COVID-19 Vaccination Effectiveness in the General Population of an Italian Province: Two Years of Follow-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
- -
- COVID-19 vaccinations (up to 31 December 2022);
- -
- SARS-CoV-2 PCR or rapid antigenic tests and COVID-19 cases (up to 15 February 2023, in order to allow a minimum of 45 days of follow-up);
- -
- Demographic (Italian “Anagrafica”, up to 15 February 2023).
2.2. Outcomes
- (a)
- infection with SARS-CoV-2, evaluated using RT-PCR; tested through nasopharyngeal swabs by the laboratories accredited by the region (throughout the follow-up), or using rapid antigen tests, performed by accredited laboratories or local pharmacies (only after January 2021);
- (b)
- COVID-19 severe disease, diagnosed by a specialist physician, virologically confirmed, and requiring hospital admission;
- (c)
- COVID-19-related death (inside or outside the hospital);
- (d)
- all-cause death.
2.3. Exposure—Vaccinations
- (a)
- subjects who received no dose of vaccine were included in the reference group “unvaccinated”;
- (b)
- subjects who received only one dose of vaccine—including mRNA-1273, BNT162b2, ChAdOx1 nCoV-19, or NVX-CoV2373—were assigned to the group “1 dose”;
- (c)
- subjects who received only one dose of JNJ-78436735 vaccine, or only two doses of the other vaccines—BNT162b2, mRNA-1273, ChAdOx1 nCoV-19 or NVX-CoV2373—were assigned to the group “2 doses”;
- (d)
- subjects who received two or more doses of vaccines, if one of the administered vaccines was JNJ-78436735, or ≥3 doses of the other vaccines—BNT162b2, mRNA-1273, ChAdOx1 nCoV-19, or NVX-CoV2373—were assigned to the group “≥3 doses”.
2.4. Follow-Up
- (a)
- 16 January 2021, for the group “unvaccinated”;
- (b)
- two weeks after the single vaccination (to account for seroconversion time) for the group “1 dose”;
- (c)
- two weeks after the second dose of vaccine for the group “2 doses”;
- (d)
- two weeks after the third dose of vaccine for the group “≥3 doses”.
2.5. Statistical Analyses
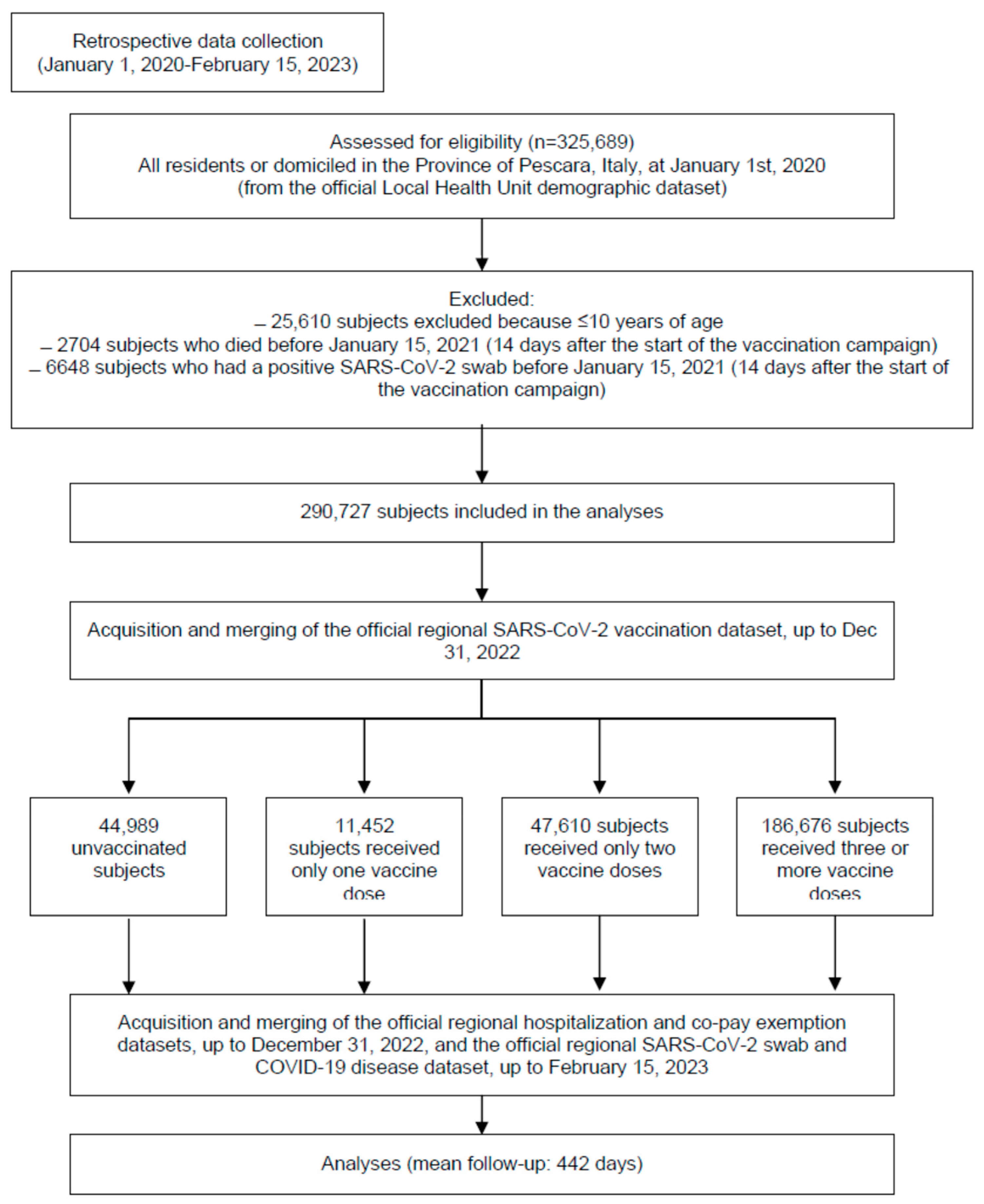
3. Results
3.1. Characteristics of the Sample
3.2. SARS-CoV-2 Infection (Table 2)
| Unvaccinated | 1 Dose A | 2 Doses B | 3/4 Doses C | p-Value G | Total Sample | |
|---|---|---|---|---|---|---|
| Overall sample D, n | 52,106 | 6341 | 50,359 | 181,921 | 290,727 | |
| Positive swabs, % (n) | 38.9 (20,283) | 34.4 (2181) | 60.0 (30,221) | 35.7 (64,874) | 40.4 (117,559) | |
| Gender | ||||||
| Males, n | 26,966 | 3229 | 24,830 | 86,932 | 141,957 | |
| Positive swabs, % (n) | 35.7 (9627) | 31.8 (1027) | 56.7 (14,072) | 33.7 (29,306) | 38.1 (54,032) | |
| Females, n | 25,140 | 3112 | 25,529 | 94,989 | 148,770 | |
| Positive swabs, % (n) | 42.4 (10,656) | 37.1 (1154) | 63.3 (16,149) | 37.4 (35,568) | V | 42.7 (63,527) |
| Age-class | ||||||
| 10–29 years, n | 13,652 | 2160 | 17,249 | 30,113 | 63,174 | |
