Heterologous Booster with BNT162b2 Induced High Specific Antibody Levels in CoronaVac Vaccinees
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Aspects
2.2. Participants and Sample Collection Periods
2.3. SARS-CoV-2 RNA Extraction and RT-qPCR
2.4. Enzyme-Linked Immunosorbent Assay for Anti-Receptor-Binding Domain (RBD) IgA and IgG and Anti-Spike IgG
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Participants
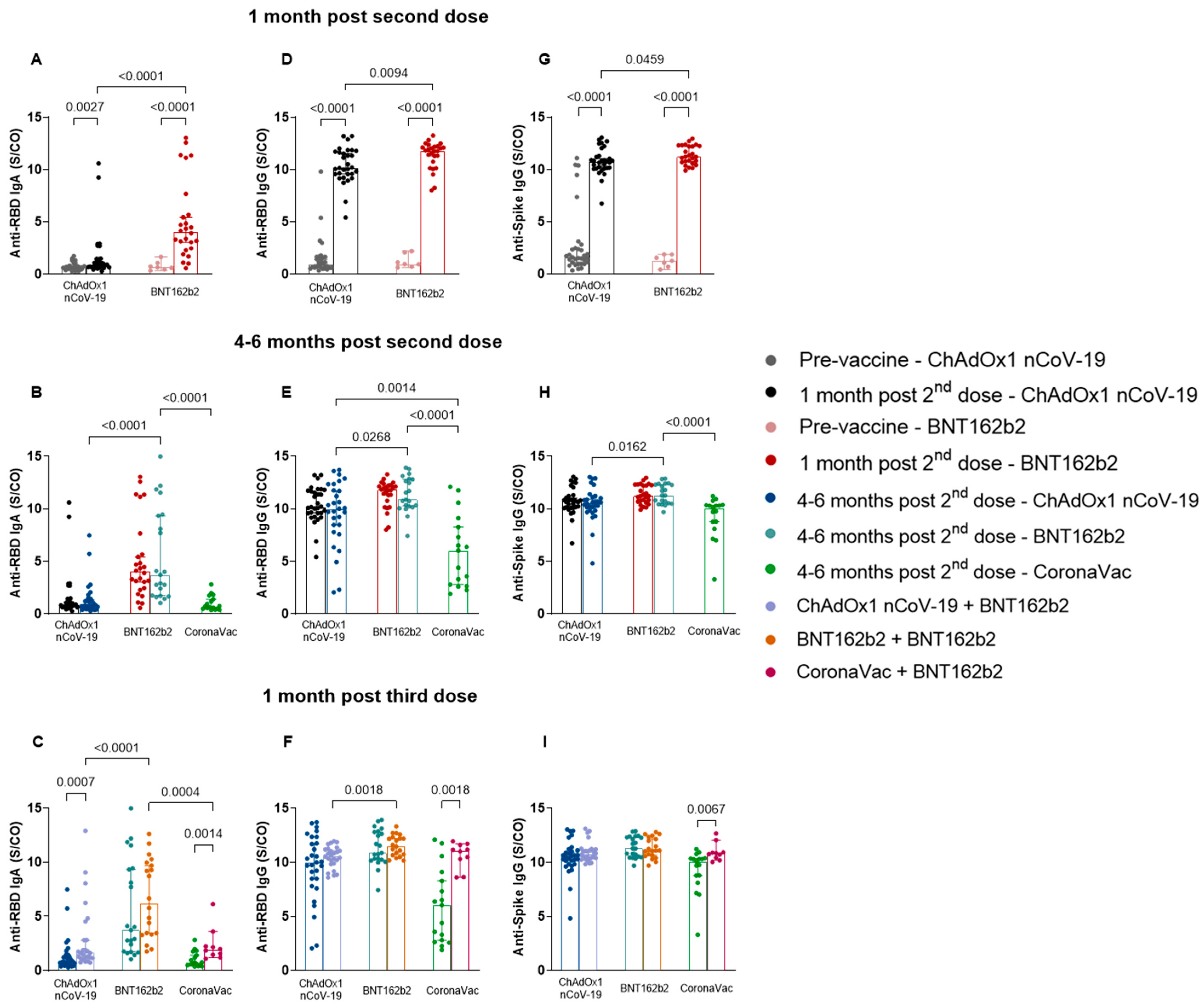
