Decreased Expression of CD314 by NK Cells Correlates with Their Ability to Respond by Producing IFN-γ after BCG Moscow Vaccination and Is Associated with Distinct Early Immune Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Protocol Registration
2.3. Innate Immune Response after BCG Vaccination
2.3.1. NK and Neutrophil Evaluation
2.3.2. Lymphocyte Evaluation
2.3.3. Flow Cytometry
2.4. Statistical Analysis
3. Results
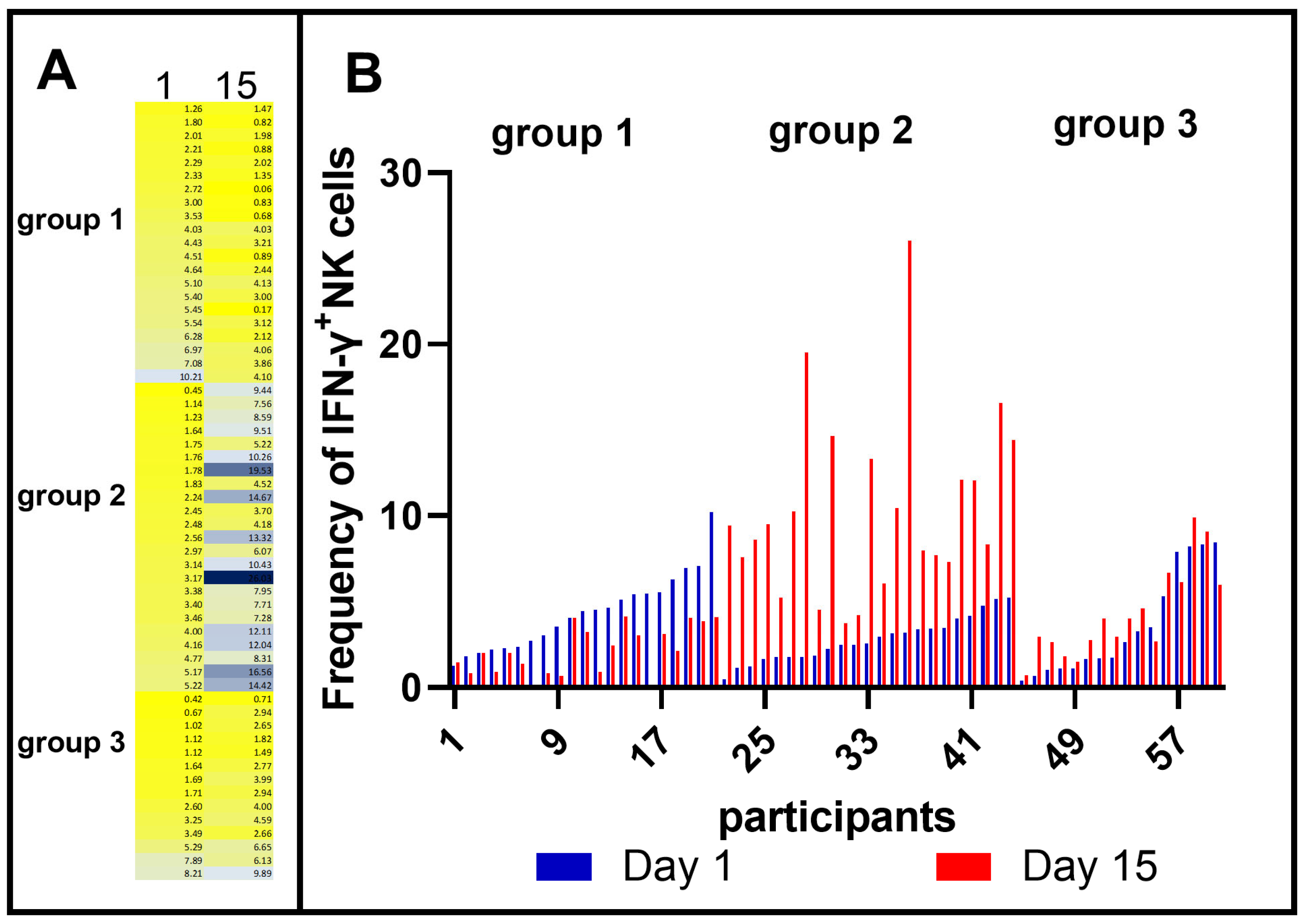
3.1. Heterogeneity of IFN-γ-Producing NK Cell Responses after Vaccination with BCG Moscow
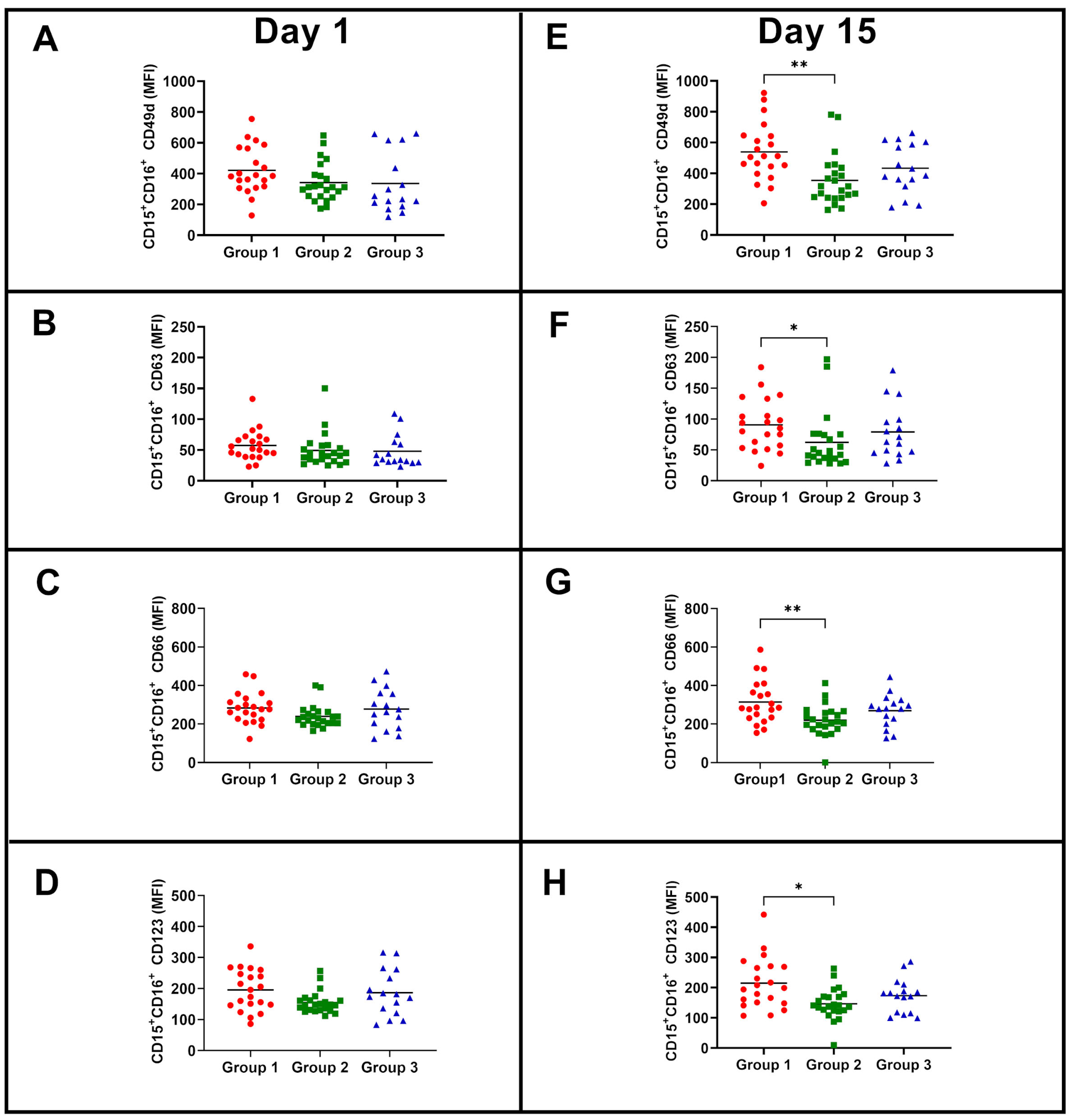
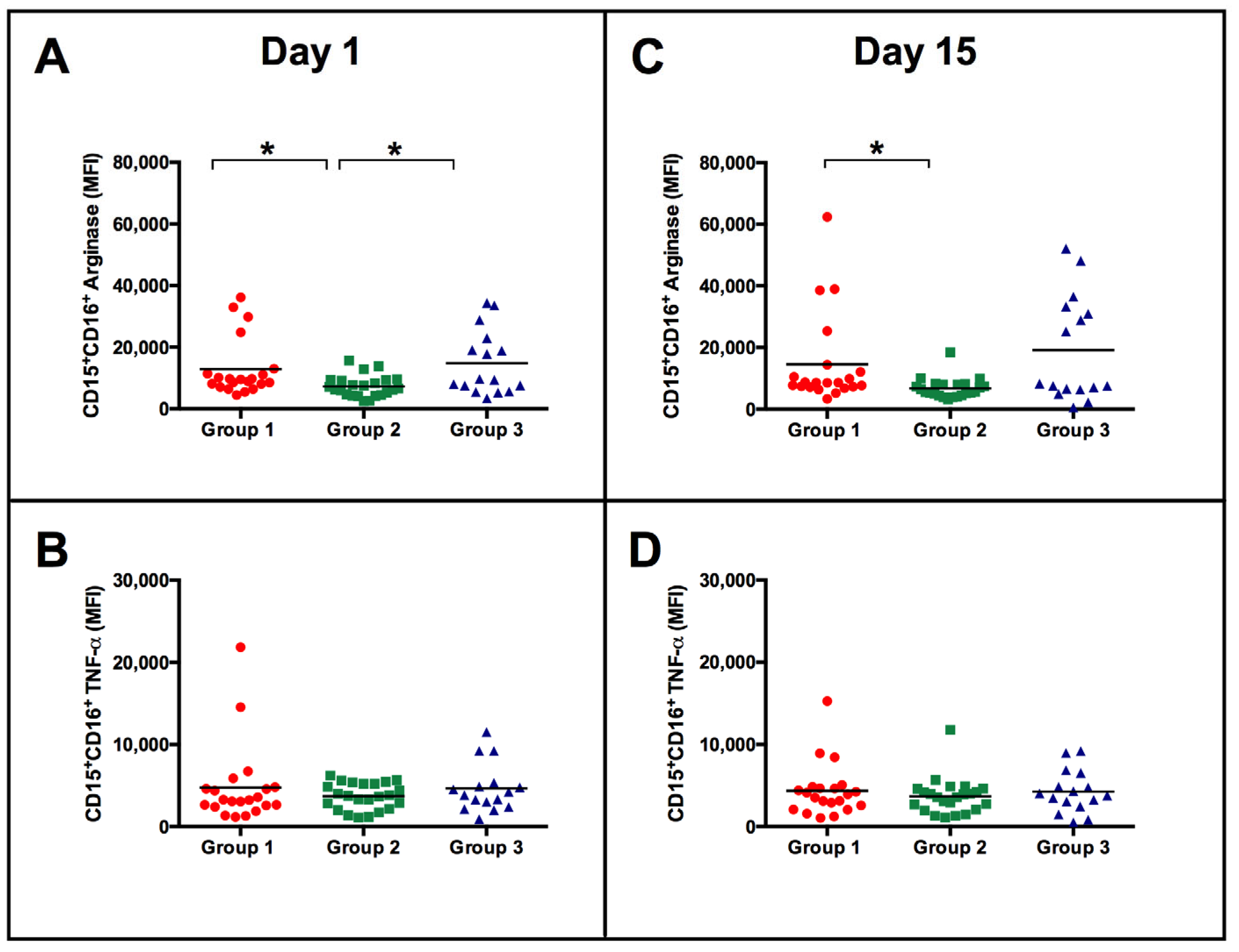
3.2. Similar Neutrophil Surface Phenotypes but Different Responses to BCG Vaccination according to the Magnitude of NK Cell IFN-γ Response
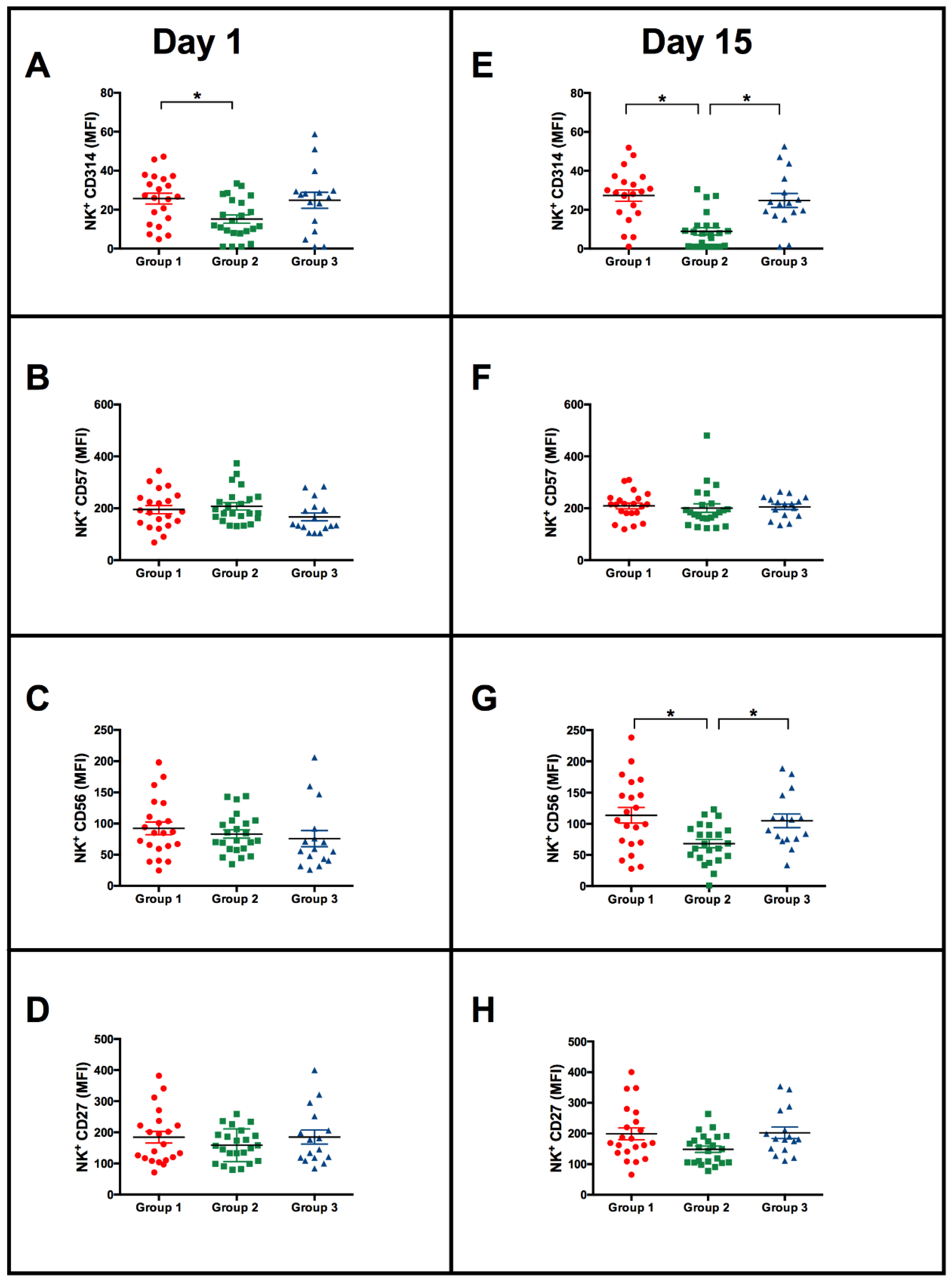
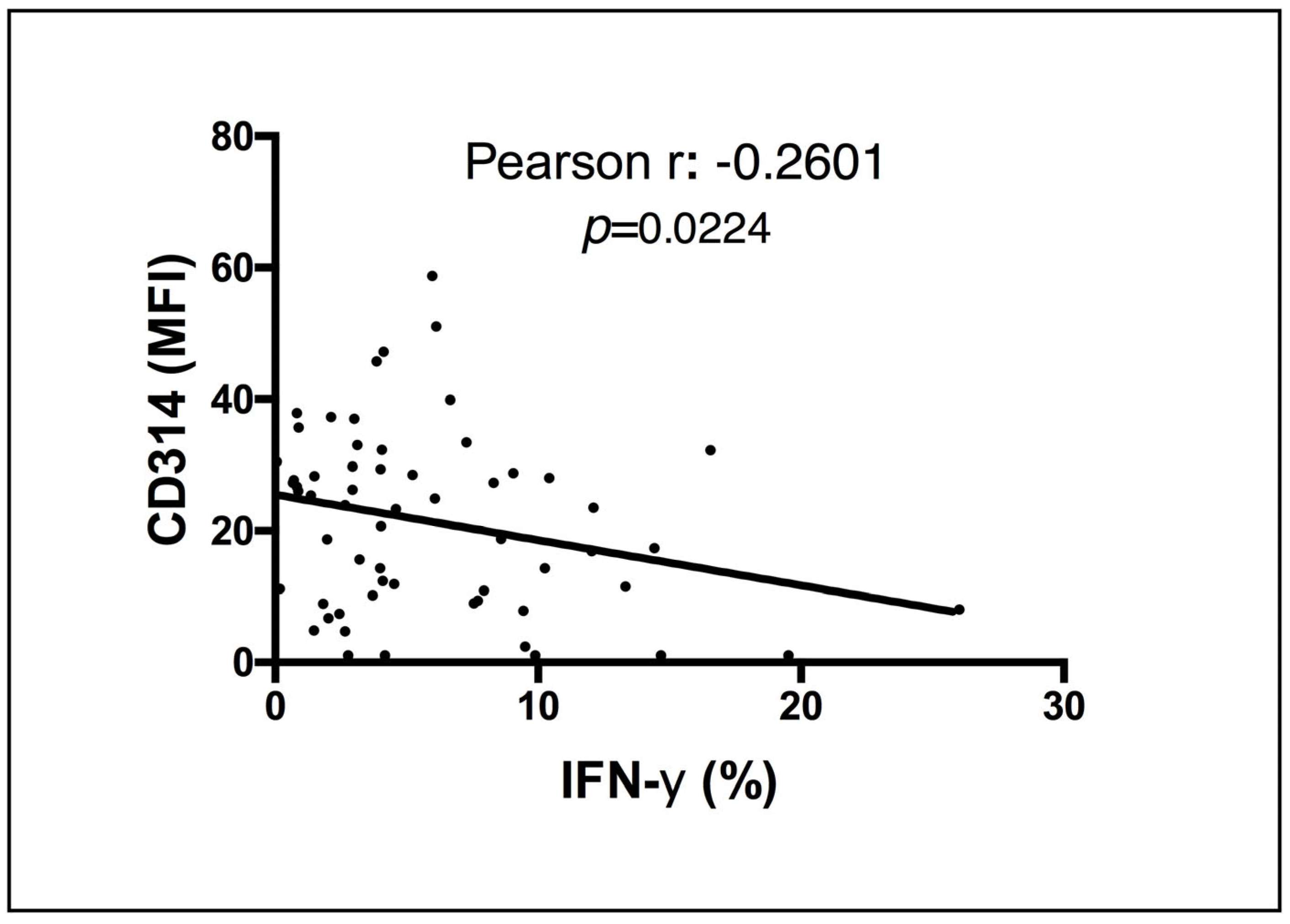
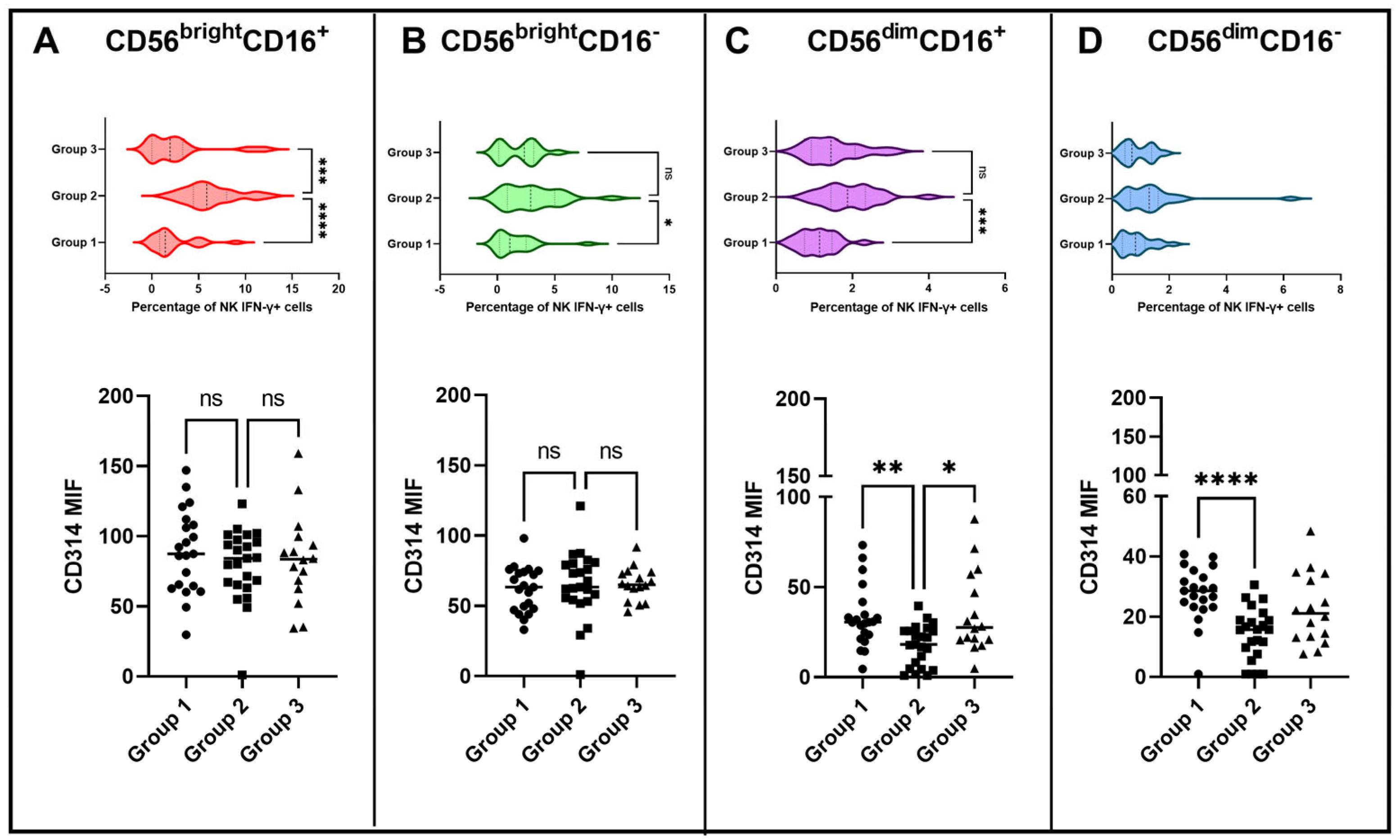
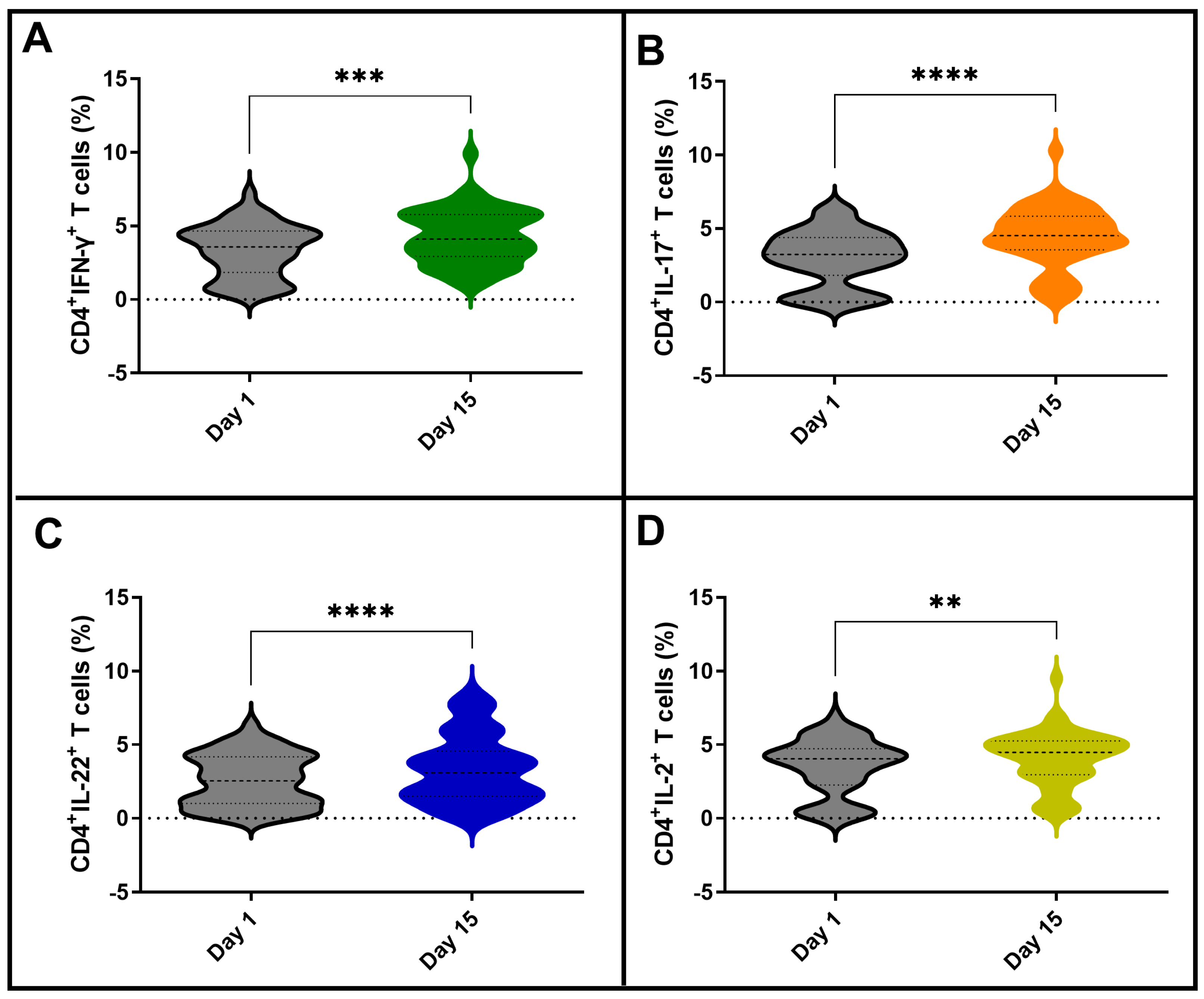
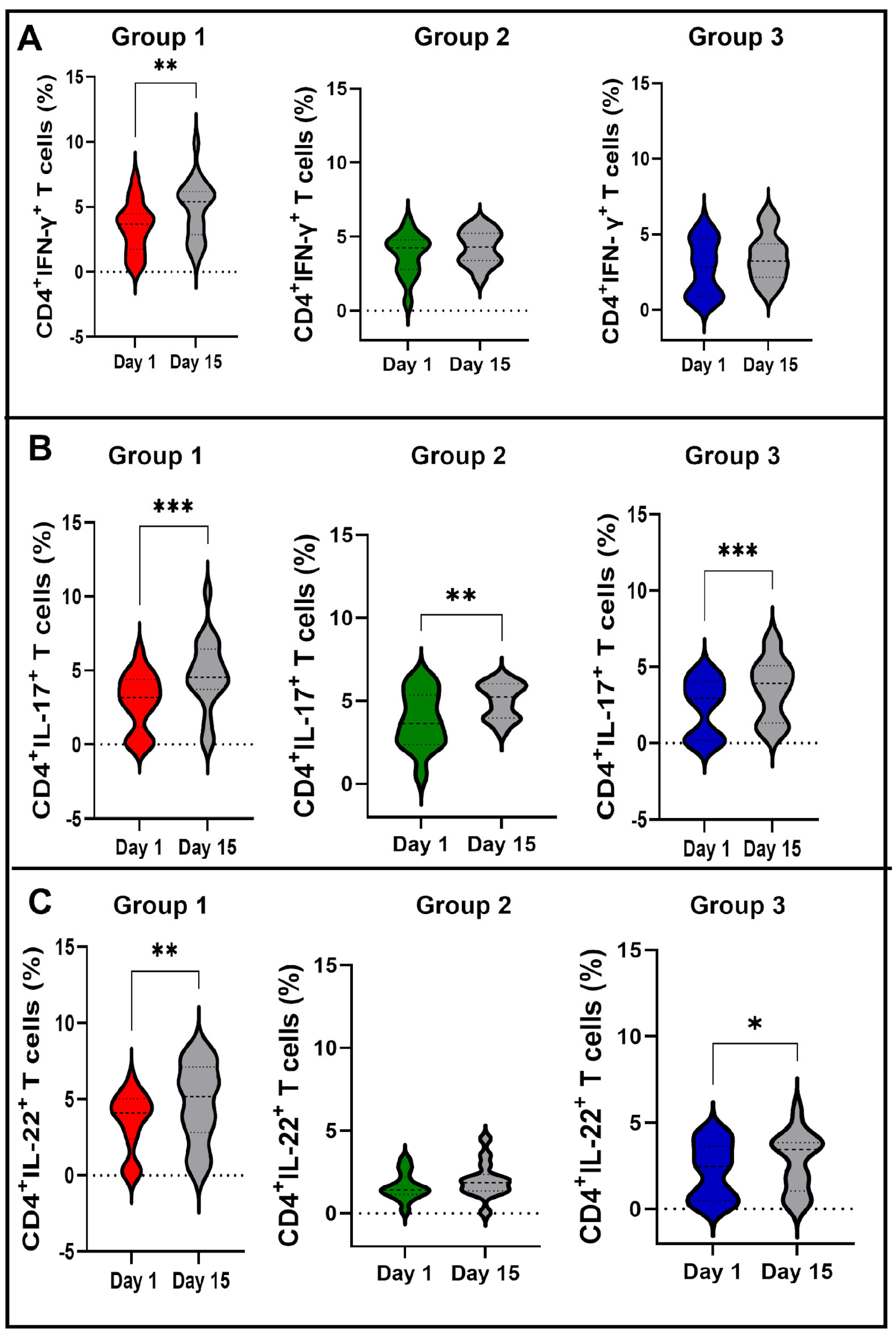
3.3. Vaccination with BCG Moscow Induces Specific T-Cell Responses but Heterogenic Phenotypes according to NK IFN-γ Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaible, U.E.; Linnemann, L.; Redinger, N.; Patin, E.C.; Dallenga, T. Strategies to Improve Vaccine Efficacy against Tuberculosis by Targeting Innate Immunity. Front. Immunol. 2017, 8, 1755. [Google Scholar] [CrossRef] [PubMed]
- Abebe, F. Immunological basis of early clearance of Mycobacterium tuberculosis infection: The role of natural killer cells. Clin. Exp. Immunol. 2021, 204, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Hanekom, W.A. The Immune Response to BCG Vaccination of Newborns. Ann. N. Y. Acad. Sci. 2005, 1062, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.P.; Scriba, T.J.; Joseph, S.; Harbacheuski, R.; Murray, R.A.; Gelderbloem, S.J.; Hawkridge, A.; Hussey, G.D.; Maecker, H.; Kaplan, G.; et al. Bacillus Calmette-Guérin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J. Immunol. 2008, 180, 3569–3577. [Google Scholar] [CrossRef]