| Positive swabs, % (n) | 43.7 (5964) | 34.9 (754) | 62.2 (10,722) | 33.6 (10,106) | V | 43.6 (27,546) |
| 30–59 years, n | 27,310 | 2978 | 24,485 | 82,102 | 136,875 | |
| Positive swabs, % (n) | 38.3 (10,447) | 38.1 (1133) | 63.8 (15,617) | 39.4 (32,382) | I; V | 43.5 (59,578) |
| 60+ years, n | 11,144 | 1203 | 8625 | 69,706 | 90,678 | |
| Positive swabs, % (n) | 34.8 (3872) | 24.4 (294) | 45.0 (3882) | 32.1 (22,387) | 33.6 (30,435) | |
| Vaccine type E | ||||||
| BNT162b2, n | -- | 3522 | 33,591 | 70,110 | 107,223 | |
| Positive swabs, % (n) | -- | 35.1 (1237) | 60.8 (20,406) | 35.7 (24,991) | V | 43.5 (46,634) |
| mRNA-1273, n | -- | 2423 | 10,583 | 20,150 | 33,156 | |
| Positive swabs, % (n) | -- | 30.1 (729) | 49.6 (5248) | 34.2 (6886) | 38.8 (12,863) | |
| Mixed vaccines F, n | -- | -- | 3789 | 91,661 | 95,450 | |
| Positive swabs, % (n) | -- | -- | 76.6 (2904) | 36.0 (32,997) | 37.6 (35,901) |
3.3. Severe COVID-19 (Table 4)
| Unvaccinated | 1 Dose A | 2 Doses B | 3/4 Doses C | p-Value G | Total Sample | |
|---|---|---|---|---|---|---|
| Overall sample * D, n | 20,283 | 2180 | 30,219 | 64,877 | 117,559 | |
| COVID-19, % (n) | 7.96 (1615) | 3.07 (67) | 1.59 (481) | 1.64 (1063) | 2.74 (3226) | |
| Gender | ||||||
| Males, n | ||||||
| COVID-19, % (n) | 9627 | 1027 | 14,072 | 29,306 | 54,032 | |
| 9.40 (905) | 2.83 (29) | 1.71 (240) | 1.84 (539) | 3.17 (1713) | ||
| Females, n | ||||||
| COVID-19, % (n) | 10,656 | 1154 | 16,149 | 35,568 | 63,527 | |
| 6.66 (710) | 3.29 (38) | 1.29 (241) | 1.47 (524) | 2.38 (1513) | ||
| Age-class | ||||||
| 10–29 years, n | 5964 | 754 | 10,722 | 10,106 | 27,546 | |
| COVID-19, % (n) | 0.57 (34) | 0.13 (1) | 0.12 (13) | 0.11 (11) | IV; V | 0.21 (59) |
| 30–59 years, n | 10,447 | 1133 | 15,617 | 32,382 | 59,578 | |
| COVID-19, % (n) | 5.34 (558) | 1.50 (17) | 0.77 (121) | 0.49 (159) | 1.44 (855) | |
| 60+ years, n | 3872 | 294 | 3882 | 22,387 | 30,435 | |
| COVID-19, % (n) | 26.4 (1023) | 16.7 (49) | 8.94 (347) | 3.99 (893) | 7.60 (2312) | |
| Vaccine type E | ||||||
| BNT162b2, n | -- | 1237 | 20,406 | 24,991 | 46,634 | |
| COVID-19, % (n) | -- | 3.56 (44) | 1.68 (342) | 2.48 (621) | 2.16 (1007) | |
| mRNA-1273, n | -- | 729 | 5248 | 6886 | 12,863 | |
| COVID-19, % (n) | -- | 1.65 (12) | 0.99 (52) | 1.07 (74) | 1.07 (138) | |
| Mixed vaccines F, n | -- | -- | 3789 | 91,661 | 95,450 | |
| COVID-19, % (n) | -- | -- | 1.32 (50) | 0.40 (368) | 0.44 (418) |
3.4. COVID-19-Related Death (Table 5)
| Unvaccinated | 1 Dose A | 2 Doses B | 3/4 Doses C | p-Value G | Total Sample | |
|---|---|---|---|---|---|---|
| Overall sample * D, n | 13,166 | 7292 | 27,472 | 69,629 | 117,559 | |
| Deaths, % (n) | 4.38 (577) | 0.88 (64) | 0.84 (232) | 0.93 (650) | 1.30 (1523) | |
| Gender | ||||||
| Males, n | 5998 | 3648 | 12,771 | 31,615 | 54,032 | |
| Deaths, % (n) | 4.87 (292) | 0.85 (31) | 0.94 (120) | 0.99 (314) | 1.40 (757) | |
