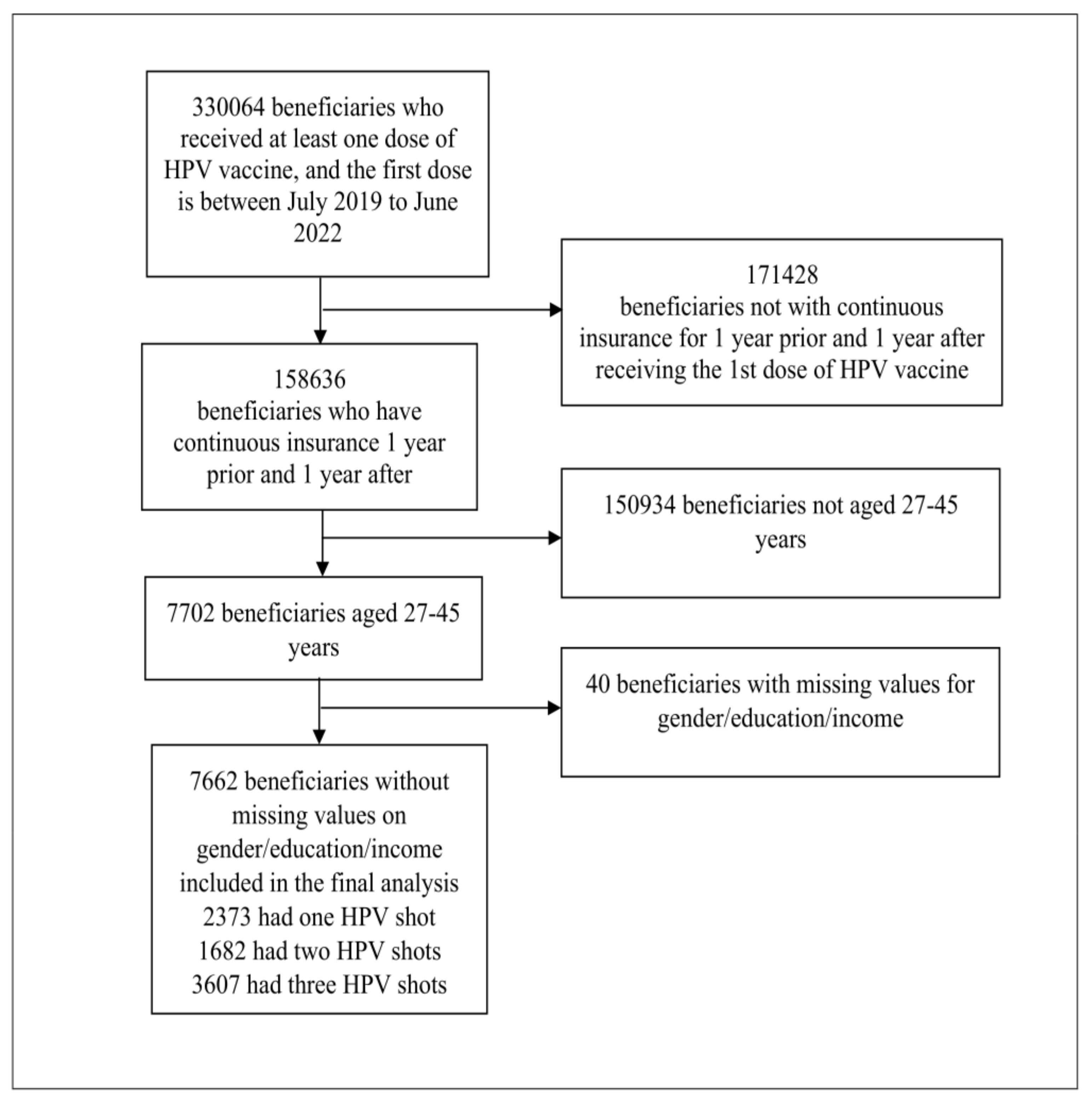
Incomplete HPV Vaccination among Individuals Aged 27–45 Years in the United States: A Mixed-Effect Analysis of Individual and Contextual Factors
Abstract
1. Background
2. Methods
2.1. Participants and Data
2.2. Ethical Considerations
2.3. Independent Variables
Individual-Level Factors
2.4. Contextual-Level Factors
2.5. Outcome Variable
3. Statistical Analysis
3.1. Descriptive Analyses
3.2. Modeling Approaches
3.3. Fixed Effects (Measures of Association)
3.4. Random Effects (Measures of Variation)
3.5. Model Fit and Specifications
4. Results
4.1. Descriptive Statistics
4.2. Multilevel Analysis of the Factors Associated with Incomplete HPV Vaccination
4.3. Random Effects Measures
5. Discussion
Limitations and Strengths
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, C.-I.; Francoeur, A.A.; Kapp, D.S.; Caesar, M.A.P.; Huh, W.K.; Chan, J.K. Trends in Human Papillomavirus–Associated Cancers, Demographic Characteristics, and Vaccinations in the US, 2001–2017. JAMA Netw. Open 2022, 5, e222530. [Google Scholar] [CrossRef] [PubMed]
- Cancer Stat Facts: Cervical Cancer. 2022. Available online: https://seer.cancer.gov/statfacts/html/cervix.html (accessed on 9 December 2022).
