Combination Immunotherapy. Nat. Rev. Cancer 2018, 18, 419–432. [Google Scholar] [CrossRef]
- Chaberek, K.; Mrowiec, M.; Kaczmarek, M.; Dutsch-Wicherek, M. The Creation of the Suppressive Cancer Microenvironment in Patients with HPV-Positive Cervical Cancer. Diagnostics 2022, 12, 1906. [Google Scholar] [CrossRef] [PubMed]
- Ghouse, S.M.; Polikarpova, A.; Muhandes, L.; Dudeck, J.; Tantcheva-Poór, I.; Hartmann, K.; Lesche, M.; Dahl, A.; Eming, S.; Müller, W.; et al. Although Abundant in Tumor Tissue, Mast Cells Have No Effect on Immunological Micro-Milieu or Growth of HPV-Induced or Transplanted Tumors. Cell Rep. 2018, 22, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Bisheshar, S.K.; Van Der Kamp, M.F.; De Ruiter, E.J.; Ruiter, L.N.; Van Der Vegt, B.; Breimer, G.E.; Willems, S.M. The Prognostic Role of Tumor Associated Macrophages in Squamous Cell Carcinoma of the Head and Neck: A Systematic Review and Meta-Analysis. Oral Oncol. 2022, 135, 106227. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, J.P.; Sánchez-Canteli, M.; Triantafyllou, A.; De Bree, R.; Mäkitie, A.A.; Franchi, A.; Hellquist, H.; Saba, N.F.; Stenman, G.; Takes, R.P.; et al. Neutrophil to Lymphocyte Ratio in Oropharyngeal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 802. [Google Scholar] [CrossRef]
- Mytilineos, D.; Ezić, J.; Von Witzleben, A.; Mytilineos, J.; Lotfi, R.; Fürst, D.; Tsamadou, C.; Theodoraki, M.-N.; Oster, A.; Völkel, G.; et al. Peripheral Cytokine Levels Differ by HPV Status and Change Treatment-Dependently in Patients with Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 21, 5990. [Google Scholar] [CrossRef]
- Barros, M.R.; De Oliveira, T.H.A.; De Melo, C.M.L.; Venuti, A.; De Freitas, A.C. Viral Modulation of TLRs and Cytokines and the Related Immunotherapies for HPV-Associated Cancers. J. Immunol. Res. 2018, 2018, 2912671. [Google Scholar] [CrossRef]
- Grabowska, A.K. The Invisible Enemy—How Human Papillomaviruses Avoid Recognition and Clearance by the Host Immune System. Open Virol. J. 2012, 6, 249–256. [Google Scholar] [CrossRef]
- Scott, M.E.; Shvetsov, Y.B.; Thompson, P.J.; Hernandez, B.Y.; Zhu, X.; Wilkens, L.R.; Killeen, J.; Vo, D.D.; Moscicki, A.-B.; Goodman, M.T. Cervical Cytokines and Clearance of Incident Human Papillomavirus Infection: Hawaii HPV Cohort Study: Mucosal Cytokines and Cervical HPV Clearance. Int. J. Cancer 2013, 133, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Torres-Poveda, K. Role of IL-10 and TGF-Β1 in Local Immunosuppression in HPV-Associated Cervical Neoplasia. World J. Clin. Oncol. 2014, 5, 753. [Google Scholar] [CrossRef] [PubMed]
- Bleotu, C.; Chifiriuc, M.C.; Grigore, R.; Grancea, C.; Popescu, C.R.; Anton, G.; Cernescu, C. Investigation of Th1/Th2 Cytokine Profiles in Patients with Laryngo-Pharyngeal, HPV-Positive Cancers. Eur. Arch. Otorhinolaryngol. 2013, 270, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, N.; Mizuno-Kamiya, M. The Role of Immune Modulatory Cytokines in the Tumor Microenvironments of Head and Neck Squamous Cell Carcinomas. Cancers 2022, 14, 2884. [Google Scholar] [CrossRef]
- Jebreel, A.; Mistry, D.; Loke, D.; Dunn, G.; Hough, V.; Oliver, K.; Stafford, N.; Greenman, J. Investigation of Interleukin 10, 12 and 18 Levels in Patients with Head and Neck Cancer. J. Laryngol. Otol. 2007, 121, 246–252. [Google Scholar] [CrossRef]
- Peghini, B.C.; Abdalla, D.R.; Barcelos, A.C.M.; Teodoro, L.D.G.V.L.; Murta, E.F.C.; Michelin, M.A. Local Cytokine Profiles of Patients with Cervical Intraepithelial and Invasive Neoplasia. Hum. Immunol. 2012, 73, 920–926. [Google Scholar] [CrossRef]
- Otani, S.; Fujii, T.; Kukimoto, I.; Yamamoto, N.; Tsukamoto, T.; Ichikawa, R.; Nishio, E.; Iwata, A. Cytokine Expression Profiles in Cervical Mucus from Patients with Cervical Cancer and Its Precursor Lesions. Cytokine 2019, 120, 210–219. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hasimu, A.; Ge, L.; Li, Q.-Z.; Zhang, R.-P.; Guo, X. Expressions of Toll-like Receptors 3, 4, 7, and 9 in Cervical Lesions and Their Correlation with HPV16 Infection in Uighur Women. Chin. J. Cancer 2011, 30, 344–350. [Google Scholar] [CrossRef]
- Stevanović, S.; Draper, L.M.; Langhan, M.M.; Campbell, T.E.; Kwong, M.L.; Wunderlich, J.R.; Dudley, M.E.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; et al. Complete Regression of Metastatic Cervical Cancer After Treatment With Human Papillomavirus–Targeted Tumor-Infiltrating T Cells. J. Clin. Oncol. 2015, 33, 1543–1550. [Google Scholar] [CrossRef]
- Karim, R.; Tummers, B.; Meyers, C.; Biryukov, J.L.; Alam, S.; Backendorf, C.; Jha, V.; Offringa, R.; Van Ommen, G.-J.B.; Melief, C.J.M.; et al. Human Papillomavirus (HPV) Upregulates the Cellular Deubiquitinase UCHL1 to Suppress the Keratinocyte’s Innate Immune Response. PLoS Pathog. 2013, 9, e1003384. [Google Scholar] [CrossRef] [PubMed]
- Artaza-Irigaray, C.; Molina-Pineda, A.; Aguilar-Lemarroy, A.; Ortiz-Lazareno, P.; Limón-Toledo, L.P.; Pereira-Suárez, A.L.; Rojo-Contreras, W.; Jave-Suárez, L.F. E6/E7 and E6* From HPV16 and HPV18 Upregulate IL-6 Expression Independently of P53 in Keratinocytes. Front. Immunol. 2019, 10, 1676. [Google Scholar] [CrossRef] [PubMed]
- Tasic-Tomic, D.; Pravica, V.; Tasic, L.; Lukac, A.; Sacic, M.; Cupic, M. Cytokine Gene Polymorphisms of TNF, IFN-γ, and IL-12 as Potential Predictors in the Onset of Cervical Disease in HR HPV-Positive Women with Behavioral Risk Cofactors. Srp. Arh. Celok. Lek. 2022, 150, 551–557. [Google Scholar] [CrossRef]