- Covián, C.; Fernández-Fierro, A.; Retamal-Díaz, A.; Díaz, F.E.; Vasquez, A.E.; Lay, M.K.; Riedel, C.A.; González, P.A.; Bueno, S.M.; Kalergis, A.M. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front. Immunol. 2019, 10, 2806. [Google Scholar] [CrossRef]
- Butkeviciute, E.; Jones, C.E.; Smith, S.G. Heterologous effects of infant BCG vaccination: Potential mechanisms of immunity. Future Microbiol. 2018, 13, 1193–1208. [Google Scholar] [CrossRef]
- Koeken, V.A.C.M.; Verrall, A.J.; Netea, M.G.; Hill, P.C.; van Crevel, R. Trained innate immunity and resistance to Mycobacterium tuberculosis infection. Clin. Microbiol. Infect. 2019, 25, 1468–1472. [Google Scholar] [CrossRef]
- Moorlag, S.J.C.F.M.; Arts, R.J.W.; van Crevel, R.; Netea, M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019, 25, 1473–1478. [Google Scholar] [CrossRef]
- Stensballe, L.G.; Nante, E.; Jensen, I.P.; Kofoed, P.E.; Poulsen, A.; Jensen, H.; Newport, M.; Marchant, A.; Aaby, P. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: A beneficial effect of BCG vaccination for girls community based case-control study. Vaccine 2005, 23, 1251–1257. [Google Scholar] [CrossRef]
- Ritz, N.; Mui, M.; Balloch, A.; Curtis, N. Non-specific effect of Bacille Calmette-Guérin vaccine on the immune response to routine immunisations. Vaccine 2013, 31, 3098–3103. [Google Scholar] [CrossRef]
- Biering-Sørensen, S.; Jensen, K.J.; Aamand, S.H.; Blok, B.; Andersen, A.; Monteiro, I.; Netea, M.G.; Aaby, P.; Benn, C.S.; Hasløv, K.H. Variation of growth in the production of the BCG vaccine and the association with the immune response. An observational study within a randomised trial. Vaccine 2015, 33, 2056–2065. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Jacobs, C.; Xavier, R.J.; van der Meer, J.W.M.; van Crevel, R.; Netea, M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014, 155, 213–219. [Google Scholar] [CrossRef]