- Cervical Cancer Statistics. 2022. Available online: https://www.cdc.gov/cancer/cervical/statistics/index.htm (accessed on 9 December 2022).
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 2007, 356, 1915–1927. [Google Scholar] [CrossRef]
- Arbyn, M.; Xu, L.; Simoens, C.; Martin-Hirsch, P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018, 5, Cd009069. [Google Scholar] [CrossRef] [PubMed]
- Paavonen, J.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.N.; Apter, D.; Kitchener, H.; Castellsague, X.; Teixeira, J.C.; Skinner, S.R.; et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double-blind, randomised study in young women. Lancet 2009, 374, 301–314. [Google Scholar] [CrossRef]
- Boersma, P.; Black, L.I. Human Papillomavirus Vaccination Among Adults Aged 18–26, 2013–2018. NCHS Data Brief. 2020, 354, 1–8. [Google Scholar]
- Prabhu, V.S.; Roberts, C.S.; Kothari, S.; Niccolai, L. Median Age at HPV Infection Among Women in the United States: A Model-Based Analysis Informed by Real-world Data. Open Forum Infect. Dis. 2021, 8, ofab111. [Google Scholar] [CrossRef]
- Meites, E.; Szilagyi, P.G.; Chesson, H.W.; Unger, E.R.; Romero, J.R.; Markowitz, L.E. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal Wkly. Rep. 2019, 68, 698–702. [Google Scholar] [CrossRef]
- National Vaccine Advisory Committee. Strengthening the Effectiveness of National, State, and Local Efforts to Improve HPV Vaccination Coverage in the United States: Recommendations from the National Vaccine Advisory Committee. Public Health Rep. 2018, 133, 543–550. [Google Scholar] [CrossRef]
- Matsuno, R.K.; Seay, J.; Porter, B.; Tannenbaum, K.; Warner, S.; Wells, N. Factors Associated with Human Papillomavirus Vaccine Initiation and Compliance Among U.S. Military Service Members. Mil. Med. 2022, usab562. [Google Scholar] [CrossRef]
- Rodriguez, S.A.; Mullen, P.D.; Lopez, D.M.; Savas, L.S.; Fernández, M.E. Factors associated with adolescent HPV vaccination in the U.S.: A systematic review of reviews and multilevel framework to inform intervention development. Prev Med. 2020, 131, 105968. [Google Scholar] [CrossRef] [PubMed]
- Sims, A.; Archie-Booker, E.; Waldrop, R.T.; Claridy, M.; Gerbi, G. Factors Associated with Human Papillomavirus Vaccination among Women in the United States. ARC J. Public Health Community Med. 2018, 3, 6–12. [Google Scholar] [PubMed]
- Kepka, D.; Bodson, J.; Lai, D.; Sanchez-Birkhead, A.; Villalta, J.; Mukundente, V.; Tavake-Pasi, F.; Davis, F.A.; Lee, D.; Napia, E.; et al. Factors Associated with Human Papillomavirus Vaccination Among Diverse Adolescents in a Region with Low Human Papillomavirus Vaccination Rates. Health Equity 2018, 2, 223–232. [Google Scholar] [CrossRef]
- Wilson, A.R.; Hashibe, M.; Bodson, J.; Gren, L.H.; Taylor, B.A.; Greenwood, J.; Jackson, B.R.; She, R.; Egger, M.J.; Kepka, D. Factors related to HPV vaccine uptake and 3-dose completion among women in a low vaccination region of the USA: An observational study. BMC Womens Health 2016, 16, 41. [Google Scholar] [CrossRef]
- McLendon, L.; Puckett, J.; Green, C.; James, J.; Head, K.J.; Yun Lee, H.; Young Pierce, J.; Beasley, M.; Daniel, C.L. Factors associated with HPV vaccination initiation among United States college students. Hum. Vaccines Immunother. 2021, 17, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Gebremeskel, R.H.; Haney, C.J.; Coyne-Beasley, T. Social and Behavioral Factors Associated with HPV Vaccination Uptake in Adolescents. J. Adolesc. Health 2014, 54 (Suppl. S2), S31. [Google Scholar] [CrossRef]