- Jayshree, R.S. The Immune Microenvironment in Human Papilloma Virus-Induced Cervical Lesions—Evidence for Estrogen as an Immunomodulator. Front. Cell. Infect. Microbiol. 2021, 11, 649815. [Google Scholar] [CrossRef]
- Monnier-Benoit, S.; Mauny, F.; Riethmuller, D.; Guerrini, J.-S.; Căpîlna, M.; Félix, S.; Seillès, E.; Mougin, C.; Prétet, J.-L. Immunohistochemical Analysis of CD4+ and CD8+ T-Cell Subsets in High Risk Human Papillomavirus-Associated Pre-Malignant and Malignant Lesions of the Uterine Cervix. Gynecol. Oncol. 2006, 102, 22–31. [Google Scholar] [CrossRef]
- Tindle, R.W. Immune Evasion in Human Papillomavirus-Associated Cervical Cancer. Nat. Rev. Cancer 2002, 2, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Gu, R.; Yu, X.; Hu, Y.; Yu, J.; Xue, X.; Zhu, X. Characteristics of Infiltrating Immune Cells and a Predictive Immune Model for Cervical Cancer. J. Cancer 2021, 12, 3501–3514. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ding, J.; Pang, N.; Du, R.; Meng, W.; Zhu, Y.; Zhang, Y.; Ma, C.; Ding, Y. The Th17/Treg Balance and the Expression of Related Cytokines in Uygur Cervical Cancer Patients. Diagn. Pathol. 2013, 8, 61. [Google Scholar] [CrossRef]
- Tang, A.; Dadaglio, G.; Oberkampf, M.; Di Carlo, S.; Peduto, L.; Laubreton, D.; Desrues, B.; Sun, C.-M.; Montagutelli, X.; Leclerc, C. B Cells Promote Tumor Progression in a Mouse Model of HPV-Mediated Cervical Cancer: B Cells Promote Tumor Progression in a Mouse Model of Cervical Cancer. Int. J. Cancer 2016, 139, 1358–1371. [Google Scholar] [CrossRef]
- Wieland, A.; Patel, M.R.; Cardenas, M.A.; Eberhardt, C.S.; Hudson, W.H.; Obeng, R.C.; Griffith, C.C.; Wang, X.; Chen, Z.G.; Kissick, H.T.; et al. Defining HPV-Specific B Cell Responses in Patients with Head and Neck Cancer. Nature 2021, 597, 274–278. [Google Scholar] [CrossRef]
- Guo, B.; Fu, S.; Zhang, J.; Liu, B.; Li, Z. Targeting Inflammasome/IL-1 Pathways for Cancer Immunotherapy. Sci. Rep. 2016, 6, 36107. [Google Scholar] [CrossRef] [PubMed]
- Azizi, E.; Carr, A.J.; Plitas, G.; Cornish, A.E.; Konopacki, C.; Prabhakaran, S.; Nainys, J.; Wu, K.; Kiseliovas, V.; Setty, M.; et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 2018, 174, 1293–1308.e36. [Google Scholar] [CrossRef] [PubMed]
- Cillo, A.R.; Kürten, C.H.L.; Tabib, T.; Qi, Z.; Onkar, S.; Wang, T.; Liu, A.; Duvvuri, U.; Kim, S.; Soose, R.J.; et al. Immune Landscape of Viral- and Carcinogen-Driven Head and Neck Cancer. Immunity 2020, 52, 183–199.e9. [Google Scholar] [CrossRef] [PubMed]
- Domnich, M.; Riedesel, J.; Pylaeva, E.; Kürten, C.H.L.; Buer, J.; Lang, S.; Jablonska, J. Oral Neutrophils: Underestimated Players in Oral Cancer. Front. Immunol. 2020, 11, 565683. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, L.; Wang, Q.; Ma, S.; Sun, J.; Ma, C.; Liu, J.; Jing, X.; Ai, D.; Nan, Z.; et al. Neutrophils Infiltration and Its Correlation with Human Papillomavirus Status in the Oral Squamous Cell Carcinoma. Cancer Manag. Res. 2019, 11, 5171–5185. [Google Scholar] [CrossRef] [PubMed]
- Al-Sahaf, S.; Hendawi, N.B.; Ollington, B.; Bolt, R.; Ottewell, P.D.; Hunter, K.D.; Murdoch, C. Increased Abundance of Tumour-Associated Neutrophils in HPV-Negative Compared to HPV-Positive Oropharyngeal Squamous Cell Carcinoma Is Mediated by IL-1R Signalling. Front. Oral Health 2021, 2, 604565. [Google Scholar] [CrossRef]
- Huang, S.H.; Waldron, J.N.; Milosevic, M.; Shen, X.; Ringash, J.; Su, J.; Tong, L.; Perez-Ordonez, B.; Weinreb, I.; Bayley, A.J.; et al. Prognostic Value of Pretreatment Circulating Neutrophils, Monocytes, and Lymphocytes in Oropharyngeal Cancer Stratified by Human Papillomavirus Status: Leukocyte and Oropharyngeal Cancer Outcomes. Cancer 2015, 121, 545–555. [Google Scholar] [CrossRef]
- Justesen, M.M.; Jakobsen, K.K.; Bendtsen, S.K.; Garset-Zamani, M.; Mordhorst, C.; Carlander, A.-L.F.; Gothelf, A.B.; Grønhøj, C.; Von Buchwald, C. Pretreatment Neutrophil-to-Lymphocyte Ratio as a Prognostic Marker for the Outcome of HPV-Positive and HPV-Negative Oropharyngeal Squamous Cell Carcinoma. Viruses 2023, 15, 198. [Google Scholar] [CrossRef]
- Fanetti, G.; Alterio, D.; Marvaso, G.; Gandini, S.; Rojas, D.P.; Gobitti, C.; Minatel, E.; Revelant, A.; Caroli, A.; Francia, C.M.; et al. Prognostic Significance of Neutrophil-to-lymphocyte Ratio in HPV Status Era for Oropharyngeal Cancer. Oral Dis. 2020, 26, 1384–1392. [Google Scholar] [CrossRef]
- Gungor, N.; Knaapen, A.M.; Munnia, A.; Peluso, M.; Haenen, G.R.; Chiu, R.K.; Godschalk, R.W.L.; Van Schooten, F.J. Genotoxic Effects of Neutrophils and Hypochlorous Acid. Mutagenesis 2010, 25, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.; Robbins, Y.; Mydlarz, W.K.; Huynh, A.P.; Schmitt, N.C.; Friedman, J.; Horn, L.A.; Palena, C.; Schlom, J.; Maeda, D.Y.; et al. Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clin. Cancer Res. 2020, 26, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Linde, I.L.; Prestwood, T.R.; Qiu, J.; Pilarowski, G.; Linde, M.H.; Zhang, X.; Shen, L.; Reticker-Flynn, N.E.; Chiu, D.K.-C.; Sheu, L.Y.; et al. Neutrophil-Activating Therapy for the Treatment of Cancer. Cancer Cell 2023, 41, 356–372.e10. [Google Scholar] [CrossRef] [PubMed]