- Williamson, S.L.; Gadd, E.; Pillay, T.; Toldi, G. Non-specific effects of BCG vaccination on neutrophil and lymphocyte counts of healthy neonates from a developed country. Vaccine 2021, 39, 1887–1891. [Google Scholar] [CrossRef]
- Di Vito, C.; Mikulak, J.; Mavilio, D. On the Way to Become a Natural Killer Cell. Front. Immunol. 2019, 10, 1812. [Google Scholar] [CrossRef]
- Madera, S.; Rapp, M.; Firth, M.A.; Beilke, J.N.; Lanier, L.L.; Sun, J.C. Type I IFN promotes NK cell expansion during viral infection by protecting NK cells against fratricide. J. Exp. Med. 2016, 213, 225–233. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Fauci, A.S.; Mavilio, D.; Kottilil, S. NK cells in HIV infection: Paradigm for protection or targets for ambush. Nat. Rev. Immunol. 2005, 5, 835–843. [Google Scholar] [CrossRef]
- Brunetta, E.; Hudspeth, K.L.; Mavilio, D. Pathologic natural killer cell subset redistribution in HIV-1 infection: New insights in pathophysiology and clinical outcomes. J. Leukoc. Biol. 2010, 88, 1119–1130. [Google Scholar] [CrossRef]
- Parra, J.A.C.; Zúñiga, N.M.; Zamudio, L.A.J.; Álvarez, L.A.J.; Lara, C.S.; Zúñiga, J. Memory of Natural Killer Cells: A New Chance against Mycobacterium tuberculosis? Front. Immunol. 2017, 8, 967. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Tsilika, M.; Moorlag, S.; Antonakos, N.; Kotsaki, A.; Domínguez-Andrés, J.; Kyriazopoulou, E.; Gkavogianni, T.; Adami, M.E.; Damoraki, G.; et al. Activate: Randomized Clinical Trial of BCG Vaccination against Infection in the Elderly. Cell 2020, 183, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Brodin, P.; Davis, M. Human immune system variation. Nat. Rev. Immunol. 2017, 17, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Damond, N.; Engler, S.; Zanotelli, V.R.T.; Schapiro, D.; Wasserfall, C.H.; Kusmartseva, I.; Nick, H.S.; Thorel, F.; Herrera, P.L.; Atkinson, M.A.; et al. A Map of Human Type 1 Diabetes Progression by Imaging Mass Cytometry. Cell Metab. 