Abstract
Understanding the waning of vaccine-induced protection is important for both immunology and public health. Population heterogeneities in underlying (pre-vaccination) susceptibility and vaccine response can cause measured vaccine effectiveness (mVE) to change over time, even in the absence of pathogen evolution and any actual waning of immune responses. We use multi-scale agent-based models parameterized using epidemiological and immunological data, to investigate the effect of these heterogeneities on mVE as measured by the hazard ratio. Based on our previous work, we consider the waning of antibodies according to a power law and link it to protection in two ways: (1) motivated by correlates of risk data and (2) using a within-host model of stochastic viral extinction. The effect of the heterogeneities is given by concise and understandable formulas, one of which is essentially a generalization of Fisher’s fundamental theorem of natural selection to include higher derivatives. Heterogeneity in underlying susceptibility accelerates apparent waning, whereas heterogeneity in vaccine response slows down apparent waning. Our models suggest that heterogeneity in underlying susceptibility is likely to dominate. However, heterogeneity in vaccine response offsets <10% to >100% (median of 29%) of this effect in our simulations. Our study suggests heterogeneity is more likely to ‘bias’ mVE downwards towards the faster waning of immunity but a subtle bias in the opposite direction is also plausible.
1. Introduction
Providing accurate estimates of vaccine-induced protection is essential in guiding public health policy. However, many factors complicate our ability to estimate population-level vaccine effectiveness (VE) such as prior immunity, underlying health risks, timing of vaccination, inconsistent hazards in different locations, and other confounders. Further adding to uncertainty is the common presence of observed waning which may reflect the actual waning of immune responses, introduction of different strains, or may be an artifact coming from heterogeneity among individuals. Many studies report the fast, intraseasonal waning of vaccine-induced protection, particularly for viruses such as influenza and SARS-CoV-2 [1,2,3]; however, various effects can bias this conclusion. The depletion of susceptible individuals (also called the frailty effect in biostatistics) can bias estimates [4,5]. Heterogeneity in exposure risk, even if exactly the same in the vaccinated and unvaccinated groups, tends to bias the vaccine effectiveness estimates downwards potentially leading to spurious claims of waning [6,7]. If natural immunity is not taken into account, merely having a “leaky” vaccine (i.e., a vaccine that provides partial protection) can bias estimates downwards [5,7]. This complicates the estimation of the actual waning of vaccine-induced protection, which is expected to occur as many correlates of protection, e.g., neutralizing antibodies, have been shown to decrease over time [8,9,10,11].
Although discussing heterogeneity in an abstract mathematical sense is common [4,5,6,7,12] (usually assuming heterogeneity to follow known statistical distributions), understanding this more concretely, along with certain nuances, is also important. Individual-to-individual heterogeneity in the risk of acquiring infection can conceptually be decomposed into two factors: heterogeneity in underlying susceptibility and heterogeneity in vaccine-induced protection. The former can arise because of behavioral pattern differences affecting the amount of contact with potentially infectious individuals (e.g., frequently riding the subway may increase such contacts), whereas the latter can arise from a variability in antibody and T-cell responses [11,13,14,15]. We assume that these behavioral patterns persist over the relevant time scale (see also the Discussion section on regression towards the mean). While our results apply to heterogeneity in underlying susceptibility and heterogeneity in vaccine-induced protection from all sources, we specifically focus on two sources—the amount of contact with potentially infectious individuals, which contributes to underlying susceptibility, and antibody level, which contributes to vaccine-induced protection.
In this paper, we focus on the hazard ratio as the measure of vaccine effectiveness, as the hazard ratio corresponds to relative risk at a particular moment in time. To determine the direction and magnitude of bias, we simulate an epidemic in a population under various frailty and vaccine protection distributions in the absence and presence of waning and evaluate the interplay between these factors. Commonly, hazard ratios are estimated with the Cox proportional hazards model and there are several standard extensions utilized in real-world studies [16,17,18,19,20,21,22,23]. While Cox proportional hazard models were not intended to be time-varying, several approaches exist in order to make it applicable for measuring waning vaccine effectiveness. We utilize time category–vaccine interactions (henceforth referred to as TVI) which, in contrast to the commonly used Cox method utilizing the scaled Schoenfeld residuals, should accurately measure the hazard ratios even in the presence of extreme (observed) waning [24].