- Mysore, V.; Cullere, X.; Mears, J.; Rosetti, F.; Okubo, K.; Liew, P.X.; Zhang, F.; Madera-Salcedo, I.; Rosenbauer, F.; Stone, R.M.; et al. FcγR Engagement Reprograms Neutrophils into Antigen Cross-Presenting Cells That Elicit Acquired Anti-Tumor Immunity. Nat. Commun. 2021, 12, 4791. [Google Scholar] [CrossRef]
- Polak, D.; Bohle, B. Neutrophils-Typical Atypical Antigen Presenting Cells? Immunol. Lett. 2022, 247, 52–58. [Google Scholar] [CrossRef]
- Vono, M.; Lin, A.; Norrby-Teglund, A.; Koup, R.A.; Liang, F.; Loré, K. Neutrophils Acquire the Capacity for Antigen Presentation to Memory CD4+ T Cells in Vitro and Ex Vivo. Blood 2017, 129, 1991–2001. [Google Scholar] [CrossRef]
- Manzo-Merino, J.; del-Toro-Arreola, S.; Rocha-Zavaleta, L.; Peralta-Zaragoza, Ó.; Jiménez-Lima, R.; Madrid-Marina, V. Immunology of Cervical Cancer. Rev. Investig. Clin. 2020, 72, 4048. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Y.; Shi, C. Targeting Natural Killer Cells for Tumor Immunotherapy. Front. Immunol. 2020, 11, 60. [Google Scholar] [CrossRef]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.-W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The Head and Neck Cancer Immune Landscape and Its Immunotherapeutic Implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef]
- Gorvel, L.; Olive, D. Tumor Associated Macrophage in HPV+ Tumors: Between Immunosuppression and Inflammation. Semin. Immunol. 2023, 65, 101671. [Google Scholar] [CrossRef]
- Chen, X.; Fu, E.; Lou, H.; Mao, X.; Yan, B.; Tong, F.; Sun, J.; Wei, L. IL-6 Induced M1 Type Macrophage Polarization Increases Radiosensitivity in HPV Positive Head and Neck Cancer. Cancer Lett. 2019, 456, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Fu, E.; Liu, T.; Yu, S.; Chen, X.; Song, L.; Lou, H.; Ma, F.; Zhang, S.; Hussain, S.; Guo, J.; et al. M2 Macrophages Reduce the Radiosensitivity of Head and Neck Cancer by Releasing HB-EGF. Oncol. Rep. 2020, 44, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Descamps, G.; Seminerio, I.; Furgiuele, S.; Dequanter, D.; Mouawad, F.; Badoual, C.; Journe, F.; Saussez, S. HPV Involvement in the Tumor Microenvironment and Immune Treatment in Head and Neck Squamous Cell Carcinomas. Cancers 2020, 12, 1060. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, Z.; Lai, Y.; Wan, T.; Zhang, K.; Zhou, B. A Review of the Research Progress in T-Lymphocyte Immunity and Cervical Cancer. Transl. Cancer Res. 2020, 9, 2026–2036. [Google Scholar] [CrossRef]
- Long, D.-L.; Song, H.-L.; Qu, P.-P. Cytokines Profiles in Cervical Mucosa in Patients with Cervical High-Risk Human Papillomavirus Infection. J. Infect. Dev. Ctries. 2021, 15, 719–725. [Google Scholar] [CrossRef]
- Ault, K.A. Effect of Prophylactic Human Papillomavirus L1 Virus-like-Particle Vaccine on Risk of Cervical Intraepithelial Neoplasia Grade 2, Grade 3, and Adenocarcinoma in Situ: A Combined Analysis of Four Randomised Clinical Trials. Lancet 2007, 369, 1861–1868. [Google Scholar] [CrossRef]
- Diniz, M.D.O.; Ferreira, L.C.D.S. Biotecnologia Aplicada Ao Desenvolvimento de Vacinas. Estud. Av. 2010, 24, 19–30. [Google Scholar] [CrossRef][Green Version]
- Kajitani, N.; Satsuka, A.; Kawate, A.; Sakai, H. Productive Lifecycle of Human Papillomaviruses That Depends Upon Squamous Epithelial Differentiation. Front. Microbiol. 2012, 3, 152. [Google Scholar] [CrossRef]
- Skeate, J.G.; Woodham, A.W.; Einstein, M.H.; Da Silva, D.M.; Kast, W.M. Current Therapeutic Vaccination and Immunotherapy Strategies for HPV-Related Diseases. Hum. Vaccines Immunother. 2016, 12, 1418–1429. [Google Scholar] [CrossRef]
- Rupar, M.J.; Golusinski, P.; Golusinski, W.; Masternak, M.M. Human Papillomavirus and the Use of Nanoparticles for Immunotherapy in HPV-Related Cancer: A Review. Rep. Pract. Oncol. Radiother. 2019, 24, 544–550. [Google Scholar] [CrossRef]
- Shibata, T.; Lieblong, B.J.; Sasagawa, T.; Nakagawa, M. The Promise of Combining Cancer Vaccine and Checkpoint Blockade for Treating HPV-Related Cancer. Cancer Treat. Rev. 2019, 78, 8–16. [Google Scholar] [CrossRef]
- Papa, S.; Adami, A.; Metoudi, M.; Achkova, D.; Van Schalkwyk, M.; Parente Pereira, A.; Bosshard-Carter, L.; Whilding, L.; Van Der Stegen, S.; Davies, D.; et al. A Phase I Trial of T4 CAR T-Cell Immunotherapy in Head and Neck Squamous Cancer (HNSCC). J. Clin. Oncol. 2018, 36, 3046. [Google Scholar] [CrossRef]
- Blank, C.; Mackensen, A. Contribution of the PD-L1/PD-1 Pathway to T-Cell Exhaustion: An Update on Implications for Chronic Infections and Tumor Evasion. Cancer Immunol. Immunother. 2007, 56, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Moy, J.; Ferris, R.L. Immunotherapy for Head and Neck Squamous Cell Carcinoma. Curr. Oncol. Rep. 2018, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Peggs, K.S.; Quezada, S.A.; Korman, A.J.; Allison, J.P. Principles and Use of Anti-CTLA4 Antibody in Human Cancer Immunotherapy. Curr. Opin. Immunol. 2006, 18, 206–213. [Google Scholar] [CrossRef]
- Yang, W.; Song, Y.; Lu, Y.-L.; Sun, J.-Z.; Wang, H.-W. Increased Expression of Programmed Death (PD)-1 and Its Ligand PD-L1 Correlates with Impaired Cell-Mediated Immunity in High-Risk Human Papillomavirus-Related Cervical Intraepithelial Neoplasia. Immunology 2013, 139, 513–522. [Google Scholar] [CrossRef]