- Franco, M.; Mazzucca, S.; Padek, M.; Brownson, R.C. Going beyond the individual: How state-level characteristics relate to HPV vaccine rates in the United States. BMC Public Health 2019, 19, 246. [Google Scholar] [CrossRef]
- Braveman, P.; Gottlieb, L. The social determinants of health: It’s time to consider the causes of the causes. Public Health Rep. 2014, 129 (Suppl. S2), 19–31. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Communities in Action: Pathways to Health Equity; Baciu, A., Negussie, Y., Geller, A., Weinstein, J.N., Eds.; National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- Rasbash, J.; Steele, F.; Browne, W.; Prosser, B. A User’s Guide to MLwiN, version 3.06; Centre for Multilevel Modelling, University of Bristol: Bristol, UK, 2022. [Google Scholar]
- StataCorp. Stata Statistics Software (Version 17 SE for Windows); StataCorp LP: College Station, TX, USA, 2017. [Google Scholar]
- Gold, R.; Naleway, A.; Riedlinger, K. Factors Predicting Completion of the Human Papillomavirus Vaccine Series. J. Adolesc. Health 2013, 52, 427–432. [Google Scholar] [CrossRef]
- Adjei Boakye, E.; Lew, D.; Muthukrishnan, M.; Tobo, B.B.; Rohde, R.L.; Varvares, M.A.; Osazuwa-Peters, N. Correlates of human papillomavirus (HPV) vaccination initiation and completion among 18–26 year olds in the United States. Hum. Vaccines Immunother. 2018, 14, 2016–2024. [Google Scholar] [CrossRef]
- Chao, C.; Velicer, C.; Slezak, J.M.; Jacobsen, S.J. Correlates for completion of 3-dose regimen of HPV vaccine in female members of a managed care organization. Mayo Clin. Proc. 2009, 84, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Conway, D. Uninsured Rates Highest for Young Adults Aged 19 to 34. 2020. Available online: https://www.census.gov/library/stories/2020/10/uninsured-rates-highest-for-young-adults-aged-19-to-34.html (accessed on 21 October 2022).
- Gabrani, J.; Schindler, C.; Wyss, K. Health Seeking Behavior Among Adults and Elderly with Chronic Health Condition(s) in Albania. Front. Public Health 2021, 9, 616014. [Google Scholar] [CrossRef]
- Deeks, A.; Lombard, C.; Michelmore, J.; Teede, H. The effects of gender and age on health related behaviors. BMC Public Health 2009, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Omaduvie, U.; Bachuwa, G.; Ayo-Yusuf, O.A.; Vardavas, C.I.; Agaku, I. State specific estimates of vaccine hesitancy among US adults. Popul. Med. 2021, 3, 1–9. [Google Scholar] [CrossRef]
- Hu, S.; Xiong, C.; Li, Q.; Wang, Z.; Jiang, Y. COVID-19 vaccine hesitancy cannot fully explain disparities in vaccination coverage across the contiguous United States. Vaccine 2022, 40, 5471–5482. [Google Scholar] [CrossRef]
- Mohammed, K.A.; Subramaniam, D.S.; Geneus, C.J.; Henderson, E.R.; Dean, C.A.; Subramaniam, D.P.; Burroughs, T.E. Rural-urban differences in human papillomavirus knowledge and awareness among US adults. Prev. Med. 2018, 109, 39–43. [Google Scholar] [CrossRef]
- Swiecki-Sikora, A.L.; Henry, K.A.; Kepka, D. HPV Vaccination Coverage Among US Teens Across the Rural-Urban Continuum. J. Rural Health. 2019, 35, 506–517. [Google Scholar] [CrossRef]