If vaccine effectiveness is assessed via the hazard ratio and the outcome of interest is the first infection post-vaccination, heterogeneity in baseline (pre-vaccination) susceptibility causes measured vaccine effectiveness (mVE) to decline over time, whereas heterogeneity in response to vaccination causes mVE to increase over time. Hence, any apparent change in mVE may reflect any combination of the selection on these heterogeneities in addition to the biological processes of pathogen evolution and waning of immune responses. In this paper, we first illustrate the problem using standard statistical distributions for both of the heterogeneities. We then provide concise formulas that give the net effect of these heterogeneities on the hazard rates and hazard ratio. Next, using parameter values based on epidemiological and immunological data incorporating the waning of antibodies, we use agent-based simulations to investigate the magnitude of these opposing effects. Our models suggest that the larger effect is from heterogeneity in baseline susceptibility but that heterogeneity in vaccine response may offset a substantial fraction of that effect. This exacerbation of observed waning may explain the negative VE reported in some studies [25,26].
2. Methods
We consider an agent-based model of acute viral infection with a constant background force of infection where we introduce heterogeneity in individual infection risk, heterogeneity in vaccine-induced protection, or both. For most scenarios to be described, vaccine protection follows the “leaky” model, wherein each vaccinated individual experiences a certain percent reduction in risk. Additionally, we model 40% out of a cohort of 100,000 to be vaccinated, in line with the CDC estimate for influenza vaccine coverage [27]. Since we model an acute viral infection, we assume sterilizing immunity upon infection for the remainder of the one-year time period considered. All simulations were run in Julia version 1.3.1 (Julia Project, github.com/JuliaLang) and statistical analysis was completed in R software version 4.2.1 (R Foundation, Vienna, Austria).
For heterogeneity in underlying risk (risk in the absence of vaccination), we select a daily risk rate for both the vaccinated and unvaccinated groups from a single gamma distribution. For heterogeneity in vaccine protection, we select vaccinated individuals’ protection from a variety of distributions including beta distributions, in contrast to leaky, homogeneous vaccination where all individuals’ protection would be the same. To establish a comparison, we use the mean vaccine efficacy in the context of no epidemic, VENE, which thereby removes the effect of selection. We then calculate vaccine effectiveness using a time category–vaccine interaction (TVI) as the independent variable of the Cox proportional hazards model in order to find a time-varying estimate of protection. The TVI method has been shown to behave accurately in circumstances where waning occurs rapidly [24].
3. Results
3.1. Underlying Susceptibility to Infection (Frailty) Distribution
When considering only heterogeneity in underlying frailty, the given gamma distributions (parameterized as Gamma(αγ, βγ) where αγ/βγ is the mean and αγ−0.5 is the coefficient of variation (CoV)) can produce the appearance of waning vaccine effectiveness, though as αγ increases this effect lessens. This appearance of waning corresponds to what many statistical studies have posited would occur, and is termed the “frailty effect” or “frailty phenomena” [28,29,30,31]; in epidemiological studies, this is sometimes alternatively called “survivor bias” or the “depletion of susceptibles” effect. Some studies have also simulated this effect but have not compared the qualitative effect of different distributions [5,6]. In Figure 1, we show how different gamma distributions with the same mean can cause differing amounts of perceived waning (waning in mVE) when the true vaccine protection is in fact constant and leaky.
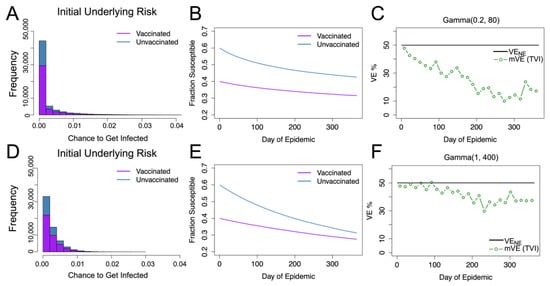
Figure 1.
Gamma distributed underlying risk with vaccine protection (VE) homogeneous at 50% protection. For underlying risk described by Gamma (0.2, 80), the distribution of the unvaccinated and vaccinated population’s daily risk is given in blue and purple as shown in (A). (B) shows the fractions of the susceptible individuals in each group. (C) shows the estimated vaccine effectiveness (mVE), which drops markedly below the given level we expect of the leaky vaccine (black), decreasing to 17% from the original 50%. (D–F) display the same results for Gamma (1, 400). As seen in (F), the estimated vaccine effectiveness is below the true value but is not as severe as (C), only decreasing to 38.5%.
3.2. Vaccine Efficacy Distribution
In simulated studies, two modes of vaccine efficacy are often compared: “leaky” vaccination, where protection is incomplete but reduces each individual’s chance to become infected by a specified amount (e.g., 50%), or “all-or-nothing” vaccination where a fraction of individuals receive complete protection from the vaccine and others receive no protection whatsoever. A limited number of studies have also considered normal-like distributions for vaccine protection [7]. We consider two main beta distributions parameterized by Beta(αβ, ββ) where αβ/(αβ + ββ) is the mean (held here at 0.5): the normal-like Beta(2, 2) and the U-shaped Beta(0.5, 0.5). These distributions, as well as their resultant dynamics and mVE estimates, are compared in Figure 2, which shows that for both of these cases, distributions in vaccine protection bias VE estimates upwards. Non-symmetric vaccine protection distributions were also tested (see Figure S1 in Supplementary Materials) but did not change the direction of bias, continuing to show increase in mVE.