- Julian, R.; Savani, M.; Bauman, J.E. Immunotherapy Approaches in HPV-Associated Head and Neck Cancer. Cancers 2021, 13, 5889. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Rehmani, H.S.; Issaeva, N. EGFR in Head and Neck Squamous Cell Carcinoma: Exploring Possibilities of Novel Drug Combinations. Ann. Transl. Med. 2020, 8, 813. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Trigo, J.; Hitt, R.; Koralewski, P.; Diaz-Rubio, E.; Rolland, F.; Knecht, R.; Amellal, N.; Schueler, A.; Baselga, J. Open-Label, Uncontrolled, Multicenter Phase II Study to Evaluate the Efficacy and Toxicity of Cetuximab As a Single Agent in Patients With Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck Who Failed to Respond to Platinum-Based Therapy. J. Clin. Oncol. 2007, 25, 2171–2177. [Google Scholar] [CrossRef]
- Feliu, J.; Garcia-Carbonero, R.; Capdevila, J.; Guasch, I.; Alonso-Orduna, V.; Lopez, C.; Garcia-Alfonso, P.; Castanon, C.; Sevilla, I.; Cerezo, L.; et al. VITAL Phase 2 Study: Upfront 5-fluorouracil, Mitomycin-C, Panitumumab and Radiotherapy Treatment in Nonmetastatic Squamous Cell Carcinomas of the Anal Canal (GEMCAD 09-02). Cancer Med. 2020, 9, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Price, T.J.; Cervantes, A.; Sobrero, A.F.; Ducreux, M.; Hotko, Y.; André, T.; Chan, E.; Lordick, F.; Punt, C.J.A.; et al. Randomized Phase III Study of Panitumumab With Fluorouracil, Leucovorin, and Irinotecan (FOLFIRI) Compared With FOLFIRI Alone As Second-Line Treatment in Patients With Metastatic Colorectal Cancer. J. Clin. Oncol. 2010, 28, 4706–4713. [Google Scholar] [CrossRef] [PubMed]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A MAb Is a Checkpoint Inhibitor That Promotes Anti-Tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e13. [Google Scholar] [CrossRef] [PubMed]
- Van Hall, T.; André, P.; Horowitz, A.; Ruan, D.F.; Borst, L.; Zerbib, R.; Narni-Mancinelli, E.; Van Der Burg, S.H.; Vivier, E. Monalizumab: Inhibiting the Novel Immune Checkpoint NKG2A. J. Immunother. Cancer 2019, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Li, N.; Lin, A.; Wang, X.; Cong, J. Efficacy and Safety of Pembrolizumab on Cervical Cancer: A Systematic Review and Single-Arm Meta-Analysis. Front. Oncol. 2022, 12, 910486. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Wolchok, J.D.; Hodi, F.S.; Hamid, O.; Kefford, R.; Weber, J.S.; Joshua, A.M.; Hwu, W.-J.; Gangadhar, T.C.; et al. Anti-Programmed-Death-Receptor-1 Treatment with Pembrolizumab in Ipilimumab-Refractory Advanced Melanoma: A Randomised Dose-Comparison Cohort of a Phase 1 Trial. Lancet 2014, 384, 1109–1117. [Google Scholar] [CrossRef]
- Youn, J.W.; Hur, S.-Y.; Woo, J.W.; Kim, Y.-M.; Lim, M.C.; Park, S.Y.; Seo, S.S.; No, J.H.; Kim, B.-G.; Lee, J.-K.; et al. Pembrolizumab plus GX-188E Therapeutic DNA Vaccine in Patients with HPV-16-Positive or HPV-18-Positive Advanced Cervical Cancer: Interim Results of a Single-Arm, Phase 2 Trial. Lancet Oncol. 2020, 21, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Ferris, R.L.; Spanos, W.C.; Leidner, R.; Gonçalves, A.; Martens, U.M.; Kyi, C.; Sharfman, W.; Chung, C.H.; Devriese, L.A.; Gauthier, H.; et al. Neoadjuvant Nivolumab for Patients with Resectable HPV-Positive and HPV-Negative Squamous Cell Carcinomas of the Head and Neck in the CheckMate 358 Trial. J. Immunother. Cancer 2021, 9, e002568. [Google Scholar] [CrossRef]
- Gillison, M.; Williams, M.; Johnson, J.; Leung, C.; Reddy, J.; Garden, A.; Ferrarotto, R.; Mott, F.; Le, X.; Gunn, G.; et al. 568 Phase 2 Trial of Induction and Concomitant CTLA4 (Ipilimumab) and PD-1 (Nivolumab) Immune Checkpoint Blockade and Intensity Modulated Radiation Therapy (IMRT) in HPV-Positive Oropharyngeal Squamous Cell Carcinoma (HPV-OPSCC). In Regular and Young Investigator Award Abstracts; BMJ Publishing Group Ltd.: London, UK, 2022; pp. A594–A595. [Google Scholar]
- Lheureux, S.; Butler, M.O.; Clarke, B.; Cristea, M.C.; Martin, L.P.; Tonkin, K.; Fleming, G.F.; Tinker, A.V.; Hirte, H.W.; Tsoref, D.; et al. Association of Ipilimumab With Safety and Antitumor Activity in Women With Metastatic or Recurrent Human Papillomavirus–Related Cervical Carcinoma. JAMA Oncol. 2018, 4, e173776. [Google Scholar] [CrossRef]
- Byrne, K.T.; Betts, C.B.; Mick, R.; Sivagnanam, S.; Bajor, D.L.; Laheru, D.A.; Chiorean, E.G.; O’Hara, M.H.; Liudahl, S.M.; Newcomb, C.; et al. Neoadjuvant Selicrelumab, an Agonist CD40 Antibody, Induces Changes in the Tumor Microenvironment in Patients with Resectable Pancreatic Cancer. Clin. Cancer Res. 2021, 27, 4574–4586. [Google Scholar] [CrossRef]
- Awad, M.M.; Govindan, R.; Balogh, K.N.; Spigel, D.R.; Garon, E.B.; Bushway, M.E.; Poran, A.; Sheen, J.H.; Kohler, V.; Esaulova, E.; et al. Personalized Neoantigen Vaccine NEO-PV-01 with Chemotherapy and Anti-PD-1 as First-Line Treatment for Non-Squamous Non-Small Cell Lung Cancer. Cancer Cell 2022, 40, 1010–1026.e11. [Google Scholar] [CrossRef] [PubMed]
- Jou, J.; Harrington, K.J.; Zocca, M.-B.; Ehrnrooth, E.; Cohen, E.E.W. The Changing Landscape of Therapeutic Cancer Vaccines—Novel Platforms and Neoantigen Identification. Clin. Cancer Res. 2021, 27, 689–703. [Google Scholar] [CrossRef]
- Harrington, K.J.; Ferris, R.L.; Gillison, M.; Tahara, M.; Argiris, A.; Fayette, J.; Schenker, M.; Bratland, Å.; Walker, J.W.T.; Grell, P.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab vs Nivolumab Alone for Treatment of Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: The Phase 2 CheckMate 714 Randomized Clinical Trial. JAMA Oncol. 2023, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- Towner, M.; Novak, K.; Chae, Y.K.; Matei, D. Ipilimumab and Nivolumab for Recurrent Neuroendocrine Cervical Carcinoma. Gynecol. Oncol. Rep. 2022, 42, 101039. [Google Scholar] [CrossRef]