| Females, n | 7168 | 3644 | 14,701 | 38,014 | 63,527 | |
| Deaths, % (n) | 3.98 (285) | 0.91 (33) | 0.76 (112) | 0.88 (336) | 1.21 (766) | |
| Age-class | ||||||
| 10–29 years, n | 3983 | 2286 | 10,023 | 11,254 | 27,546 | |
| Deaths, % (n) | 0.10 (4) | 0.00 (0) | 0.00 (0) | 0.02 (2) | I; V; VI | 0.02 (6) |
| 30–59 years, n | 6854 | 3867 | 14,179 | 34,678 | 59,578 | |
| Deaths, % (n) | 0.88 (60) | 0.23 (9) | 0.09 (13) | 0.11 (38) | 0.20 (120) | |
| 60+ years, n | 2329 | 1139 | 3270 | 23,697 | 30,435 | |
| Deaths, % (n) | 22.0 (513) | 4.83 (55) | 6.70 (219) | 2.57 (610) | 4.59 (1397) | |
| Vaccine type E | ||||||
| BNT162b2, n | -- | 3679 | 19,426 | 27,026 | 50,131 | |
| Deaths, % (n) | -- | 1.20 (44) | 0.90 (175) | 1.41 (380) | 1.19 (599) | |
| mRNA-1273, n | -- | 3532 | 5163 | 7271 | 15,966 | |
| Deaths, % (n) | -- | 0.54 (19) | 0.81 (42) | 0.92 (67) | IV; V | 0.80 (128) |
| Mixed vaccines F, n | -- | -- | 1219 | 35,332 | 36,551 | |
| Deaths, % (n) | -- | -- | 0.49 (6) | 0.57 (203) | 0.57 (209) |
3.5. All-Cause Death (Table 6)
| Unvaccinated | 1 Dose A | 2 Doses B | 3/4 Doses C | p-Value G | Total Sample | |
|---|---|---|---|---|---|---|
| Overall sample D, n | 44,989 | 11,452 | 47,610 | 186,676 | 290,727 | |
| Deaths, % (n) | 4.41 (1986) | 2.84 (325) | 4.13 (1964) | 1.36 (2546) | 2.35 (6821) | |
| Gender | ||||||
| Males, n | 23,337 | 5850 | 23,529 | 89,241 | 141,957 | |
| Deaths, % (n) | 3.93 (918) | 2.85 (167) | 4.18 (984) | 1.44 (1287) | II | 2.36 (3356) |
| Females, n | 21,652 | 5602 | 24,081 | 97,435 | 148,770 | |
| Deaths, % (n) | 4.93 (1068) | 2.82 (158) | 4.07 (980) | 1.29 (1259) | 2.33 (3465) | |
| Age-class | ||||||
| 10–29 years, n | 11,671 | 3692 | 16,550 | 31,261 | 63,174 | |
| Deaths, % (n) | 0.11 (13) | 0.11 (4) | 0.01 (2) | 0.02 (6) | I; VI | 0.04 (25) |
| 30–59 years, n | 23,717 | 5712 | 23,047 | 94,399 | 136,875 | |
| Deaths, % (n) | 0.74 (175) | 0.65 (37) | 0.46 (107) | 0.18 (153) | I; IV | 0.34 (472) |
| 60+ years, n | 9601 | 2048 | 8013 | 71,016 | 90,678 | |
| Deaths, % (n) | 18.7 (1798) | 13.9 (284) | 23.2 (1855) | 3.4 (2387) | 7.0 (6324) | |
| Vaccine type E | ||||||
| BNT162b2, n | -- | 5964 | 32,611 | 72,145 | 110,720 | |
| Deaths, % (n) | -- | 3.44 (205) | 4.28 (1397) | 1.92 (1383) | 2.70 (2985) | |
| mRNA-1273, n | -- | 5226 | 10,498 | 20,535 | 36,259 | |
| Deaths, % (n) | -- | 1.84 (96) | 4.43 (465) | 1.50 (308) | V | 2.40 (869) |
| Mixed vaccines F, n | -- | -- | 2104 | 93,996 | 96,100 | |
| Deaths, % (n) | -- | -- | 0.81 (17) | 0.91 (855) | VI | 0.91 (872) |
3.6. Additional Analyses: Omicron Predominance and Vaccine Types
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization [WHO]. Director-General’s Opening Remarks at the Media Briefing—5 May 2023. Available online: https://www.who.int/news-room/speeches/item/who-director-general-s-opening-remarks-at-the-media-briefing---5-may-2023 (accessed on 5 July 2023).