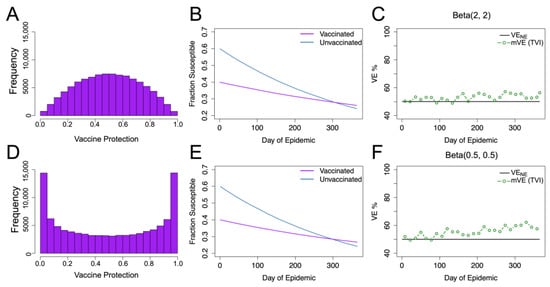
Figure 2.
Beta distributed vaccine protection with homogeneous underlying risk. (A–C) give the results for vaccine protection distributed Beta(2, 2), where (A) displays the resultant distribution in the vaccinated population, (B) shows the change in susceptible populations (as a fraction of total population) over time, and (C) shows the vaccine effectiveness (mVE) estimate which increased by 6%. Keeping the mean the same but changing the distribution shape, as seen in (D), to Beta(0.5, 0.5), we, likewise, see similar infection dynamics (E) but a higher mVE (F), increasing by approximately 10%. Here, VENE is the vaccine efficacy if there were no epidemic, and it remains constant at 50%.
As seen in Figure 1 and Figure 2, singly, the two types of heterogeneity appear to contribute in opposite directions; beta distributed heterogeneity in vaccine protection tends to skew the estimate upwards while gamma distributed heterogeneity in underlying risk tends to skew the estimates downwards and to a greater extent. As these effects compete when combined, we constructed a predictor to determine which direction, if any, the competing distributions change mVE away from VENE.
3.3. Effect of Selection on Heterogeneities
Assuming hazard rates for a given individual are not time-varying and ignoring stochasticity, , the average hazard rate in not-yet-infected individuals is given by the following equation:
where is the probability density function for the hazard rates at time 0; we allow to be a generalized function (e.g., a delta function) so the formula also applies to discrete probability distributions. Let denote the random variable for Let and be the moment-generating and cumulant-generating functions of , respectively. Since the denominator of Equation (1) is and the numerator is and , we obtain the following relation:
Hence, the first derivative of is the second cumulant (i.e., the variance) of , the second derivative of is the third cumulant of (related to the skewness), the third derivative is the fourth cumulant (related to excess kurtosis), and so on. Since can be viewed as fitness, the above is essentially equivalent to a generalization of Fisher’s fundamental theorem of natural selection, according to which the n-th derivative of mean fitness over time is the n + 1 cumulant [32,33].
Since in most cases the force of infection is not constant, we further extended this relation between the hazard rates and cumulants. If we let where is an individual i’s hazard rate, FOI is the force of infection at time t, and qi is the individual’s relative hazard, we can recover the above relation in terms of a transformation of time ,
where is the cumulant generating function for the distribution of −qi.
We now consider the hazard ratio comparing vaccinated to unvaccinated individuals, , where is the average hazard rate in not-yet-infected vaccinated individuals and is the analogous for unvaccinated individuals. To find the rate of change of the hazard ratio and recalling that the first derivative of the mean hazard rate is the second cumulant (i.e., variance) of the hazard rates, we apply the quotient rule (or the quotient and chain rules for the case of the time-varying FOI), which yields the following equation:
where is the variance of the vaccinated group’s hazard rates and is the variance of the unvaccinated group’s hazard rates.
If at time t = 0 the underlying risk is distributed Gamma(αγ, βγ) and vaccine protection is distributed Beta(αβ, ββ), and the two are independent, determines the direction in which these heterogeneities affect HR(t) as
Hence, even in this simple scenario, heterogeneity can cause either an increase or decrease in mVE.
3.4. Vaccine Effectiveness under Competing Heterogeneities
We considered the interplay of heterogeneities in both underlying frailty and vaccine protection and compared vaccine effectiveness estimates to our predicted value based on Equation (4). We found that overall estimates tended to oscillate around the predicted value (purple dashed), as seen in Figure 3. Here, we found that, depending on the underlying distributions, the mVE can increase, decrease, or remain steady around VENE, the vaccine efficacy under no epidemic. Likewise, the extent of observed change was dependent on the interplay of both distributions, with some estimated VE values changing a negligible amount and others shown here changing greater than 20% compared to the VENE value. Furthermore, for all of the combinations shown in Figure 3, we maintained a VENE of 50%, but the initial protection level given also mediates how far from VENE a given distribution can change, as seen in Figure S2 in Supplementary Materials.