- Khairkhah, N.; Bolhassani, A.; Najafipour, R. Current and Future Direction in Treatment of HPV-Related Cervical Disease. J. Mol. Med. 2022, 100, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, H.; Wang, W.; Fu, Y.-X.; Zhu, M. A Novel Dendritic Cell Targeting HPV16 E7 Synthetic Vaccine in Combination with PD-L1 Blockade Elicits Therapeutic Antitumor Immunity in Mice. OncoImmunology 2016, 5, e1147641. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, P.; Ganeshrajah, S.; Raghanvan, R.K.; Singh, S.S.; Thangarajan, R. Development and Clinical Evaluation of Dendritic Cell Vaccines for HPV Related Cervical Cancer—A Feasibility Study. Asian Pac. J. Cancer Prev. 2014, 15, 5909–5916. [Google Scholar] [CrossRef]
- Santin, A.; Bellone, S.; Roman, J.; Burnett, A.; Cannon, M.; Pecorelli, S. Therapeutic Vaccines for Cervical Cancer: Dendritic Cell-Based Immunotherapy. Curr. Pharm. Des. 2005, 11, 3485–3500. [Google Scholar] [CrossRef]
- Santin, A.D.; Bellone, S.; Palmieri, M.; Zanolini, A.; Ravaggi, A.; Siegel, E.R.; Roman, J.J.; Pecorelli, S.; Cannon, M.J. Human Papillomavirus Type 16 and 18 E7-Pulsed Dendritic Cell Vaccination of Stage IB or IIA Cervical Cancer Patients: A Phase I Escalating-Dose Trial. J. Virol. 2008, 82, 1968–1979. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, Y.; Mei, L.; Liu, M.; Li, S.; Xiao, H.; Zhu, H.; Wu, S.; Chen, H.; Huang, L. Enhanced Immunotherapeutic Effect of Modified HPV16 E7-Pulsed Dendritic Cell Vaccine by an Adeno-ShRNA-SOCS1 Virus. Int. J. Oncol. 2013, 43, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Hoya, A.; Soto-Cruz, I. NK Cell Regulation in Cervical Cancer and Strategies for Immunotherapy. Cells 2021, 10, 3104. [Google Scholar] [CrossRef] [PubMed]
- Veluchamy, J.P.; Heeren, A.M.; Spanholtz, J.; Van Eendenburg, J.D.H.; Heideman, D.A.M.; Kenter, G.G.; Verheul, H.M.; Van Der Vliet, H.J.; Jordanova, E.S.; De Gruijl, T.D. High-Efficiency Lysis of Cervical Cancer by Allogeneic NK Cells Derived from Umbilical Cord Progenitors Is Independent of HLA Status. Cancer Immunol. Immunother. 2017, 66, 51–61. [Google Scholar] [CrossRef]
- Jin, B.Y.; Campbell, T.E.; Draper, L.M.; Stevanović, S.; Weissbrich, B.; Yu, Z.; Restifo, N.P.; Rosenberg, S.A.; Trimble, C.L.; Hinrichs, C.S. Engineered T Cells Targeting E7 Mediate Regression of Human Papillomavirus Cancers in a Murine Model. JCI Insight 2018, 3, e99488. [Google Scholar] [CrossRef]
- Stevanović, S.; Helman, S.R.; Wunderlich, J.R.; Langhan, M.M.; Doran, S.L.; Kwong, M.L.M.; Somerville, R.P.T.; Klebanoff, C.A.; Kammula, U.S.; Sherry, R.M.; et al. A Phase II Study of Tumor-Infiltrating Lymphocyte Therapy for Human Papillomavirus–Associated Epithelial Cancers. Clin. Cancer Res. 2019, 25, 1486–1493. [Google Scholar] [CrossRef]
- Wang, X.; Sandberg, M.L.; Martin, A.D.; Negri, K.R.; Gabrelow, G.B.; Nampe, D.P.; Wu, M.-L.; McElvain, M.E.; Toledo Warshaviak, D.; Lee, W.-H.; et al. Potent, Selective CARs as Potential T-Cell Therapeutics for HPV-Positive Cancers. J. Immunother. 2021, 44, 292–306. [Google Scholar] [CrossRef] [PubMed]
- El-Kadiry, A.E.-H.; Rafei, M.; Shammaa, R. Cell Therapy: Types, Regulation, and Clinical Benefits. Front. Med. 2021, 8, 756029. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Yasinjan, F.; Du, Y.; Geng, H.; Zhang, Y.; He, M.; Guo, R.; Yang, L.; Cui, J.; Mu, D.; et al. Immunotherapy in Cervical Cancer: From the View of Scientometric Analysis and Clinical Trials. Front. Immunol. 2023, 14, 1094437. [Google Scholar] [CrossRef]
- Mollanoori, H.; Shahraki, H.; Rahmati, Y.; Teimourian, S. CRISPR/Cas9 and CAR-T Cell, Collaboration of Two Revolutionary Technologies in Cancer Immunotherapy, an Instruction for Successful Cancer Treatment. Hum. Immunol. 2018, 79, 876–882. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Z.; Ma, X. Description of CRISPR-Cas9 Development and Its Prospects in Human Papillomavirus-Driven Cancer Treatment. Front. Immunol. 2022, 13, 1037124. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, H.; Wang, T.; He, D.; Tian, R.; Cui, Z.; Tian, X.; Gao, Q.; Ma, X.; Yang, J.; et al. In Vitro and in Vivo Growth Inhibition of Human Cervical Cancer Cells via Human Papillomavirus E6/E7 MRNAs’ Cleavage by CRISPR/Cas13a System. Antivir. Res. 2020, 178, 104794. [Google Scholar] [CrossRef]
- Zhen, S.; Qiang, R.; Lu, J.; Tuo, X.; Yang, X.; Li, X. CRISPR/Cas9-HPV-liposome Enhances Antitumor Immunity and Treatment of HPV Infection-associated Cervical Cancer. J. Med. Virol. 2023, 95, e28144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Huang, J.; Ma, W.; Yang, W.; Hu, B. The Antitumor Activity of CAR-T-PD1 Cells Enhanced by HPV16mE7-Pulsed and SOCS1-Silenced DCs in Cervical Cancer Models. Cancer Manag. Res. 2021, 13, 6045–6053. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, A.X.J.; Chen, G.; Wu, Y.; Gu, W. Prognostic and Therapeutic TILs of Cervical Cancer—Current Advances and Future Perspectives. Mol. Ther. Oncolytics 2021, 22, 410–430. [Google Scholar] [CrossRef]
- Yu, L.; Lanqing, G.; Huang, Z.; Xin, X.; Minglin, L.; Fa-hui, L.; Zou, H.; Min, J. T Cell Immunotherapy for Cervical Cancer: Challenges and Opportunities. Front. Immunol. 2023, 14, 1105265. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, N.; Jain, A.; Campana, D. NK Cells for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef]