2019, 29, 755–768.e5. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.S.; Schwartzberg, P.L.; Kotliarov, Y.; Biancotto, A.; Xie, Z.; Germain, R.N.; Wang, E.; Olnes, M.J.; Narayanan, M.; Golding, H.; et al. Global analyses of human immune variation reveal baseline predictors of postvaccination responses. Cell 2014, 157, 499–513. [Google Scholar] [CrossRef]
- Debisarun, P.A.; Kilic, G.; de Bree, L.C.J.; Pennings, L.J.; van Ingen, J.; Benn, C.S.; Aaby, P.; Dijkstra, H.; Lemmers, H.; Domínguez-Andrés, J.; et al. The impact of BCG dose and revaccination on trained immunity. Clin. Immunol. 2023, 246, 109208. [Google Scholar] [CrossRef]
- Cirovic, B.; de Bree, L.C.J.; Groh, L.; Blok, B.A.; Chan, J.; van der Velden, W.J.F.M.; Bremmers, M.E.J.; van Crevel, R.; Handler, K.; Picelli, S.; et al. BCG Vaccination in Humans Elicits Trained Immunity via the Hematopoietic Progenitor Compartment. Cell Host Microbe 2020, 28, 322–334.e5. [Google Scholar] [CrossRef]
- Jaeger, B.N.; Donadieu, J.; Cognet, C.; Bernat, C.; Ordoñez-Rueda, D.; Barlogis, V.; Mahlaoui, N.; Fenis, A.; Narni-Mancinelli, E.; Beaupain, B.; et al. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J. Exp. Med. 2012, 209, 565–580. [Google Scholar] [CrossRef]
- Moorlag, S.J.C.F.M.; Rodriguez-Rosales, Y.A.; Gillard, J.; Fanucchi, S.; Theunissen, K.; Novakovic, B.; de Bont, C.M.; Negishi, Y.; Fok, E.T.; Kalafati, L.; et al. BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils. Cell Rep. 2020, 33, 108387. [Google Scholar] [CrossRef]
- Junqueira-Kipnis, A.P.; dos Anjos, L.R.B.; Barbosa, L.C.S.; Costa, A.C.; Borges, K.C.M.; Cardoso, A.C.O.; Ribeiro, K.M.; Rosa, S.B.A.; Souza, C.C.; Neves, R.C.; et al. BCG revaccination of health workers in Brazil to improve innate immune responses against COVID-19: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 881. [Google Scholar] [CrossRef]