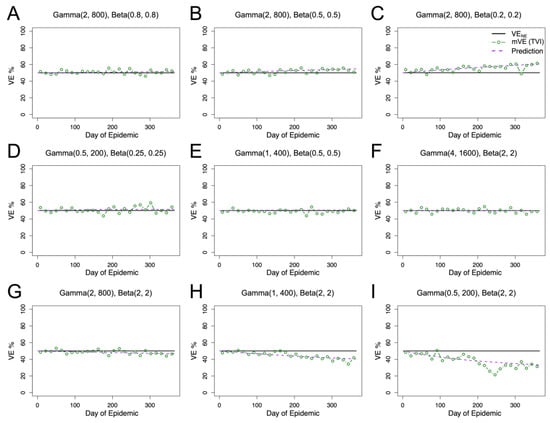
Figure 3.
Competing heterogeneities allow for diverse outcomes in mVE. Per Equation (5), we predicted an increase in observed vaccine effectiveness in (A–C), no change in (D–F), and a decrease in (G–I). For all panels, our predicted value (purple dashed) closely matches the mVE (green). Given that an individual’s vaccine protection is constant for these simulations (black), this reiterates the difficulty in interpreting changes in mVE.
Even larger changes in mVE can be found by either extending the study period, allowing for more individuals to get infected and thus contributing to the over- or underestimation, or by considering a more extreme distribution. Additionally, it is theoretically possible to generate non-monotonic behavior, as shown in Figure S3 in Supplementary Materials, where mVE can go both up and down relative to VENE.
3.5. Modeling Waning Protection
Without direct challenge studies, estimating heterogeneity in vaccine effectiveness can be fraught. However, many studies use antibody titers as a correlate of protection, including those for SARS-CoV-2 [34,35,36]. Using data on waning SARS-CoV-2 antibodies, we created a distribution for initial protection in a population that then wanes over time. We modeled the waning of antibodies as a power law of the form
in line with our previous studies [9,10,11]. The exponent −1 corresponds to relatively fast waning. Here, C for each individual is drawn from a log normal distribution with a standard deviation of 0.75–1.5 natural log in line with [11,13,14]. In previous studies, we analyzed antibodies and waning starting at day 42 post-infection or vaccination. We assumed here that all individuals in the vaccinated group were fully vaccinated before the study began. After this, we correlated an individual’s antibody level to their individual VE using
where the antibody-to-VE conversion exponent was based on an adjustment to the relationships given in [14] for HAI titers and the risk of infection, with exponents of approximately −0.35. Because HAI titer is only one component of the antibody response, we slightly increased the strength of the relationship and used −0.5. We call this the risk-correlate model.
We also considered a different relationship between the VE and antibody based on a within-host stochastic extinction model
where a = 10 is the death rate of virions, R0 = 10 is the basic reproductive number at the between-cell level [37], Ab represents the scaled level of antibodies, and m = 0.5 is the product of the number of viral particles per inoculum and a virion’s probability of successfully infecting a cell in the absence of antibodies (see Supplementary Materials for the derivation and additional details). This relationship gives qualitatively similar results to the risk-correlate model.
Antibody is scaled to give an approximately 90% initial vaccine protection in the population when the standard deviation for the natural log of the antibody is equal to 1, again in line with [11,13,14]; inherently, as standard deviation is varied, this causes the initial average of vaccine-induced protection in the population to vary slightly. This distribution replaces the beta distributions used in Section 3.4 for vaccine protection, while the underlying frailty in both groups continues to be modeled with gamma distributions, with the CoV based on a study [38] that estimated a CoV of 0.7–1.5 (mean of 0.9) based on contact surveys of a very short duration (e.g., two days) and a CoV of 0.3–0.9 (mean of 0.5) based on contact surveys when aggregating by 1-year age categories. As elaborated by [38], the former is likely an overestimate whereas the latter is likely an underestimate; hence, we considered a CoV of 0.5–1.
Using the risk-correlate model, Figure 4 compares a simulation without any heterogeneity (Figure 4A) to simulations with just heterogeneity in (antibody-induced) vaccine protection or underlying frailty and simulations, with heterogeneity in both protection and underlying frailty, where underlying frailty is characterized by the coefficient of variation (CoV) of the gamma distribution where CoV = , and the mean of the gamma distributions are held the same as the previous figure. Recapitulating the earlier simulations, heterogeneity in vaccine protection results in an increase relative to VENE. However, heterogeneity in underlying frailty overwhelms this positive trend and causes VE estimates to be underestimated. Similar qualitative results are also given using the unadjusted power law exponent estimated from the HAI titers (a likely underestimate) as shown in the Supplementary Materials.