- Grana, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef]
- Ratajczak, P.; Banach, Z.; Kopciuch, D.; Paczkowska, A.; Zaprutko, T.; Krawczyk, J.; Maciuszek-Bartkowska, B.; Kus, K. Tozinameran (Pfizer, BioNTech) and Elasomeran (Moderna) Efficacy in COVID-19—A Systematic Review of Randomised Controlled Trial Studies. Healthcare 2023, 11, 1532. [Google Scholar] [CrossRef]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2022, 114, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ren, W.; Wu, T.; Wang, Q.; Luo, M.; Yi, Y.; Li, J. Real-World Safety of COVID-19 mRNA Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2023, 11, 1118. [Google Scholar] [CrossRef]
- Wu, N.; Joyal-Desmarais, K.; Ribeiro, P.A.B.; Vieira, A.M.; Stojanovic, J.; Sanuade, C.; Yip, D.; Bacon, S.L. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: Findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir. Med. 2023, 11, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef] [PubMed]
- Pather, S.; Madhi, S.A.; Cowling, B.J.; Moss, P.; Kamil, J.P.; Ciesek, S.; Muik, A.; Tureci, O. SARS-CoV-2 Omicron variants: Burden of disease, impact on vaccine effectiveness and need for variant-adapted vaccines. Front. Immunol. 2023, 14, 1130539. [Google Scholar] [CrossRef]
- Kulper-Schiek, W.; Piechotta, V.; Pilic, A.; Batke, M.; Dreveton, L.S.; Geurts, B.; Koch, J.; Koppe, S.; Treskova, M.; Vygen-Bonnet, S.; et al. Facing the Omicron variant-how well do vaccines protect against mild and severe COVID-19? Third interim analysis of a living systematic review. Front. Immunol. 2022, 13, 940562. [Google Scholar] [CrossRef] [PubMed]
- Meggiolaro, A.; Sane Schepisi, M.; Farina, S.; Castagna, C.; Mammone, A.; Siddu, A.; Stefanelli, P.; Boccia, S.; Rezza, G. Effectiveness of vaccination against SARS-CoV-2 Omicron variant infection, symptomatic disease, and hospitalization: A systematic review and meta-analysis. Expert Rev. Vaccines 2022, 21, 1831–1841. [Google Scholar] [CrossRef]
- Acuti Martellucci, C.; Flacco, M.E.; Soldato, G.; Di Martino, G.; Carota, R.; Caponetti, A.; Manzoli, L. Effectiveness of COVID-19 Vaccines in the General Population of an Italian Region before and during the Omicron Wave. Vaccines 2022, 10, 662. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Gram, M.A.; Emborg, H.D.; Schelde, A.B.; Friis, N.U.; Nielsen, K.F.; Moustsen-Helms, I.R.; Legarth, R.; Lam, J.U.H.; Chaine, M.; Malik, A.Z.; et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the Alpha, Delta, or Omicron SARS-CoV-2 variant: A nationwide Danish cohort study. PLoS Med. 2022, 19, e1003992. [Google Scholar] [CrossRef]
- Grewal, R.; Nguyen, L.; Buchan, S.A.; Wilson, S.E.; Nasreen, S.; Austin, P.C.; Brown, K.A.; Fell, D.B.; Gubbay, J.B.; Schwartz, K.L.; et al. Effectiveness of mRNA COVID-19 vaccine booster doses against Omicron severe outcomes. Nat. Commun. 2023, 14, 1273. [Google Scholar] [CrossRef]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 2022, 327, 639–651. [Google Scholar] [CrossRef]
- Atanasov, V.; Barreto, N.; Whittle, J.; Meurer, J.; Weston, B.W.; Luo, Q.E.; Franchi, L.; Yuan, A.Y.; Zhang, R.; Black, B. Understanding COVID-19 Vaccine Effectiveness against Death Using a Novel Measure: COVID Excess Mortality Percentage. Vaccines 2023, 11, 379. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Frankland, T.B.; Ackerson, B.K.; Takhar, H.S.; Ogun, O.A.; Simmons, S.R.; Zamparo, J.M.; et al. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: A retrospective cohort study. Lancet Reg. Health Am. 2022, 9, 100198. [Google Scholar] [CrossRef]
- Thompson, M.G.; Natarajan, K.; Irving, S.A.; Rowley, E.A.; Griggs, E.P.; Gaglani, M.; Klein, N.P.; Grannis, S.J.; DeSilva, M.B.; Stenehjem, E.; et al. Effectiveness of a Third Dose of mRNA Vaccines against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations among Adults during Periods of Delta and Omicron Variant Predominance—VISION Network, 10 States, August 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 139–145. [Google Scholar] [CrossRef]
- Mateo-Urdiales, A.; Sacco, C.; Fotakis, E.A.; Del Manso, M.; Bella, A.; Riccardo, F.; Bressi, M.; Rota, M.C.; Petrone, D.; Siddu, A.; et al. Relative effectiveness of monovalent and bivalent mRNA boosters in preventing severe COVID-19 due to omicron BA.5 infection up to 4 months post-administration in people aged 60 years or older in Italy: A retrospective matched cohort study. Lancet Infect. Dis. 2023. in print. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Lopez Bernal, J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat. Med. 2022, 28, 831–837. [Google Scholar] [CrossRef]
- Cocchio, S.; Zabeo, F.; Facchin, G.; Piva, N.; Venturato, G.; Marcon, T.; Saia, M.; Tonon, M.; Mongillo, M.; Da Re, F.; et al. Differences in Immunological Evasion of the Delta (B.1.617.2) and Omicron (B.1.1.529) SARS-CoV-2 Variants: A Retrospective Study on the Veneto Region’s Population. Int. J. Environ. Res. Public Health 2022, 19, 8179. [Google Scholar] [CrossRef]
- Flacco, M.E.; Soldato, G.; Acuti Martellucci, C.; Di Martino, G.; Carota, R.; Caponetti, A.; Manzoli, L. Risk of SARS-CoV-2 Reinfection 18 Months After Primary Infection: Population-Level Observational Study. Front. Public Health 2022, 10, 884121. [Google Scholar] [CrossRef]
- Paul, P.; El-Naas, A.; Hamad, O.; Salameh, M.A.; Mhaimeed, N.; Laswi, I.; Abdelati, A.A.; AlAnni, J.; Khanjar, B.; Al-Ali, D.; et al. Effectiveness of the pre-Omicron COVID-19 vaccines against Omicron in reducing infection, hospitalization, severity, and mortality compared to Delta and other variants: A systematic review. Hum. Vaccines Immunother. 2023, 19, 2167410. [Google Scholar] [CrossRef] [PubMed]
- Flacco, M.E.; Soldato, G.; Acuti Martellucci, C.; Carota, R.; Di Luzio, R.; Caponetti, A.; Manzoli, L. Interim Estimates of COVID-19 Vaccine Effectiveness in a Mass Vaccination Setting: Data from an Italian Province. Vaccines 2021, 9, 628. [Google Scholar] [CrossRef]
- Riccardo, F.; Andrianou, X.; Bella, A.; Del Manso, M.; Urdiales, A.M.; Fabiani, M.; Bellino, S.; Boros, S.; D’Ancona, F.; Rota, M.C.; et al. COVID-19 Integrated Surveillance System. Available online: https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza (accessed on 5 July 2023).