- Shin, M.H.; Kim, J.; Lim, S.A.; Kim, J.; Kim, S.-J.; Lee, K.-M. NK Cell-Based Immunotherapies in Cancer. Immune Netw. 2020, 20, e14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Deng, J.; Rao, S.; Guo, S.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Chen, M.; et al. Tumor Infiltrating Lymphocyte (TIL) Therapy for Solid Tumor Treatment: Progressions and Challenges. Cancers 2022, 14, 4160. [Google Scholar] [CrossRef] [PubMed]
- Zmievskaya, E.; Valiullina, A.; Ganeeva, I.; Petukhov, A.; Rizvanov, A.; Bulatov, E. Application of CAR-T Cell Therapy beyond Oncology: Autoimmune Diseases and Viral Infections. Biomedicines 2021, 9, 59. [Google Scholar] [CrossRef]
- Hernández-López, A.; Téllez-González, M.A.; Mondragón-Terán, P.; Meneses-Acosta, A. Chimeric Antigen Receptor-T Cells: A Pharmaceutical Scope. Front. Pharmacol. 2021, 12, 720692. [Google Scholar] [CrossRef]
- Kumar, A.; Watkins, R.; Vilgelm, A.E. Cell Therapy With TILs: Training and Taming T Cells to Fight Cancer. Front. Immunol. 2021, 12, 690499. [Google Scholar] [CrossRef]
- Leko, V.; Rosenberg, S.A. Identifying and Targeting Human Tumor Antigens for T Cell-Based Immunotherapy of Solid Tumors. Cancer Cell 2020, 38, 454–472. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef]
- Westrich, J.A.; Warren, C.J.; Pyeon, D. Evasion of Host Immune Defenses by Human Papillomavirus. Virus Res. 2017, 231, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Minn, A.J. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity 2018, 48, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Romero, K.; Rodríguez, R.M.; Amedei, A.; Barceló-Coblijn, G.; Lopez, D.H. Immune Landscape in Tumor Microenvironment: Implications for Biomarker Development and Immunotherapy. Int. J. Mol. Sci. 2020, 21, 5521. [Google Scholar] [CrossRef]
- Petrillo, M.; Zannoni, G.F.; Martinelli, E.; Pedone Anchora, L.; Ferrandina, G.; Tropeano, G.; Fagotti, A.; Scambia, G. Polarisation of Tumor-Associated Macrophages toward M2 Phenotype Correlates with Poor Response to Chemoradiation and Reduced Survival in Patients with Locally Advanced Cervical Cancer. PLoS ONE 2015, 10, e0136654. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Li, Y.; Zhao, X. Research Progress on Tumor-Associated Macrophages and Inflammation in Cervical Cancer. BioMed Res. Int. 2020, 2020, 6842963. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ye, Z.; Song, F.; He, Y.; Liu, J. The Role of TAMs in Tumor Microenvironment and New Research Progress. Stem Cells Int. 2022, 2022, 6842963. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Zhang, P.; Xing, H.; Zhao, S.; Song, Y.; Wan, D.; Yu, J. Targeting Toll-like Receptor 7/8 for Immunotherapy: Recent Advances and Prospectives. Biomark. Res. 2022, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Kim, S.; Kim, J.-E.; Lee, S.N.; Shin, I.W.; Shin, H.S.; Jin, S.M.; Noh, Y.-W.; Kang, Y.J.; Kim, Y.S.; et al. Lyophilizable and Multifaceted Toll-like Receptor 7/8 Agonist-Loaded Nanoemulsion for the Reprogramming of Tumor Microenvironments and Enhanced Cancer Immunotherapy. ACS Nano 2019, 13, 12671–12686. [Google Scholar] [CrossRef]
- Rodell, C.B.; Arlauckas, S.P.; Cuccarese, M.F.; Garris, C.S.; Li, R.; Ahmed, M.S.; Kohler, R.H.; Pittet, M.J.; Weissleder, R. TLR7/8-Agonist-Loaded Nanoparticles Promote the Polarization of Tumour-Associated Macrophages to Enhance Cancer Immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.H.; Espinosa, I.; Edris, B.; Li, R.; Montgomery, K.; Zhu, S.; Varma, S.; Marinelli, R.J.; Van De Rijn, M.; West, R.B. The Macrophage Colony-Stimulating Factor 1 Response Signature in Breast Carcinoma. Clin. Cancer Res. 2009, 15, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.C.; Ruffell, B.; Oei, Y.; Bissell, M.J.; Coussens, L.M.; Pryer, N.; Daniel, D. CSF1R Inhibition Delays Cervical and Mammary Tumor Growth in Murine Models by Attenuating the Turnover of Tumor-Associated Macrophages and Enhancing Infiltration by CD8 + T Cells. OncoImmunology 2013, 2, e26968. [Google Scholar] [CrossRef] [PubMed]
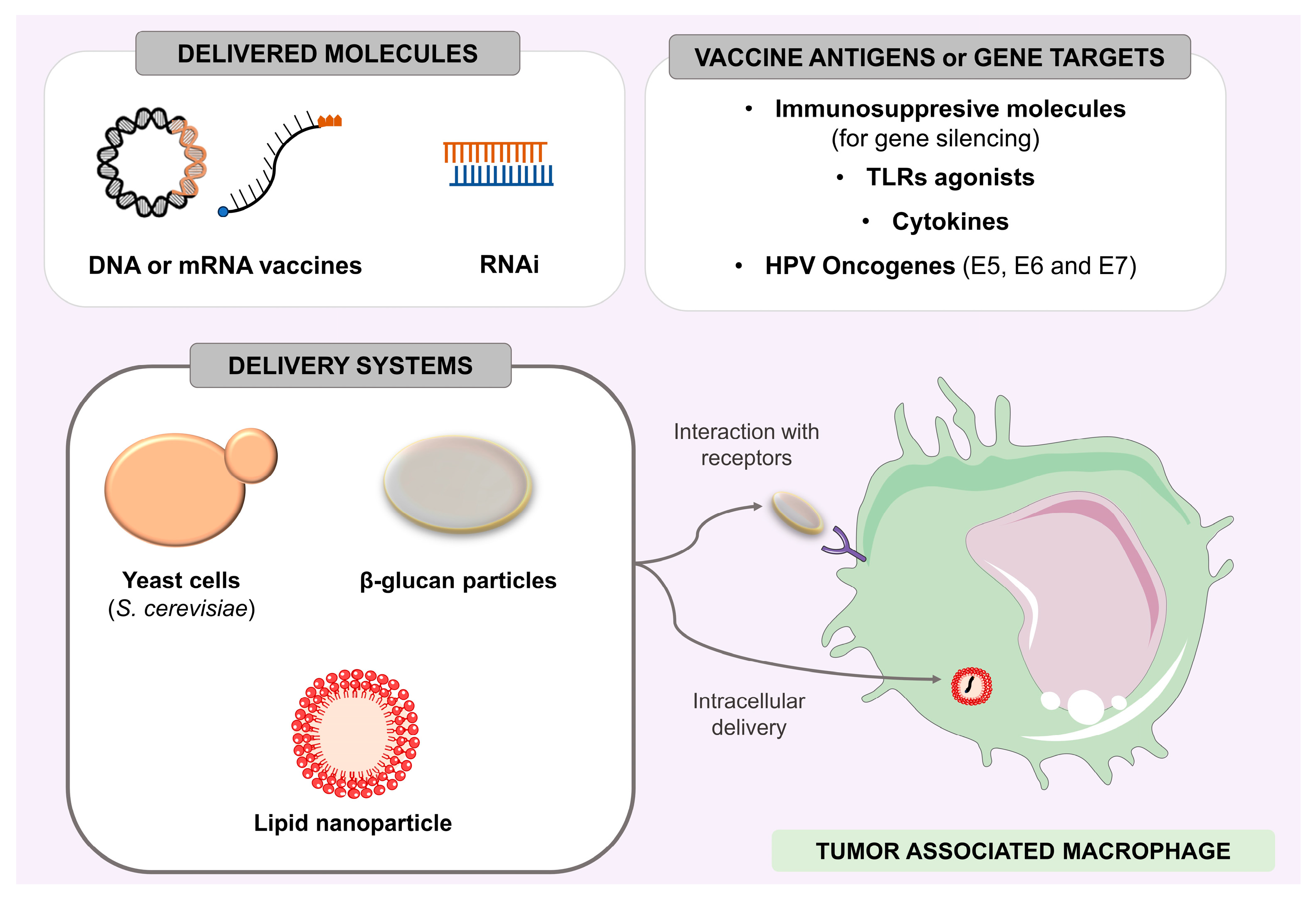
- de Moura, I.A.; Silva, A.J.D.; de Macêdo, L.S.; da Conceição Viana Invenção, M.; de Sousa, M.M.G.; de Freitas, A.C. Enhancing the Effect of Nucleic Acid Vaccines in the Treatment of HPV-Related Cancers: An Overview of Delivery Systems. Pathogens 2022, 11, 1444. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Bai, Y.; Xia, L.; Pan, J.; Sun, X.; Zhu, Z.; Ding, J.; Qi, C.; Tang, C. Oral Administration of a Whole Glucan Particle (WGP)-Based Therapeutic Cancer Vaccine Targeting Macrophages Inhibits Tumor Growth. Cancer Immunol. Immunother. 2022, 71, 2007–2028. [Google Scholar] [CrossRef]
- Liu, M.; Luo, F.; Ding, C.; Albeituni, S.; Hu, X.; Ma, Y.; Cai, Y.; McNally, L.; Sanders, M.A.; Jain, D.; et al. Dectin-1 Activation by a Natural Product β-Glucan Converts Immunosuppressive Macrophages into an M1-like Phenotype. J. Immunol. 2015, 195, 5055–5065. [Google Scholar] [CrossRef]
- Seif, M.; Philippi, A.; Breinig, F.; Kiemer, A.K.; Hoppstädter, J. Yeast (Saccharomyces Cerevisiae) Polarizes Both M-CSF- and GM-CSF-Differentiated Macrophages Toward an M1-Like Phenotype. Inflammation 2016, 39, 1690–1703. [Google Scholar] [CrossRef]
- Silva, A.J.D.; de Macêdo, L.S.; Leal, L.R.S.; de Jesus, A.L.S.; Freitas, A.C. Yeasts as a Promising Delivery Platform for DNA and RNA Vaccines. FEMS Yeast Res. 2021, 21, foab018. [Google Scholar] [CrossRef]