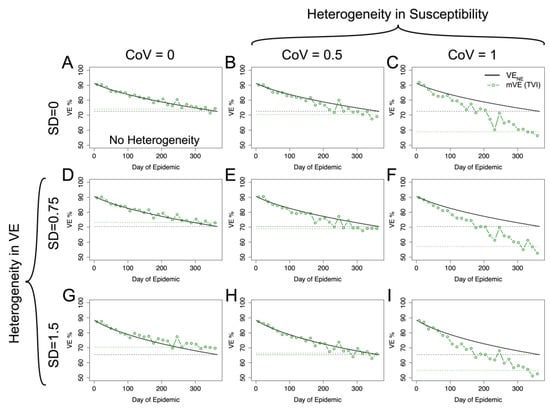
Figure 4.
mVE for plausible acute infectious disease parameters is likely to underestimate VENE but not necessarily in all circumstances. Under no heterogeneities, as in (A), mVE is extremely close to VENE; however, the introduction of heterogeneity biases the estimate. In the first column, showing simulations lacking heterogeneity in underlying susceptibility, heterogeneity in antibody biases the estimate upwards (D,G). In the first row, without any heterogeneity in antibody, the bias is downwards (B,C). With both, the underlying heterogeneity in susceptibility outcompetes heterogeneity in VE and leads to an underestimate relative to VENE (E,F,I), though not as extreme as would be seen if a vaccine was purely homogeneously leaky, except in (H) where the two effects approximately cancel each other out. SD indicates the standard deviation of antibody (at a given time) in natural logs; higher SD in antibody translates to higher variability in vaccine protection via Equation (6). CoV indicates the coefficient of variation in underlying susceptibility at the beginning of the simulation. The black dashed line shows the final VENE level reached in the population, while the green dashed line shows the final mVE as a mean of the last three estimated points.
Using the within-host stochastic model given by Equation (8) yields similar results, as seen in Figure 5, to the risk-correlate model. For both models, the degree of the over- or underestimation (relative to VENE) at the end of the season is given in Table S1 in Supplementary Materials. Again, for plausible acute infectious disease parameters, mVE tends to be approximately the same as or underestimates VENE.
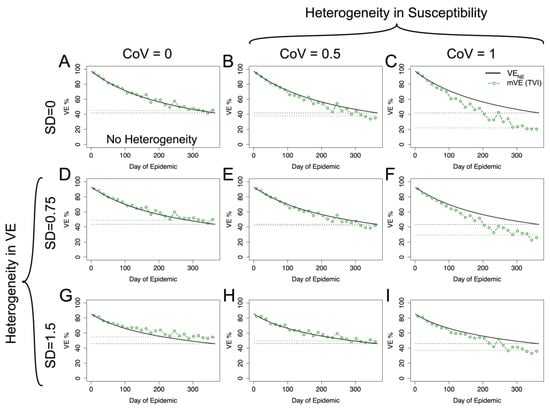
Figure 5.
Within-host stochastic model. Utilizing the within-host stochastic model (Equation (8)) for antibody-mediated, vaccine-induced protection yields similar results to the risk-correlate model (Equation (7)). As expected, increasing heterogeneity in underlying susceptibility (moving left to right) pushes mVE downwards while increasing heterogeneity in vaccine-induced protection (moving top to bottom) pushes mVE upwards. When these effects are mixed, as in (E,F,H,I), the heterogeneities compete. (A) shows the no heterogeneity case, (B,C) heterogeneity in underlying susceptibility only, and (D,G) heterogeneity in vaccine-induced protection only. The black dashed line shows the final VENE level reached in the population, while the green dashed line shows the final mVE as a mean of the last three estimated points.
4. Discussion
Heterogeneity complicates the ability to accurately estimate the extent of vaccine-induced protection in a population, as well as if protection is truly waning or merely appears to be waning. While this has been extensively investigated for underlying frailty [4,5,6], the confounding effect of heterogeneity in vaccine protection has been less-thoroughly explored. In many cases [7,12,39], only all-or-nothing and leaky vaccines are investigated but we argue that these are edge cases that are wonderful for illustrating theory but are unlikely to accurately model real-world responses to vaccination. In particular, this study considers a much wider array of distributions and shows that the net effect of selection on these heterogeneities can cause either an increase or a decrease in mVE, with the effect given by concise and interpretable formulas.
We parameterized our model using data from epidemiological and immunological studies and also incorporated within-host modeling of the immune system and potential pathogens. We found that, within the estimated ranges, mVE is likely to be underestimated; however, the degree of underestimation is quite varied with heterogeneity in vaccine response, offsetting anywhere from less than 10% to greater than 100% (median of 29%) of the effect of heterogeneity in underlying susceptibility alone (Table S1 in the Supplementary Materials). Therefore, vaccine effectiveness estimates should be interpreted with caution, especially over time as the heterogeneities continue to accumulate differential outcomes. While mVE seems more likely to underestimate than overestimate VENE, underestimation should not be assumed, as our range of plausible parameters includes cases without any underestimation.