- Adjei Boakye, E.; Babatunde, O.A.; Wang, M.; Osazuwa-Peters, N.; Jenkins, W.; Lee, M.; Kim, M. Geographic Variation in Human Papillomavirus Vaccination Initiation and Completion Among Young Adults in the U.S. Am. J. Prev. Med. 2021, 60, 387–396. [Google Scholar] [CrossRef]
- Firenze, A.; Marsala, M.G.; Bonanno, V.; Maranto, M.; Ferrara, C.; Giovannelli, L.; Restivo, V. Facilitators and barriers HPV unvaccinated girls after 5 years of program implementation. Hum. Vaccines Immunother. 2015, 11, 240–244. [Google Scholar] [CrossRef]
- Zimet, G.D.; Weiss, T.W.; Rosenthal, S.L.; Good, M.B.; Vichnin, M.D. Reasons for non-vaccination against HPV and future vaccination intentions among 19–26 year-old women. BMC Womens Health 2010, 10, 27. [Google Scholar] [CrossRef]
- Patty, N.J.S.; van Dijk, H.M.; Wallenburg, I.; Bal, R.; Helmerhorst, T.J.M.; van Exel, J.; Cramm, J.M. To vaccinate or not to vaccinate? Perspectives on HPV vaccination among girls, boys, and parents in the Netherlands: A Q-methodological study. BMC Public Health 2017, 17, 872. [Google Scholar] [CrossRef] [PubMed]
- Giambi, C.; D’Ancona, F.; Del Manso, M.; De Mei, B.; Giovannelli, I.; Cattaneo, C.; Possenti, V.; Declich, S. Local Representatives for VALORE. Exploring reasons for non-vaccination against human papillomavirus in Italy. BMC Infect. Dis. 2014, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Liddon, N.C.; Hood, J.E.; Leichliter, J.S. Intent to receive HPV vaccine and reasons for not vaccinating among unvaccinated adolescent and young women: Findings from the 2006–2008 National Survey of Family Growth. Vaccine 2012, 30, 2676–2682. [Google Scholar] [CrossRef] [PubMed]
| Variables | Incomplete (N, %) | Complete (N, %) | Total (N, %) | p-Value |
|---|---|---|---|---|
| Individual-level factors | 4055 (52.92) | 3607 (47.08) | 7662 (100) | |
| Sex | ||||
| Male | 1425 (54.85) | 1173 (45.15) | 2598 (100) | |
| Female | 2630 (51.94) | 2434 (48.06) | 5064 (100) | <0.05 |
| Age in years | ||||
| 27–30 | 1174 (64.33) | 651 (35.67) | 1825 (100) | |
| 31–40 | 2144 (50.88) | 2070 (49.12) | 4214 (100) | |
| 41–45 | 737 (45.41) | 886 (54.59) | 1623 (100) | <0.0001 |
| Obesity | ||||
| Normal | 3910 (52.94) | 3476 (47.06) | 7386 (100) | |
| Obese | 145 (52.54) | 131 (47.46) | 276 (100) | 0.896 |
| Smoking | ||||
| Non-smoker | 3900 (52.76) | 3492 (47.24) | 7392 (100) | |
| Smoker | 155 (57.41) | 115 (42.59) | 270 (100) | 0.133 |
| Charlson comorbidity index | ||||
| None | 3729 (52.65) | 3353 (47.35) | 7082 (100) | |
| One | 189 (55.92) | 149 (44.08) | 338 (100) | |
| Two or more | 137 (56.61) | 105 (43.39) | 242 (100) | 0.254 |
| Contextual-level factors | N = 3839 | |||
| Percentage of high school graduate | ||||
| ≤88.2 | 1048 (54.53) | 874 (45.47) | 1922 (100) | |
| 88.2–93.1 | 1039 (53.17) | 915 (46.83) | 1954 (100) | |
| 93.1–95.9 | 1020 (53.12) | 900 (46.88) | 1920 (100) | |
| ≥95.9 | 948 (50.80) | 918 (49.20) | 1866 (100) | 0.143 |
| Household income | ||||
| ≤USD 58,782 | 1051 (54.85) | 865 (45.15) | 1916 (100) | |
| USD 58,782–76,288.5 | 1029 (53.73) | 886 (46.27) | 1915 (100) | |
| USD 76,288.5–97,944 | 1008 (52.61) | 908 (47.39) | 1916 (100) | |
| ≥USD 97,944 | 967 (50.50) | 948 (49.50) | 1915 (100) | <0.05 |
| Region | ||||
| Northeast | 489 (50.62) | 477 (49.38) | 966 (100) | |
| Mid-west | 994 (52.96) | 883 (47.04) | 1877 (100) | |