- Dos Anjos, L.R.B.; da Costa, A.C.; Cardoso, A.D.R.O.; Guimarães, R.A.; Rodrigues, R.L.; Ribeiro, K.M.; Borges, K.C.M.; Carvalho, A.C.O.; Dias, C.I.S.; Rezende, A.O.; et al. Efficacy and Safety of BCG Revaccination with M. bovis BCG Moscow to Prevent COVID-19 Infection in Health Care Workers: A Randomized Phase II Clinical Trial. Front. Immunol. 2022, 13, 841–868. [Google Scholar] [CrossRef]
- Angelo, L.S.; Banerjee, P.P.; Monaco-Shawver, L.; Rosen, J.B.; Makedonas, G.; Forbes, L.R.; Mace, E.M.; Orange, J.S. Practical NK cell phenotyping and variability in healthy adults. Immunol. Res. 2015, 62, 341–356. [Google Scholar] [CrossRef]
- Poli, A.; Michel, T.; Thérésine, M.; Andrès, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef]
- Amand, M.; Iserentant, G.; Poli, A.; Sleiman, M.; Fievez, V.; Sanchez, I.P.; Sauvageot, N.; Michel, T.; Aouali, N.; Janji, B.; et al. Human CD56dimCD16dim Cells as an Individualized Natural Killer Cell Subset. Front. Immunol. 2017, 8, 699. [Google Scholar] [CrossRef]
- Esin, S.; Batoni, G. Natural killer cells: A coherent model for their functional role in Mycobacterium tuberculosis infection. J. Innate Immun. 2015, 7, 11–24. [Google Scholar] [CrossRef]
- Sivori, S.; Pende, D.; Quatrini, L.; Pietra, G.; Della Chiesa, M.; Vacca, P.; Tumino, N.; Moretta, F.; Mingari, M.C.; Locatelli, F.; et al. NK cells and ILCs in tumor immunotherapy. Mol. Asp. Med. 2021, 80, 100870. [Google Scholar] [CrossRef]
- Sivori, S.; Vacca, P.; Del Zotto, G.; Munari, E.; Mingari, M.C.; Moretta, L. Human NK cells: Surface receptors, inhibitory checkpoints, and translational applications. Cell. Mol. Immunol. 2019, 16, 430–441. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Jin, T.; Tian, Y.; Dai, C.; Widarma, C.; Song, R.; Xu, F. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy. Signal Transduct. Target. Ther. 2020, 5, 250. [Google Scholar] [CrossRef]