Previous studies have used all-or-nothing or beta distributions to model vaccine-induced protection [7,12,39]. However, modeling waning with such distributions is not straightforward. Our technique of modeling the decay of immune responses (in our case, antibodies) at the individual level and converting these immune responses into individual level VE is more transparent and possibly easier to implement than modeling waning by, for example, shifting a beta distribution over time.
There are some important caveats to interpreting our results. Although Equations (2)–(4) give the effect of selection on the hazard rates and hazard ratios, the hazard rates and ratios can also be affected by regression towards (or away from) the mean. Regression towards the mean would tend to mitigate the effects of both heterogeneities. Secondly, in our simulations, heterogeneity in underlying susceptibility and heterogeneity in vaccine response are uncorrelated at baseline. Allowing for correlations permits for more diverse outcomes and affects not only waning but also the initial level of mVE. It should be noted that Equations (2)–(4) are valid even in the presence of such correlations. Extending our simulations to include such correlations and also regression towards or away from the mean is straightforward. In the current study, we focused only on the hazard ratio as a measure of VE. We did not consider the effect of seasonality, spatial structure, or epidemic waves. We also did not consider all possible combinations of distributions, but rather limited ourselves to those implied by data for acute respiratory infections.
5. Conclusions
We have brought together antibody waning, evolutionary theory, immune correlates of risk, vaccine-effectiveness estimation and within-host modeling in order to understand the effect of heterogeneity on the perceived waning of vaccine effectiveness. Further exploration of potential synergies between these areas of research may prove fruitful. For example, immune correlates of risk studies may be able to inform within-host modeling, and joint data analysis of correlates of risk, vaccine effectiveness over time and the waning of immune responses may allow for the more precise estimation and deconvolution of confounding factors.
Overall, we have given concise statistical formulas for understanding the effect of selection on mVE and have given estimates of the magnitude of this effect under a variety of situations based on both antibody and frailty data [10,38]. Our results suggest that VENE is likely but not certain to be higher than mVE due to variation at the individual level, and that the level of discrepancy is dependent on the specifics of the population and vaccine, meaning that a simple overall correction cannot be applied. Further exploration for how to correct for these factors statistically or via the study design is essential to more accurately understanding vaccine-induced protection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11081312/s1, Supplementary Text S1.
Author Contributions
A.N., H.A. and V.I.Z. contributed to the conception and design of this study; A.N. performed the simulations; H.A. designed the within-host models; A.N., H.A. and V.I.Z. analyzed the simulation results; and A.N. and H.A. wrote the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Heart, Lung, and Blood Institute and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grants U01 HL139483, U01 AI150747, and U01 AI144616).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The code used to generate the simulations for this study as well as for its analysis can be found upon publication at the Zarnitsyna Lab Github (https://github.com/ZarnitsynaLab/ArielNikas-VEHeterogeneity).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ray, G.T.; Lewis, N.; Klein, N.P.; Daley, M.F.; Wang, S.V.; Kulldorff, M.; Fireman, B. Intraseason Waning of Influenza Vaccine Effectiveness. Clin. Infect. Dis. 2019, 68, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef] [PubMed]