- Acuti Martellucci, C.; Flacco, M.E.; Cappadona, R.; Bravi, F.; Mantovani, L.; Manzoli, L. SARS-CoV-2 pandemic: An overview. Adv. Biol. Regul. 2020, 77, 100736. [Google Scholar] [CrossRef]
- Italian Government. Strategic Plan for Anti-SARS-CoV-2/COVID-19 Vaccination [Piano Strategico Nazionale dei Vaccini per la Prevenzione delle Infezioni da SARS-CoV-2]; Italian Government: Rome, Italy, 2020. [Google Scholar]
- Italian Ministry of Health. Cirular Letter n. 13824 of 22 February 2022. Nuvaxovid (Novavax) COVID-19 Vaccine Indication for Use in Individuals 18 Years of Age and older I. [Indicazione di Utilizzo del Vaccino Anti COVID-19 Nuvaxovid (Novavax) nei Soggetti di età Pari o Superiore a 18 Anni]; Italian Ministry of Health: Rome Italy, 2022. [Google Scholar]
- Italian Institute of Health. Characteristics of COVID-19 Patients Dying in Italy Report Based on Available Data on 10 January 2022. Available online: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-analysis-of-deaths-10-2-2022 (accessed on 5 July 2023).
- Italian Government. Interim recommendations on the target groups for SARS-CoV-2/COVID-19 vaccination [Raccomandazioni ad-interim sui gruppi target della vaccinazione anti SARS-CoV-2/COVID-19]. Gazz. Uff. Serie Generale. 2021, 72, 38–45. [Google Scholar]
- Flacco, M.E.; Acuti Martellucci, C.; Soldato, G.; Di Martino, G.; Carota, R.; De Benedictis, M.; Di Marco, G.; Parruti, G.; Di Luzio, R.; Caponetti, A.; et al. COVID-19 Vaccination Did Not Increase the Risk of Potentially Related Serious Adverse Events: 18-Month Cohort Study in an Italian Province. Vaccines 2022, 11, 31. [Google Scholar] [CrossRef]
- Song, S.; Madewell, Z.J.; Liu, M.; Longini, I.M.; Yang, Y. Effectiveness of SARS-CoV-2 vaccines against Omicron infection and severe events: A systematic review and meta-analysis of test-negative design studies. Front. Public Health 2023, 11, 1195908. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef]
- Singhal, S.; Kumar, P.; Singh, S.; Saha, S.; Dey, A.B. Clinical features and outcomes of COVID-19 in older adults: A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 321. [Google Scholar] [CrossRef]
- Li, Z.; Liu, S.; Li, F.; Li, Y.; Peng, P.; Li, S.; He, L.; Liu, T. Efficacy, immunogenicity and safety of COVID-19 vaccines in older adults: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 965971. [Google Scholar] [CrossRef]
- Yang, X.H.; Bao, W.J.; Zhang, H.; Fu, S.K.; Jin, H.M. The Efficacy of SARS-CoV-2 Vaccination in the Elderly: A Systemic Review and Meta-analysis. J. Gen. Intern. Med. 2023, 1–9, in print. [Google Scholar] [CrossRef]
- World Health Organization [WHO]. COVID-19 Strategic Preparedness and Response Plan; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Bhopal, S.S.; Bagaria, J.; Olabi, B.; Bhopal, R. Children and young people remain at low risk of COVID-19 mortality. Lancet Child Adolesc. Health 2021, 5, e12–e13. [Google Scholar] [CrossRef] [PubMed]
- Katoto, P.D.; Tamuzi, J.L.; Brand, A.S.; Marangu, D.M.; Byamungu, L.N.; Wiysonge, C.S.; Gray, G. Effectiveness of COVID-19 Pfizer-BioNTech (BNT162b2) mRNA vaccination in adolescents aged 12–17 years: A systematic review and meta-analysis. Hum. Vaccines Immunother. 2023, 19, 2214495. [Google Scholar] [CrossRef]
- Cortes, J.; Aguiar, P.M.V.; Ferrinho, P. COVID-19-related adolescent mortality and morbidity in nineteen European countries. Eur. J. Pediatr. 2023. in print. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Fuller, T.; Cambou, M.C.; Segura, E.R.; Kamau, E.; Yang, S.; Garner, O.B.; Nielsen-Saines, K. Epidemiology and SARS-CoV-2 Infection Patterns among Youth Followed at a Large Los Angeles Health Network during 2020–2022: Clinical Presentation, Prevalent Strains, and Correlates of Disease Severity. Vaccines 2023, 11, 1024. [Google Scholar] [CrossRef] [PubMed]
- Mallah, N.; Pardo-Seco, J.; Lopez-Perez, L.R.; Gonzalez-Perez, J.M.; Roson, B.; Otero-Barros, M.T.; Duran-Parrondo, C.; Nartallo-Penas, V.; Miras-Carballal, S.; Rodriguez-Tenreiro, C.; et al. Effectiveness of COVID-19 vaccine booster in the general population and in subjects with comorbidities. A population-based study in Spain. Environ. Res. 2022, 215, 114252. [Google Scholar] [CrossRef]