- Jiang, Y.; He, L.; Chen, H.; Bice, T.; Zhang, Z.; Liu, J.; Ding, H.; Han, X.; Shang, H. Alteration of inhibitory and activating NK cell receptor expression on NK cells in HIV-infected Chinese. Cell Immunol. 2011, 271, 219–226. [Google Scholar] [CrossRef]
- Long, E.O.; Kim, H.S.; Liu, D.; Peterson, M.E.; Rajagopalan, S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013, 31, 227–258. [Google Scholar] [CrossRef]
- Esin, S.; Batoni, G.; Pardini, M.; Favilli, F.; Bottai, D.; Maisetta, G.; Florio, W.; Vanacore, R.; Wigzell, H.; Campa, M. Functional characterization of human natural killer cells responding to Mycobacterium bovis bacille Calmette-Guérin. Immunology 2004, 112, 143–152. [Google Scholar] [CrossRef]
- Scarno, G.; Pietropaolo, G.; Di Censo, C.; Gadina, M.; Santoni, A.; Sciumè, G. Transcriptional, Epigenetic and Pharmacological Control of JAK/STAT Pathway in NK Cells. Front. Immunol. 2019, 10, 2456. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Sciumè, G.; Davis, F.P.; Iwata, S.; Zitti, B.; Robinson, G.W.; Hennighausen, L.; Kanno, Y.; O’Shea, J.J. Subset- and tissue-defined STAT5 thresholds control homeostasis and function of innate lymphoid cells. J. Exp. Med. 2017, 214, 2999–3014. [Google Scholar] [CrossRef]
- Martin, T.C.; Ilieva, K.M.; Visconti, A.; Beaumont, M.; Kiddle, S.J.; Dobson, R.J.B.; Mangino, M.; Lim, E.M.; Pezer, M.; Steves, C.J.; et al. Dysregulated Antibody, Natural Killer Cell and Immune Mediator Profiles in Autoimmune Thyroid Diseases. Cells 2020, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, E.; Koch, J.; Cerwenka, A.; Steinle, A. New prospects on the NKG2D/NKG2DL system for oncology. Oncoimmunology 2013, 2, e26097. [Google Scholar] [CrossRef] [PubMed]
- Fauriat, C.; Long, E.O.; Ljunggren, H.G.; Bryceson, Y.T. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 2010, 115, 2167–2176. [Google Scholar] [CrossRef]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef]