| West | 1518 (52.00) | 1401 (48.00) | 2919 (100) | |
| South | 1054 (55.47) | 846 (44.53) | 1900 (100) | <0.05 |
| Variable | Model 1 a | Model 2 b | Model 3 c | Model 4 d |
|---|---|---|---|---|
| Fixed Effects | OR (CrI) | aOR (CrI) | aOR (CrI) | aOR (CrI) |
| Individual-level factors | ||||
| Sex | ||||
| Female (vs. Male) | 1.09 (0.99–1.20) | 1.09 (0.99–1.20) | ||
| Age in years | ||||
| 27–30 | 1 (reference) | 1 (reference) | ||
| 31–40 | 0.57 (0.51–0.64) | 0.57 (0.51–0.64) | ||
| 41–45 | 0.45 (0.39–0.52) | 0.46 (0.40–0.53) | ||
| Obesity | ||||
| No obese | 1 (reference) | 1 (reference) | ||
| Obese | 1.01 (0.79–1.30) | 1.01 (0.79–1.30) | ||
| Smoking status | ||||
| Non-smoker | 1 (reference) | 1 (reference) | ||
| Smoker | 1.24 (0.96–1.59) | 1.22 (0.95–1.57) | ||
| Charlson comorbidity index | ||||
| None | 1 (reference) | 1 (reference) | ||
| One | 1.21 (0.97–1.52) | 1.21 (0.97–1.52) | ||
| Two or more | 1.20 (0.92–1.57) | 1.18 (0.90–1.54) | ||
| Contextual-level factors | ||||
| Percentage of high school graduate | ||||
| ≤88.2 | 1 (reference) | 1 (reference) | ||
| 88.2–93.1 | 0.97 (0.85–1.11) | 0.99 (0.86–1.13) | ||
| 93.1–95.9 | 1.01 (0.87–1.17) | 1.01 (0.88–1.18) | ||
| ≥95.9 | 0.93 (0.79–1.09) | 0.96 (0.81–1.13) | ||
| Household income | ||||
| ≤USD 58,782 | 1 (reference) | 1 (reference) | ||
| USD 58,782–76,288.5 | 0.95 (0.83–1.09) | 0.97 (0.84–1.11) | ||
| USD 76,288.5–97,944 | 0.91 (0.79–1.06) | 0.95 (0.82–1.11) | ||
| ≥USD 97,944 | 0.85 (0.72–1.00) | 0.90 (0.76–1.07) | ||
| Region | ||||
| Northeast | 1 (reference) | 1 (reference) | ||
| Mid-west | 1.08 (0.91–1.27) | 1.07 (0.90–1.26) | ||
| West | 1.03 (0.88–1.20) | 1.03 (0.88–1.22) | ||
| South | 1.22 (1.03–1.43) | 1.21 (1.03–1.40) | ||
| Measures of variation | ||||
| Variance (SE) | 0.050 (0.223) | 0.048 (0.218) | 0.042 (0.204) | 0.040 (0.199) |
| Explained variation (%) | Reference | 3.7 | 16.3 | 19.6 |
| Intra-cluster correlation (%) | 14.9 | 14.4 | 12.5 | 12.0 |
| MOR | 1.24 | 1.23 | 1.21 | 1.21 |
| Model fit statistics | ||||
| Bayesian DIC | 10,611.5 | 10,522.0 | 10,673.7 | 10,590.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adekanmbi, V.; Guo, F.; Hsu, C.D.; Shan, Y.; Kuo, Y.-F.; Berenson, A.B. Incomplete HPV Vaccination among Individuals Aged 27–45 Years in the United States: A Mixed-Effect Analysis of Individual and Contextual Factors. Vaccines 2023, 11, 820. https://doi.org/10.3390/vaccines11040820
Adekanmbi V, Guo F, Hsu CD, Shan Y, Kuo Y-F, Berenson AB. Incomplete HPV Vaccination among Individuals Aged 27–45 Years in the United States: A Mixed-Effect Analysis of Individual and Contextual Factors. Vaccines. 2023; 11(4):820. https://doi.org/10.3390/vaccines11040820
Chicago/Turabian StyleAdekanmbi, Victor, Fangjian Guo, Christine D. Hsu, Yong Shan, Yong-Fang Kuo, and Abbey B. Berenson. 2023. "Incomplete HPV Vaccination among Individuals Aged 27–45 Years in the United States: A Mixed-Effect Analysis of Individual and Contextual Factors" Vaccines 11, no. 4: 820. https://doi.org/10.3390/vaccines11040820
APA StyleAdekanmbi, V., Guo, F., Hsu, C. D., Shan, Y., Kuo, Y.-F., & Berenson, A. B. (2023). Incomplete HPV Vaccination among Individuals Aged 27–45 Years in the United States: A Mixed-Effect Analysis of Individual and Contextual Factors. Vaccines, 11(4), 820. https://doi.org/10.3390/vaccines11040820