- Flacco, M.E.; Acuti Martellucci, C.; Soldato, G.; Carota, R.; Fazii, P.; Caponetti, A.; Manzoli, L. Rate of reinfections after SARS-CoV-2 primary infection in the population of an Italian province: A cohort study. J. Public Health 2022, 44, e475–e478. [Google Scholar] [CrossRef]
- Italian Government. Decree-Law no. 105 of 23 July 2021. Urgent Measures to Deal with the Epidemiological Emergency from COVID-19 and for the Safe Exercise of Social and Economic Activities [Misure Urgenti per Fronteggiare l’emergenza Epidemiologica da COVID-19 e per l’esercizio in Sicurezza di Attivita’ Sociali ed Economiche]; Italian Government: Rome, Italy, 2021. [Google Scholar]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Italian Ministry of Health. Circular Letter n.9498 of 4 April 2022. Update on Quarantine and Self-Surveillance Measures for Close (High-Risk) Contacts of SARS-CoV-2 Infection Cases [Aggiornamento Sulle Misure di Quarantena e Autosorveglianza per i Contatti Stretti (ad Alto Rischio) di Casi di Infezione da SARS-Cov-2]; Italian Ministry of Health: Rome, Italy, 2022. [Google Scholar]
- de Meijere, G.; Valdano, E.; Castellano, C.; Debin, M.; Kengne-Kuetche, C.; Turbelin, C.; Noel, H.; Weitz, J.S.; Paolotti, D.; Hermans, L.; et al. Attitudes towards booster, testing and isolation, and their impact on COVID-19 response in winter 2022/2023 in France, Belgium, and Italy: A cross-sectional survey and modelling study. Lancet Reg. Health Eur. 2023, 28, 100614. [Google Scholar] [CrossRef]
- Domoslawska-Zylinska, K.; Krysinska-Pisarek, M.; Czabanowska, K.; Sesa, G. Vaccinated and Unvaccinated Risk Perceptions and Motivations for COVID-19 Preventive Measures Based on EPPM-A Polish Qualitative Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 13473. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Garcia-Beltran, W.F.; Chang, C.C.; Berrios Mairena, C.; Thierauf, J.C.; Kirkpatrick, G.; Onozato, M.L.; Cheng, J.; St Denis, K.J.; Lam, E.C.; et al. Comparative Immunogenicity and Effectiveness of mRNA-1273, BNT162b2, and Ad26.COV2.S COVID-19 Vaccines. J. Infect. Dis. 2022, 225, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Mayr, F.B.; Talisa, V.B.; Shaikh, O.S.; Omer, S.B.; Butt, A.A.; Yende, S. Comparative COVID-19 Vaccine Effectiveness over Time in Veterans. Open Forum. Infect. Dis. 2022, 9, ofac311. [Google Scholar] [CrossRef]
- Hulme, W.J.; Horne, E.M.F.; Parker, E.P.K.; Keogh, R.H.; Williamson, E.J.; Walker, V.; Palmer, T.M.; Curtis, H.J.; Walker, A.J.; Andrews, C.D.; et al. Comparative effectiveness of BNT162b2 versus mRNA-1273 COVID-19 vaccine boosting in England: Matched cohort study in OpenSAFELY-TPP. BMJ 2023, 380, e072808. [Google Scholar] [CrossRef] [PubMed]
- Self, W.H.; Tenforde, M.W.; Rhoads, J.P.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults without Immunocompromising Conditions—United States, March–August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Hung Nguyen, V.; Boileau, C.; Bogdanov, A.; Sredl, M.; Bonafede, M.; Ducruet, T.; Chavers, S.; Rosen, A.; Martin, D.; Buck, P.; et al. Relative Effectiveness of BNT162b2, mRNA-1273, and Ad26.COV2.S Vaccines and Homologous Boosting in Preventing COVID-19 in Adults in the US. Open Forum. Infect. Dis. 2023, 10, ofad288. [Google Scholar] [CrossRef]
- Atanasov, V.; Barreto, N.; Whittle, J.; Meurer, J.; Weston, B.W.; Luo, Q.E.; Yuan, A.Y.; Franchi, L.; Zhang, R.; Black, B. Selection Effects and COVID-19 Mortality Risk after Pfizer vs. Moderna Vaccination: Evidence from Linked Mortality and Vaccination Records. Vaccines 2023, 11, 971. [Google Scholar] [CrossRef]
- Cheng, H.; Peng, Z.; Si, S.; Alifu, X.; Zhou, H.; Chi, P.; Zhuang, Y.; Mo, M.; Yu, Y. Immunogenicity and Safety of Homologous and Heterologous Prime-Boost Immunization with COVID-19 Vaccine: Systematic Review and Meta-Analysis. Vaccines 2022, 10, 798. [Google Scholar] [CrossRef]
- Costa Clemens, S.A.; Weckx, L.; Clemens, R.; Almeida Mendes, A.V.; Ramos Souza, A.; Silveira, M.B.V.; da Guarda, S.N.F.; de Nobrega, M.M.; de Moraes Pinto, M.I.; Gonzalez, I.G.S.; et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): A phase 4, non-inferiority, single blind, randomised study. Lancet 2022, 399, 521–529. [Google Scholar] [CrossRef]