- Jacobs, R.; Hintzen, G.; Kemper, A.; Beul, K.; Kempf, S.; Behrens, G.; Sykora, K.W.; Schmidt, R.E. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur. J. Immunol. 2001, 31, 3121–3127. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Turner, S.C.; Chen, K.S.; Ghaheri, B.A.; Ghayur, T.; Carson, T.G.W.E.; Caligiuri, M.A. Human natural killer cells: A unique innate immunoregulatory role for the CD56(bright) subset. Blood 2001, 97, 3146–3151. [Google Scholar] [CrossRef]
- Zhu, S.; Phatarpekar, P.V.; Denman, C.J.; Senyukov, V.V.; Somanchi, S.S.; Nguyen-Jackson, H.T.; Mace, E.M.; Freeman, A.F.; Watowich, S.S.; Orange, J.S.; et al. Transcription of the activating receptor NKG2D in natural killer cells is regulated by STAT3 tyrosine phosphorylation. Blood 2014, 124, 403–411. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, G.; Xiao, F.; Xie, J.; Wang, S.; Lu, L.; Cui, D. Role of Th22 Cells in the Pathogenesis of Autoimmune Diseases. Front. Immunol. 2021, 12, 688066. [Google Scholar] [CrossRef]
- Hart, O.M.; Athie-Morales, V.; O’Connor, G.M.; Gardiner, C.M. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J. Immunol. 2005, 175, 1636–1642. [Google Scholar] [CrossRef]
- Oliveira, E.S.; Marinho, J.M.; Barbosa, T. Interferon-gamma production by mononuclear cells in Bacille Calmette-Guérin-revaccinated healthy volunteers predicted long-term antimycobacterial responses in a randomized controlled trial. Vaccine 2013, 31, 3778–3782. [Google Scholar] [CrossRef]
- Mihaila, A.C.; Ciortan, L.; Macarie, R.D.; Vadana, M.; Cecoltan, S.; Preda, M.B.; Hudita, A.; Gan, A.M.; Jakobsson, G.; Tucureanu, M.M.; et al. Transcriptional Profiling and Functional Analysis of N1/N2 Neutrophils Reveal an Immunomodulatory Effect of S100A9-Blockade on the Pro-Inflammatory N1 Subpopulation. Front. Immunol. 2021, 12, 708–770. [Google Scholar] [CrossRef]