- Keehner, J.; Horton, L.E.; Binkin, N.J.; Laurent, L.C.; Alliance, S.; Pride, D.; Longhurst, C.A.; Abeles, S.R.; Torriani, F.J. Resurgence of SARS-CoV-2 Infection in a Highly Vaccinated Health System Workforce. N. Engl. J. Med. 2021, 385, 1330–1332. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.; Schrag, S.J.; Verani, J.R.; Lipsitch, M. Identifying and Alleviating Bias Due to Differential Depletion of Susceptible People in Postmarketing Evaluations of COVID-19 Vaccines. Am. J. Epidemiol. 2022, 191, 800–811. [Google Scholar] [CrossRef]
- Lipsitch, M.; Goldstein, E.; Ray, G.T.; Fireman, B. Depletion-of-susceptibles bias in influenza vaccine waning studies: How to ensure robust results. Epidemiol. Infect. 2019, 147, e306. [Google Scholar] [CrossRef]
- Lipsitch, M. Challenges of Vaccine Effectiveness and Waning Studies. Clin. Infect. Dis. 2019, 68, 1631–1633. [Google Scholar] [CrossRef]
- Tokars, J.I.; Patel, M.M.; Foppa, I.M.; Reed, C.; Fry, A.M.; Ferdinands, J.M. Waning of Measured Influenza Vaccine Effectiveness Over Time: The Potential Contribution of Leaky Vaccine Effect. Clin. Infect. Dis. 2020, 71, e633–e641. [Google Scholar] [CrossRef]
- Antia, A.; Ahmed, H.; Handel, A.; Carlson, N.E.; Amanna, I.J.; Antia, R.; Slifka, M. Heterogeneity and longevity of antibody memory to viruses and vaccines. PLoS Biol. 2018, 16, e2006601. [Google Scholar] [CrossRef]
- Zarnitsyna, V.I.; Akondy, R.S.; Ahmed, H.; McGuire, D.J.; Zarnitsyn, V.G.; Moore, M.; Johnson, P.L.F.; Ahmed, R.; Li, K.W.; Hellerstein, M.K.; et al. Dynamics and turnover of memory CD8 T cell responses following yellow fever vaccination. PLoS Comput. Biol. 2021, 17, e1009468. [Google Scholar] [CrossRef]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody Persistence through 6 Months after the Second Dose of mRNA-1273 Vaccine for COVID-19. N. Engl. J. Med. 2021, 384, 2259–2261. [Google Scholar] [CrossRef]
- Cohen, K.W.; Linderman, S.L.; Moodie, Z.; Czartoski, J.; Lai, L.; Mantus, G.; Norwood, C.; Nyhoff, L.E.; Edara, V.V.; Floyd, K.; et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep. Med. 2021, 2, 100354. [Google Scholar] [CrossRef]
- White, M.T.; Griffin, J.T.; Drakeley, C.J.; Ghani, A.C. Heterogeneity in malaria exposure and vaccine response: Implications for the interpretation of vaccine efficacy trials. Malar. J. 2010, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Zarnitsyna, V.I.; Lavine, J.; Ellebedy, A.; Ahmed, R.; Antia, R. Multi-epitope Models Explain How Pre-existing Antibodies Affect the Generation of Broadly Protective Responses to Influenza. PLoS Pathog. 2016, 12, e1005692. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Fang, V.J.; Ip, D.K.; Chan, K.H.; Leung, G.M.; Peiris, J.S.; Cowling, B.J. Estimation of the association between antibody titers and protection against confirmed influenza virus infection in children. J. Infect. Dis. 2013, 208, 1320–1324. [Google Scholar] [CrossRef]
- Joshi, D.; Nyhoff, L.E.; Zarnitsyna, V.I.; Moreno, A.; Manning, K.; Linderman, S.; Burrell, A.R.; Stephens, K.; Norwood, C.; Mantus, G.; et al. Infants and young children generate more durable antibody responses to SARS-CoV-2 infection than adults. medRxiv 2023. [Google Scholar] [CrossRef]
- Petrie, J.G.; Ohmit, S.E.; Truscon, R.; Johnson, E.; Braun, T.M.; Levine, M.Z.; Eichelberger, M.C.; Monto, A.S. Modest Waning of Influenza Vaccine Efficacy and Antibody Titers During the 2007-2008 Influenza Season. J. Infect. Dis. 2016, 214, 1142–1149. [Google Scholar] [CrossRef]
- Durham, L.K.; Longini, I.M., Jr.; Halloran, M.E.; Clemens, J.D.; Nizam, A.; Rao, M. Estimation of vaccine efficacy in the presence of waning: Application to cholera vaccines. Am. J. Epidemiol. 1998, 147, 948–959. [Google Scholar] [CrossRef]
- Haber, M.; Tate, J.E.; Lopman, B.A.; Qi, W.; Ainslie, K.E.C.; Parashar, U.D. Comparing statistical methods for detecting and estimating waning efficacy of rotavirus vaccines in developing countries. Hum. Vaccin. Immunother. 2021, 17, 4632–4635. [Google Scholar] [CrossRef]
- Rane, M.S.; Rohani, P.; Halloran, M.E. Durability of protection after 5 doses of acellular pertussis vaccine among 5–9 year old children in King County, Washington. Vaccine 2021, 39, 6144–6150. [Google Scholar] [CrossRef]
- Fong, Y.Y.; Halloran, M.E.; Park, J.K.; Marks, F.; Clemens, J.D.; Chao, D.L. Efficacy of a bivalent killed whole-cell cholera vaccine over five years: A re-analysis of a cluster-randomized trial. BMC Infect. Dis. 2018, 18, 84. [Google Scholar] [CrossRef]