- Andersson, N.W.; Thiesson, E.M.; Baum, U.; Pihlstrom, N.; Starrfelt, J.; Faksova, K.; Poukka, E.; Lund, L.C.; Hansen, C.H.; Aakjaer, M.; et al. Comparative effectiveness of heterologous third dose vaccine schedules against severe covid-19 during omicron predominance in Nordic countries: Population based cohort analyses. BMJ 2023, 382, e074325. [Google Scholar] [CrossRef]
- Doerre, A.; Doblhammer, G. The influence of gender on COVID-19 infections and mortality in Germany: Insights from age- and gender-specific modeling of contact rates, infections, and deaths in the early phase of the pandemic. PLoS ONE 2022, 17, e0268119. [Google Scholar] [CrossRef]
- Bravi, F.; Flacco, M.E.; Carradori, T.; Volta, C.A.; Cosenza, G.; De Togni, A.; Acuti Martellucci, C.; Parruti, G.; Mantovani, L.; Manzoli, L. Predictors of severe or lethal COVID-19, including Angiotensin Converting Enzyme inhibitors and Angiotensin II Receptor Blockers, in a sample of infected Italian citizens. PLoS ONE 2020, 15, e0235248. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, L.; Chen, G. Is there a difference in the efficacy of COVID-19 vaccine in males and females?—A systematic review and meta-analysis. Hum. Vaccines Immunother. 2021, 17, 4741–4746. [Google Scholar] [CrossRef]
- Heidari, S.; Palmer-Ross, A.; Goodman, T. A Systematic Review of the Sex and Gender Reporting in COVID-19 Clinical Trials. Vaccines 2021, 9, 1322. [Google Scholar] [CrossRef]
- Jensen, A.; Stromme, M.; Moyassari, S.; Chadha, A.S.; Tartaglia, M.C.; Szoeke, C.; Ferretti, M.T. COVID-19 vaccines: Considering sex differences in efficacy and safety. Contemp. Clin. Trials 2022, 115, 106700. [Google Scholar] [CrossRef] [PubMed]
- Sulis, G.; Kim, J.Y.; Rodrigue, V.; Gore, G.; Peebles, A.; Ulrich, A.K.; Horn, M.; Basta, N.E. Sex-disaggregated effectiveness data reporting in COVID-19 vaccine research: A systematic review. Commun. Med. 2023, 3, 69. [Google Scholar] [CrossRef]
- Zintel, S.; Flock, C.; Arbogast, A.L.; Forster, A.; von Wagner, C.; Sieverding, M. Gender differences in the intention to get vaccinated against COVID-19: A systematic review and meta-analysis. J. Public Health 2023, 31, 1303–1327. [Google Scholar] [CrossRef]
- Yek, C.; Warner, S.; Mancera, A.; Kadri, S.S. Misclassification bias in estimating clinical severity of SARS-CoV-2 variants. Lancet 2022, 400, 809. [Google Scholar] [CrossRef] [PubMed]
- World Health Organizatin [WHO]. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants (accessed on 5 July 2023).
- Sullivan, S.G.; Feng, S.; Cowling, B.J. Potential of the test-negative design for measuring influenza vaccine effectiveness: A systematic review. Expert Rev. Vaccines 2014, 13, 1571–1591. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhao, Y.; Wang, S.; He, H.; Chen, Z.; Zhou, Y.; Yan, R.; Tang, X.; Zhu, Y.; Xu, X. Assessing COVID-19 Vaccine Booster Hesitancy Using the Modified 5C Scale in Zhejiang Province, China: A Cross-Sectional Study. Vaccines 2023, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Whiting, P.F.; Brush, J.E. Interpreting a covid-19 test result. BMJ 2020, 369, m1808. [Google Scholar] [CrossRef] [PubMed]
- Bendavid, E.; Mulaney, B.; Sood, N.; Shah, S.; Bromley-Dulfano, R.; Lai, C.; Weissberg, Z.; Saavedra-Walker, R.; Tedrow, J.; Bogan, A.; et al. COVID-19 antibody seroprevalence in Santa Clara County, California. Int. J. Epidemiol. 2021, 50, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. Over- and under-estimation of COVID-19 deaths. Eur. J. Epidemiol. 2021, 36, 581–588. [Google Scholar] [CrossRef]
- Axfors, C.; Ioannidis, J.P.A. Infection fatality rate of COVID-19 in community-dwelling elderly populations. Eur. J. Epidemiol. 2022, 37, 235–249. [Google Scholar] [CrossRef] [PubMed]
| Unvaccinated | 1 Dose A | 2 Doses B | 3/4 Doses C | Total Sample | |
|---|---|---|---|---|---|
| (n = 44,989) | (n = 11,452) | (n = 47,610) | (n = 186,676) | (n = 290,727) | |
| Mean age in years (SD) | 44.4 (20.2) | 41.9 (19.3) | 40.7 (20.3) | 52.5 (20.2) | 48.9 (20.8) |
| Gender | % | % | % | % | % (n) |
| Females | 14.6 | 3.8 | 16.2 | 65.5 | 51.2 (148,770) |
| Males | 16.4 | 4.1 | 16.6 | 62.9 | 48.8 (141,957) |
| Age class in years | |||||
| 10–29 | 18.5 | 5.8 | 26.2 | 49.5 | 21.7 (63,174) |
| 30–59 | 17.3 | 4.2 | 16.8 | 61.7 | 47.1 (136,875) |
| 60 or more | 10.6 | 2.3 | 8.8 | 78.3 | 31.2 (90,678) |
| Risk factors and comorbidities D | |||||