- Alcantara, C.A.; Glassman, I.; Nguyen, K.H.; Parthasarathy, A.; Venketaraman, V. Neutrophils in Mycobacterium tuberculosis. Vaccines 2023, 11, 631. [Google Scholar] [CrossRef]
- Alkarni, M.; Lipman, M.; Lowe, D.M. The roles of neutrophils in non-tuberculous mycobacterial pulmonary disease. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 14. [Google Scholar] [CrossRef]
- Oberlies, J.; Watzl, C.; Giese, T.; Luckner, C.; Kropf, P.; Müller, I.; Ho, A.D.; Munder, M. Regulation of NK cell function by human granulocyte arginase. J. Immunol. 2009, 182, 5259–5267. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa, A.C.; de Souza Barbosa, L.C.; Kipnis, A.; Junqueira-Kipnis, A.P. Decreased Expression of CD314 by NK Cells Correlates with Their Ability to Respond by Producing IFN-γ after BCG Moscow Vaccination and Is Associated with Distinct Early Immune Responses. Vaccines 2023, 11, 1297. https://doi.org/10.3390/vaccines11081297
da Costa AC, de Souza Barbosa LC, Kipnis A, Junqueira-Kipnis AP. Decreased Expression of CD314 by NK Cells Correlates with Their Ability to Respond by Producing IFN-γ after BCG Moscow Vaccination and Is Associated with Distinct Early Immune Responses. Vaccines. 2023; 11(8):1297. https://doi.org/10.3390/vaccines11081297
Chicago/Turabian Styleda Costa, Adeliane Castro, Lília Cristina de Souza Barbosa, André Kipnis, and Ana Paula Junqueira-Kipnis. 2023. "Decreased Expression of CD314 by NK Cells Correlates with Their Ability to Respond by Producing IFN-γ after BCG Moscow Vaccination and Is Associated with Distinct Early Immune Responses" Vaccines 11, no. 8: 1297. https://doi.org/10.3390/vaccines11081297
APA Styleda Costa, A. C., de Souza Barbosa, L. C., Kipnis, A., & Junqueira-Kipnis, A. P. (2023). Decreased Expression of CD314 by NK Cells Correlates with Their Ability to Respond by Producing IFN-γ after BCG Moscow Vaccination and Is Associated with Distinct Early Immune Responses. Vaccines, 11(8), 1297. https://doi.org/10.3390/vaccines11081297