- Tesz, G.J.; Aouadi, M.; Prot, M.; Nicoloro, S.M.; Boutet, E.; Amano, S.U.; Goller, A.; Wang, M.; Guo, C.-A.; Salomon, W.E.; et al. Glucan Particles for Selective Delivery of SiRNA to Phagocytic Cells in Mice. Biochem. J. 2011, 436, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lu, S.; Zhang, L.; Ji, M.; Liu, S.; Wang, S.; Liu, R. An Indoleamine 2, 3-Dioxygenase SiRNA Nanoparticle-Coated and Trp2-Displayed Recombinant Yeast Vaccine Inhibits Melanoma Tumor Growth in Mice. J. Control. Release 2018, 273, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, R.; Zhang, H.; Sun, R.; Li, S.; Xia, C.; Li, Z.; Zhang, L.; Guo, Y.; Huang, J. Recombinant Hemagglutinin Protein and DNA-RNA-Combined Nucleic Acid Vaccines Harbored by Yeast Elicit Protective Immunity against H9N2 Avian Influenza Infection. Poult. Sci. 2023, 102, 102662. [Google Scholar] [CrossRef]
- Xu, T.; Yu, S.; Zhang, J.; Wu, S. Dysregulated Tumor-Associated Macrophages in Carcinogenesis, Progression and Targeted Therapy of Gynecological and Breast Cancers. J. Hematol. Oncol. J. Hematol. Oncol. 2021, 14, 181. [Google Scholar] [CrossRef] [PubMed]
- Llopiz, D.; Ruiz, M.; Silva, L.; Repáraz, D.; Aparicio, B.; Egea, J.; Lasarte, J.J.; Redin, E.; Calvo, A.; Angel, M.; et al. Inhibition of Adjuvant-Induced TAM Receptors Potentiates Cancer Vaccine Immunogenicity and Therapeutic Efficacy. Cancer Lett. 2021, 499, 279–289. [Google Scholar] [CrossRef]
- Lepique, A.P.; Daghastanli, K.R.P.; Cuccovia, I.M.; Villa, L.L. HPV16 Tumor Associated Macrophages Suppress Antitumor T Cell Responses. Clin. Cancer Res. 2009, 15, 4391–4400. [Google Scholar] [CrossRef]
- Shobaki, N.; Sato, Y.; Suzuki, Y.; Okabe, N.; Harashima, H. Manipulating the Function of Tumor-Associated Macrophages by SiRNA-Loaded Lipid Nanoparticles for Cancer Immunotherapy. J. Control. Release 2020, 325, 235–248. [Google Scholar] [CrossRef]
- Singhania, R. RNA Interference for the Treatment of Papillomavirus Disease. Open Virol. J. 2012, 6, 204–215. [Google Scholar] [CrossRef][Green Version]
- Wang, Q.; Steger, A.; Mahner, S.; Jeschke, U.; Heidegger, H. The Formation and Therapeutic Update of Tumor-Associated Macrophages in Cervical Cancer. Int. J. Mol. Sci. 2019, 20, 3310. [Google Scholar] [CrossRef]
- Wang, D.; Xue, M.; Chen, J.; Chen, H.; Liu, J.; Li, Q.; Xie, Y.; Hu, Y.; Ni, Y.; Zhou, Q. Macrophage-Derived Implantable Vaccine Prevents Postsurgical Tumor Recurrence. Biomaterials 2021, 278, 121161. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, L.; Dai, X.; Yu, H.; Wang, J.; Lei, A.; Zhu, M.; Xu, J.; Zhao, W.; Zhu, Y.; et al. Pluripotent Stem Cell-Derived CAR-Macrophage Cells with Antigen-Dependent Anti-Cancer Cell Functions. J. Hematol. Oncol. J. Hematol. Oncol. 2020, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Pan, W.; Jin, L.; Huang, W.; Li, Y.; Wu, D.; Gao, C.; Ma, D.; Liao, S. Human Papillomavirus Vaccine against Cervical Cancer: Opportunity and Challenge. Cancer Lett. 2020, 471, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Duluc, D.; Joo, H.; Oh, S. Dendritic Cell Targeting Vaccine for HPV-Associated Cancer. Cancer Cell Microenviron. 2016, 3, e1482. [Google Scholar]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-Presentation by Dendritic Cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.J.; Benike, C.; Fagnoni, F.; Liles, T.M.; Czerwinski, D.; Taidi, B.; Engleman, E.G.; Levy, R. Vaccination of Patients with B–Cell Lymphoma Using Autologous Antigen–Pulsed Dendritic Cells. Nat. Med. 1996, 2, 52–58. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D.; et al. First Results on Survival from a Large Phase 3 Clinical Trial of an Autologous Dendritic Cell Vaccine in Newly Diagnosed Glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef]
- Prue, R.L.; Vari, F.; Radford, K.J.; Tong, H.; Hardy, M.Y.; D’Rozario, R.; Waterhouse, N.J.; Rossetti, T.; Coleman, R.; Tracey, C.; et al. A Phase I Clinical Trial of CD1c (BDCA-1)+ Dendritic Cells Pulsed With HLA-A*0201 Peptides for Immunotherapy of Metastatic Hormone Refractory Prostate Cancer. J. Immunother. 2015, 38, 71–76. [Google Scholar] [CrossRef]
- Schreibelt, G.; Bol, K.F.; Westdorp, H.; Wimmers, F.; Aarntzen, E.H.J.G.; Duiveman-de Boer, T.; Van De Rakt, M.W.M.M.; Scharenborg, N.M.; De Boer, A.J.; Pots, J.M.; et al. Effective Clinical Responses in Metastatic Melanoma Patients after Vaccination with Primary Myeloid Dendritic Cells. Clin. Cancer Res. 2016, 22, 2155–2166. [Google Scholar] [CrossRef]
- Baldin, A.V.; Savvateeva, L.V.; Bazhin, A.V.; Zamyatnin, A.A. Dendritic Cells in Anticancer Vaccination: Rationale for Ex Vivo Loading or In Vivo Targeting. Cancers 2020, 12, 590. [Google Scholar] [CrossRef]
- Johnson, P.; Rosendahl, N.; Radford, K.J. Conventional Type 1 Dendritic Cells (CDC1) as Cancer Therapeutics: Challenges and Opportunities. Expert Opin. Biol. Ther. 2022, 22, 465–472. [Google Scholar] [CrossRef]
- Perez, C.R.; De Palma, M. Engineering Dendritic Cell Vaccines to Improve Cancer Immunotherapy. Nat. Commun. 2019, 10, 5408. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Komori, S.; Kotani, T.; Murata, Y.; Matozaki, T. The Role of Type-2 Conventional Dendritic Cells in the Regulation of Tumor Immunity. Cancers 2022, 14, 1976. [Google Scholar] [CrossRef] [PubMed]
- Usero, L.; Miralles, L.; Esteban, I.; Pastor-Quiñones, C.; Maleno, M.J.; Leal, L.; García, F.; Plana, M. Feasibility of Using Monocyte-Derived Dendritic Cells Obtained from Cryopreserved Cells for DC-Based Vaccines. J. Immunol. Methods 2021, 498, 113133. [Google Scholar] [CrossRef]