- Minsoko, P.A.; Lell, B.; Fernandes, J.F.; Abossolo, B.P.; Kabwende, A.L.; Adegnika, A.A.; Mordmuller, B.; Issifou, S.; Kremsner, P.G.; Loembe, M.M.; et al. Efficacy and Safety of the RTS,S/AS01 Malaria Vaccine during 18 Months after Vaccination: A Phase 3 Randomized, Controlled Trial in Children and Young Infants at 11 African Sites. PLoS Med. 2014, 11, e1001685. [Google Scholar] [CrossRef]
- Olotu, A.; Lusingu, J.; Leach, A.; Lievens, M.; Vekemans, J.; Msham, S.; Lang, T.; Gould, J.; Dubois, M.C.; Jongert, E.; et al. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5-17 months in Kenya and Tanzania: A randomised controlled trial. Lancet Infect. Dis. 2011, 11, 102–109. [Google Scholar] [CrossRef]
- Alonso, P.L.; Sacarlal, J.; Aponte, J.J.; Leach, A.; Macete, E.; Milman, J.; Mandomando, I.; Spiessens, B.; Guinovart, C.; Espasa, M.; et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: Randomised controlled trial. Lancet 2004, 364, 1411–1420. [Google Scholar] [CrossRef]
- Nikas, A.; Ahmed, H.; Zarnitsyna, V.I. Estimating Waning of Vaccine Effectiveness: A Simulation Study. Clin. Infect. Dis. 2023, 76, 479–486. [Google Scholar] [CrossRef]
- Kissling, E.; Nunes, B.; Robertson, C.; Valenciano, M.; Reuss, A.; Larrauri, A.; Cohen, J.M.; Oroszi, B.; Rizzo, C.; Machado, A.; et al. I-MOVE multicentre case-control study 2010/11 to 2014/15: Is there within-season waning of influenza type/subtype vaccine effectiveness with increasing time since vaccination? Eurosurveillance 2016, 21, 13–24. [Google Scholar] [CrossRef]
- Kissling, E.; Valenciano, M.; Larrauri, A.; Oroszi, B.; Cohen, J.M.; Nunes, B.; Pitigoi, D.; Rizzo, C.; Rebolledo, J.; Paradowska-Stankiewicz, I.; et al. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: Results from the I-MOVE multicentre case-control study. Eurosurveillance 2013, 18, 33–42. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Flu Vaccination Coverage, United States, 2018–2019 Influenza Season 2019. Updated 1/26/22. Available online: https://www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm (accessed on 23 March 2023).
- O’Hagan, J.J.; Hernan, M.A.; Walensky, R.P.; Lipsitch, M. Apparent declining efficacy in randomized trials: Examples of the Thai RV144 HIV vaccine and South African CAPRISA 004 microbicide trials. AIDS 2012, 26, 123–126. [Google Scholar] [CrossRef]
- Thernau, T.M.; Crowson, C.; Atkinson, E. Using Time Dependent Covariates and Time Dependent Coefficients in the Cox Model. Surviv. Vignettes 2021, 2, 1–25. [Google Scholar]
- Smith, P.G.; Rodrigues, L.C.; Fine, P.E. Assessment of the protective efficacy of vaccines against common diseases using case-control and cohort studies. Int. J. Epidemiol. 1984, 13, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.G.; Cowling, B.J.; Greenland, S. Frailty and influenza vaccine effectiveness. Vaccine 2016, 34, 4645–4646. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.F. Selection in Asexual Populations-an Extension of the Fundamental Theorem. J. Theor. Biol. 1992, 155, 537–544. [Google Scholar] [CrossRef]
- Gerrish, P.J.; Sniegowski, P.D. Real time forecasting of near-future evolution. J. R. Soc. Interface 2012, 9, 2268–2278. [Google Scholar] [CrossRef] [PubMed]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; Triccas, J.A.; Khoury, D.S.; Davenport, M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: A meta-analysis. Lancet Microbe 2022, 3, e52–e61. [Google Scholar] [CrossRef]
- Ke, R.; Zitzmann, C.; Ho, D.D.; Ribeiro, R.M.; Perelson, A.S. In vivo kinetics of SARS-CoV-2 infection and its relationship with a person’s infectiousness. Proc. Natl. Acad. Sci. USA 2021, 118, e2111477118. [Google Scholar] [CrossRef]
- Gomes, M.G.M.; Ferreira, M.U.; Corder, R.M.; King, J.G.; Souto-Maior, C.; Penha-Goncalves, C.; Goncalves, G.; Chikina, M.; Pegden, W.; Aguas, R. Individual variation in susceptibility or exposure to SARS-CoV-2 lowers the herd immunity threshold. J. Theor. Biol. 2022, 540, 111063. [Google Scholar] [CrossRef]
- Halloran, M.E.; Longini, I.M., Jr.; Struchiner, C.J. Estimability and interpretation of vaccine efficacy using frailty mixing models. Am. J. Epidemiol. 1996, 144, 83–97. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).