| No hypertension | 16.7 | 4.2 | 17.5 | 61.6 | 86.2 (250,472) |
| Hypertension | 8.1 | 2.1 | 9.7 | 80.1 | 13.9 (40,255) |
| No diabetes | 15.9 | 4.0 | 16.7 | 63.4 | 94.6 (275,128) |
| Diabetes | 8.8 | 2.5 | 10.0 | 78.7 | 5.4 (15,599) |
| No CVD | 16.0 | 3.9 | 16.8 | 63.3 | 92.0 (267,475) |
| CVD | 10.0 | 4.1 | 11.5 | 74.5 | 8.0 (23,252) |
| No COPD | 15.6 | 3.9 | 16.4 | 64.0 | 96.2 (279,692) |
| COPD | 11.2 | 3.7 | 15.5 | 69.6 | 3.8 (11,035) |
| No kidney disease | 15.5 | 4.0 | 16.4 | 64.1 | 98.1 (285,296) |
| Kidney disease | 13.1 | 2.8 | 14.3 | 69.8 | 1.9 (5431) |
| No cancer | 15.9 | 4.0 | 16.7 | 63.4 | 94.3 (274,147) |
| Cancer | 9.1 | 2.3 | 11.3 | 77.3 | 5.7 (16,580) |
| Type of vaccine E | |||||
| BNT162b2 | -- | 52.1 | 68.5 | 38.6 | 45.1 (110,720) |
| mRNA-1273 | -- | 45.6 | 22.0 | 11.0 | 14.8 (36,259) |
| ChAdOx1 nCoV-19 | -- | 2.0 | 3.7 | 0.0 | 0.8 (2007) |
| JNJ-78436735 | -- | -- | 1.1 | 0.0 | 0.2 (508) |
| NVX-CoV2373 | -- | 0.0 | 4.4 | 0.0 | 0.1 (144) |
| Mixed F | -- | 0.3 | 0.2 | 50.4 | 39.1 (96,100) |
| Mean follow-up in days (SD) G | 750 (123) | 404 (107) | 472 (108) | 400 (52) | 466 (148) |
| Outcomes | SARS-CoV-2 | COVID-19 B | COVID-19-Related Death B | All-Cause Death |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Vaccine doses | ||||
| Unvaccinated | 1 (Ref. cat.) | 1 (Ref. cat.) | 1 (Ref. cat.) | 1 (Ref. cat.) |
| 1 dose C | 1.26 (1.21–1.32) * | 0.47 (0.37–0.60) * | 0.36 (0.28–0.47) * | 1.40 (1.24–1.58) * |
| 2 doses D | 2.41 (2.37–2.46) * | 0.27 (0.24–0.30) * | 0.38 (0.32–0.44) * | 1.36 (1.28–1.45) * |
| 3/4 doses E | 1.27 (1.25–1.29) * | 0.12 (0.11–0.13) * | 0.15 (0.14–0.17) * | 0.22 (0.20–0.23) * |
| Age class, years | ||||
| 60 or more | ||||
| Unvaccinated | 1 (Ref. cat.) | 1 (Ref. cat.) | 1 (Ref. cat.) | 1 (Ref. cat.) |
| 1 dose C | 1.07 (0.95–1.20) | 0.58 (0.44–0.77) * | 0.36 (0.27–0.48) * | 1.40 (1.23–1.59) * |
| 2 doses D | 1.91 (1.82–2.00) * | 0.35 (0.31–0.40) * | 0.43 (0.37–0.50) * | 1.45 (1.36–1.56) * |
| 3/4 doses E | 1.09 (1.05–1.13) * | 0.14 (0.13–0.16) * | 0.16 (0.14–0.18) * | 0.22 (0.20–0.23) * |
| 30–59 | ||||
| Unvaccinated | 1 (Ref. cat.) | 1 (Ref. cat.) | 1 (Ref. cat.) | 1 (Ref. cat.) |
| 1 dose C | 1.46 (1.38–1.56) * | 0.33 (0.20–0.53) * | 0.29 (0.14–0.60) * | 1.19 (0.82–1.72) |
| 2 doses D | 2.73 (2.66–2.81) * | 0.16 (0.13–0.19) * | 0.12 (0.07–0.23) * | 0.78 (0.61–1.00) |
| 3/4 doses E | 1.46 (1.43–1.50) * | 0.08 (0.07–0.10) * | 0.11 (0.07–0.17) * | 0.20 (0.16–0.25) * |
| 10–29 | ||||
| Unvaccinated | 1 (Ref. cat.) | 1 (Ref. cat.) | 1 (Ref. cat.) | 1 (Ref. cat.) |
| 1 dose C | 1.13 (1.05–1.22) * | 0.25 (0.03–1.86) | NE | 1.57 (0.49–4.97) |
| 2 doses D | 2.10 (2.03–2.17) * | 0.21 (0.11–0.40) * | NE | 0.17 (0.04–0.76) ** |
| 3/4 doses E | 1.01 (0.98–1.04) | 0.17 (0.08–0.33) * | 0.20 (0.03–1.24) | 0.22 (0.08–0.59) ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosso, A.; Flacco, M.E.; Soldato, G.; Di Martino, G.; Acuti Martellucci, C.; Carota, R.; De Benedictis, M.; Di Marco, G.; Di Luzio, R.; Fiore, M.; et al. COVID-19 Vaccination Effectiveness in the General Population of an Italian Province: Two Years of Follow-Up. Vaccines 2023, 11, 1325. https://doi.org/10.3390/vaccines11081325
Rosso A, Flacco ME, Soldato G, Di Martino G, Acuti Martellucci C, Carota R, De Benedictis M, Di Marco G, Di Luzio R, Fiore M, et al. COVID-19 Vaccination Effectiveness in the General Population of an Italian Province: Two Years of Follow-Up. Vaccines. 2023; 11(8):1325. https://doi.org/10.3390/vaccines11081325
Chicago/Turabian StyleRosso, Annalisa, Maria Elena Flacco, Graziella Soldato, Giuseppe Di Martino, Cecilia Acuti Martellucci, Roberto Carota, Marco De Benedictis, Graziano Di Marco, Rossano Di Luzio, Matteo Fiore, and et al. 2023. "COVID-19 Vaccination Effectiveness in the General Population of an Italian Province: Two Years of Follow-Up" Vaccines 11, no. 8: 1325. https://doi.org/10.3390/vaccines11081325
APA StyleRosso, A., Flacco, M. E., Soldato, G., Di Martino, G., Acuti Martellucci, C., Carota, R., De Benedictis, M., Di Marco, G., Di Luzio, R., Fiore, M., Caponetti, A., & Manzoli, L. (2023). COVID-19 Vaccination Effectiveness in the General Population of an Italian Province: Two Years of Follow-Up. Vaccines, 11(8), 1325. https://doi.org/10.3390/vaccines11081325