- Salah, A.; Wang, H.; Li, Y.; Ji, M.; Ou, W.-B.; Qi, N.; Wu, Y. Insights Into Dendritic Cells in Cancer Immunotherapy: From Bench to Clinical Applications. Front. Cell Dev. Biol. 2021, 9, 686544. [Google Scholar] [CrossRef]
- Huang, L.; Rong, Y.; Tang, X.; Yi, K.; Qi, P.; Hou, J.; Liu, W.; He, Y.; Gao, X.; Yuan, C.; et al. Engineered Exosomes as an in Situ DC-Primed Vaccine to Boost Antitumor Immunity in Breast Cancer. Mol. Cancer 2022, 21, 45. [Google Scholar] [CrossRef]
- Wculek, S.K.; Cueto, F.J.; Mujal, A.M.; Melero, I.; Krummel, M.F.; Sancho, D. Dendritic Cells in Cancer Immunology and Immunotherapy. Nat. Rev. Immunol. 2020, 20, 7–24. [Google Scholar] [CrossRef]
- Lee, S.-J.; Yang, A.; Wu, T.-C.; Hung, C.-F. Immunotherapy for Human Papillomavirus-Associated Disease and Cervical Cancer: Review of Clinical and Translational Research. J. Gynecol. Oncol. 2016, 27, e51. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, H.; Jayakumar, H.; Seetharaman, A.; Singh, S.S.; Ganeshrajah, S.; Jagadish, N.; Suri, A.; Thangarajan, R.; Ramanathan, P. Dendritic Cells Matured with Recombinant Human Sperm Associated Antigen 9 (RhSPAG9) Induce CD4+, CD8+ T Cells and Activate NK Cells: A Potential Candidate Molecule for Immunotherapy in Cervical Cancer. Cancer Cell Int. 2021, 21, 473. [Google Scholar] [CrossRef]
- Thornburg, C.; Boczkowski, D.; Gilboa, E.; Nair, S.K. Induction of Cytotoxic T Lymphocytes With Dendritic Cells Transfected With Human Papillomavirus E6 and E7 RNA: Implications for Cervical Cancer Immunotherapy. J. Immunother. 2000, 23, 412–418. [Google Scholar] [CrossRef]
- Bolhassani, A.; Shahbazi, S.; Agi, E.; Haghighipour, N.; Hadi, A.; Asgari, F. Modified DCs and MSCs with HPV E7 Antigen and Small Hsps: Which One Is the Most Potent Strategy for Eradication of Tumors? Mol. Immunol. 2019, 108, 102–110. [Google Scholar] [CrossRef]
- Mo, Y.; Ma, J.; Zhang, H.; Shen, J.; Chen, J.; Hong, J.; Xu, Y.; Qian, C. Prophylactic and Therapeutic HPV Vaccines: Current Scenario and Perspectives. Front. Cell. Infect. Microbiol. 2022, 12, 909223. [Google Scholar] [CrossRef] [PubMed]
| Target | Antibody-Based Strategy | Mechanism of Action | Clinical Status | References |
|---|---|---|---|---|
| EGFR | Cetuximab | Monoclonal antibody targeting EGFR | FDA-approved for HNSCC | [158,159,160] |
| Panitumumab | FDA-approved for colorectal cancer | [161,162] | ||
| NKG2D | Monalizumab | Monoclonal antibody targeting NKG2D ligands | Under clinical evaluation for various cancers | [157,163,164] |
| PD-1 | Pembrolizumab | Monoclonal antibody targeting PD-1 | FDA-approved for various cancers | [165,166,167] |
| Nivolumab | [64,168,169] | |||
| CTLA-4 | Ipilimumab | Monoclonal antibody targeting CTLA-4 | FDA-approved for various cancers | [166,170,171] |
| CD40 | Selicrelumab | Monoclonal antibody targeting CD40 | Under clinical evaluation for solid tumors | [172] |
| Tumor neoantigens | NEO-PV-01 | Personalized vaccine targeting tumor neoantigens | Under clinical evaluation for various cancers | [173,174] |
| Nivolumab + Ipilimumab | Combination therapy targeting PD-1 and CTLA-4 | FDA-approved for MSI-H/dMMR solid tumors | [175,176] |
| DC Subtypes | Features | Impact on HPV+ Cancers | References |
|---|---|---|---|
| cDC1 | Express CLEC9A, CADM1, XCR1, CD141 |
| [241] |
| |||
| cDC2 | Express SIRPα, CD1c (BDCA1), CLEC10A (CD301a) |
| [242,243] |
| |||
| MoDCs | Result from inflammatory processes |
| [244] |
| |||
| pDCs | Express CD4, CD123, CD303, CD304, BDCA-2, HLA-DR, TLR7/TLR9 |
| [242,245] |
| |||
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.J.D.; Moura, I.A.d.; Gama, M.A.T.M.d.; Leal, L.R.S.; Pinho, S.S.d.; Espinoza, B.C.F.; Santos, D.L.d.; Santos, V.E.P.; Sena, M.G.A.M.D.; Invenção, M.D.C.V.; et al. Advancing Immunotherapies for HPV-Related Cancers: Exploring Novel Vaccine Strategies and the Influence of Tumor Microenvironment. Vaccines 2023, 11, 1354. https://doi.org/10.3390/vaccines11081354
Silva AJD, Moura IAd, Gama MATMd, Leal LRS, Pinho SSd, Espinoza BCF, Santos DLd, Santos VEP, Sena MGAMD, Invenção MDCV, et al. Advancing Immunotherapies for HPV-Related Cancers: Exploring Novel Vaccine Strategies and the Influence of Tumor Microenvironment. Vaccines. 2023; 11(8):1354. https://doi.org/10.3390/vaccines11081354
Chicago/Turabian StyleSilva, Anna Jéssica Duarte, Ingrid Andrêssa de Moura, Marco Antonio Turiah Machado da Gama, Lígia Rosa Sales Leal, Samara Sousa de Pinho, Benigno Cristofer Flores Espinoza, Daffany Luana dos Santos, Vanessa Emanuelle Pereira Santos, Matheus Gardini Amancio Marques De Sena, Maria Da Conceição Viana Invenção, and et al. 2023. "Advancing Immunotherapies for HPV-Related Cancers: Exploring Novel Vaccine Strategies and the Influence of Tumor Microenvironment" Vaccines 11, no. 8: 1354. https://doi.org/10.3390/vaccines11081354
APA StyleSilva, A. J. D., Moura, I. A. d., Gama, M. A. T. M. d., Leal, L. R. S., Pinho, S. S. d., Espinoza, B. C. F., Santos, D. L. d., Santos, V. E. P., Sena, M. G. A. M. D., Invenção, M. D. C. V., Macêdo, L. S. d., França Neto, P. L. d., & Freitas, A. C. d. (2023). Advancing Immunotherapies for HPV-Related Cancers: Exploring Novel Vaccine Strategies and the Influence of Tumor Microenvironment. Vaccines, 11(8), 1354. https://doi.org/10.3390/vaccines11081354