3.2. Heterologous Booster with BNT162b2 Induced Higher Specific Antibody Levels in the CoronaVac Group Compared to ChAdOx1 nCoV-19 and BNT162b2
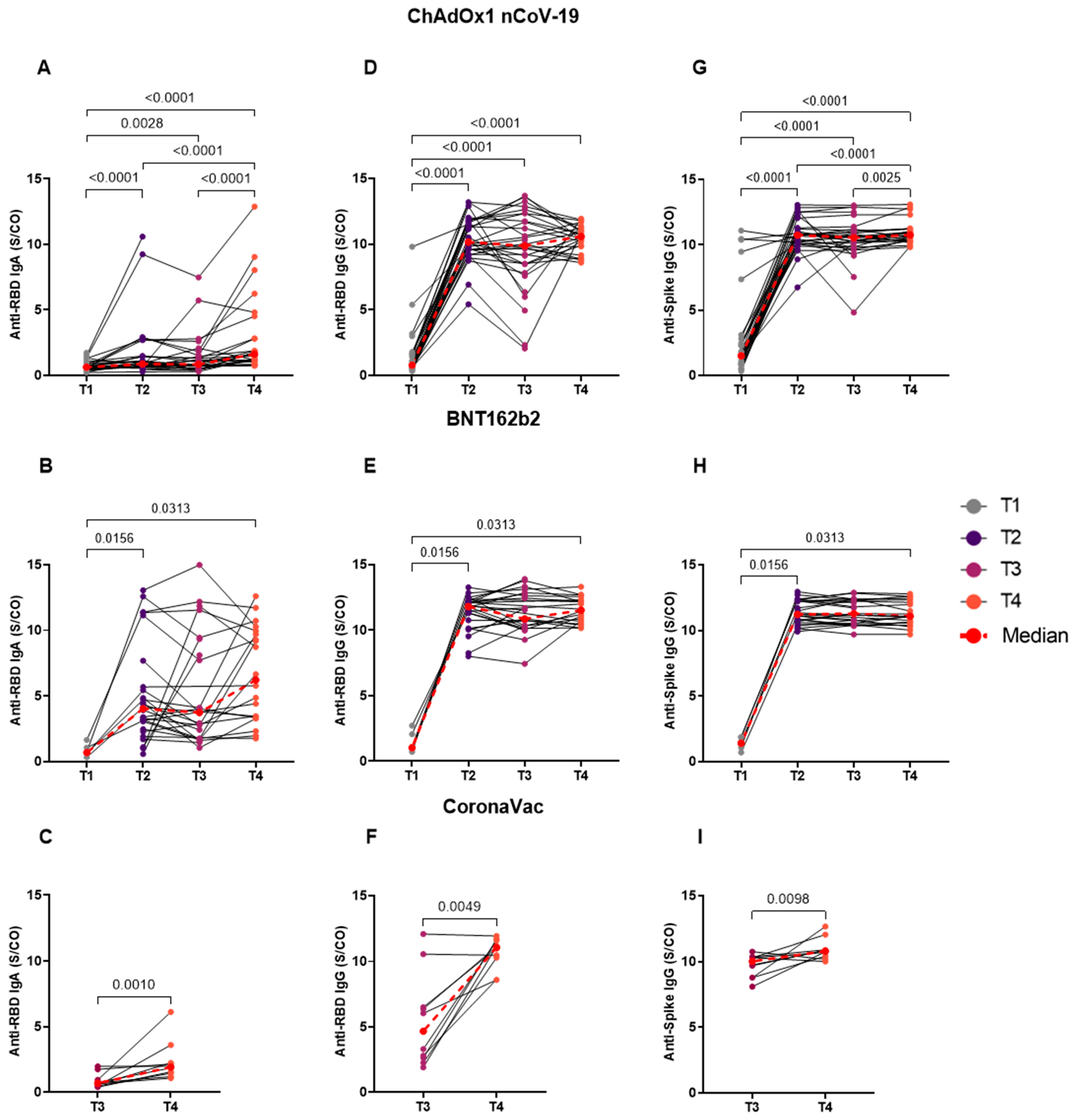
3.3. Homologous BNT162b2 Booster Reveals No Difference in the Longitudinal Analyses of Distinct Time Points
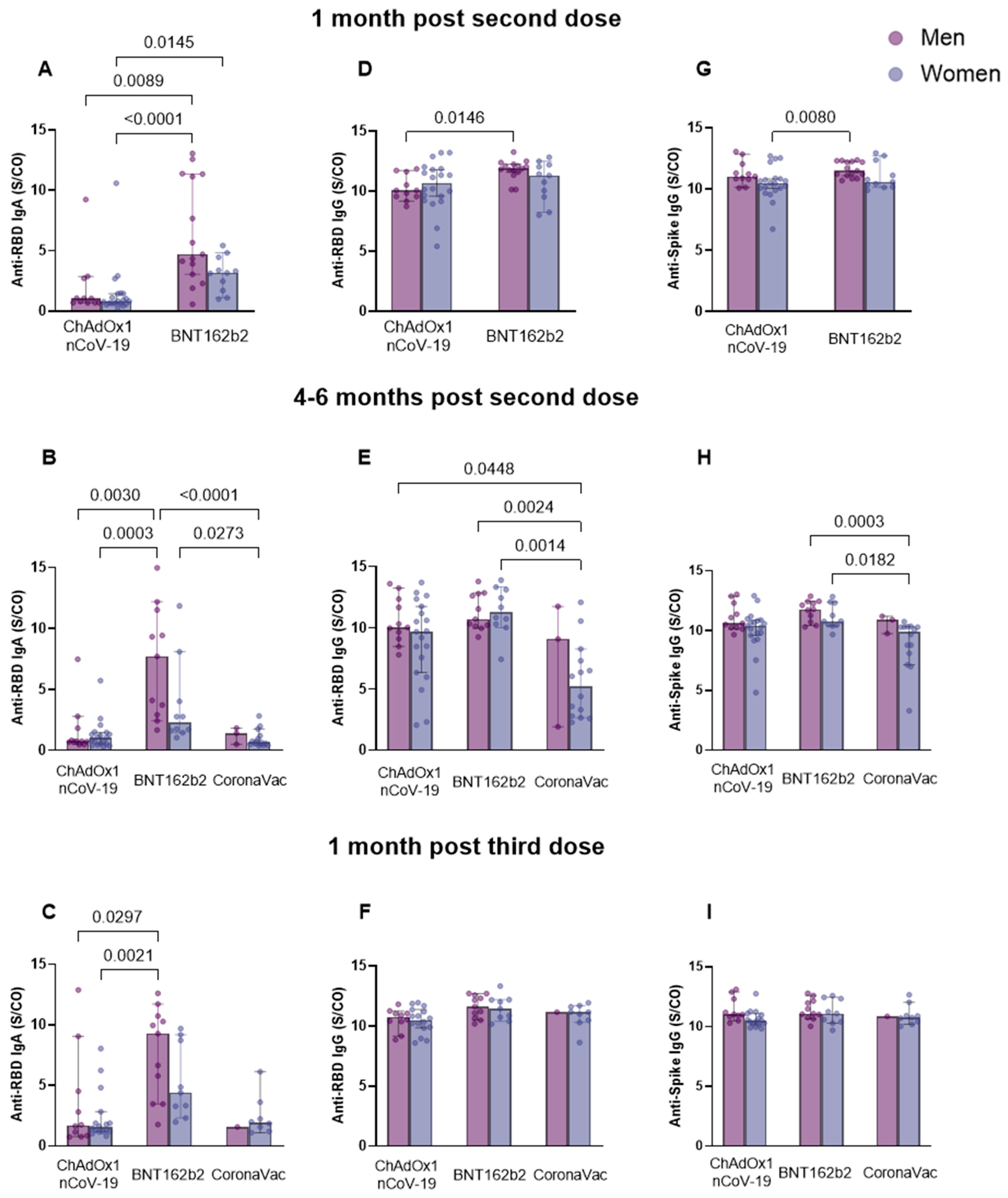
3.4. Different Patterns of Anti-Spike IgG and Anti-RBD IgG and IgA Antibody Response Associated with Sex
3.5. Age influence on Anti-RBD IgA and IgG and Anti-Spike IgG Serum Levels for Each of the Groups
3.6. Individuals without Comorbidities from the BNT162b2 Group Produced Higher Sera Levels of Anti-Spike IgG after BNT162b2 Booster
3.7. Individuals from the CoronaVac Group Previously Infected with SARS-CoV-2 Presented Higher Antibody Levels Compared to the Other Groups
3.8. Anti-Spike and Anti-RBD IgG Levels Showed a Very Similar Production, and a Positive Correlation of Both Antibodies Was Found for ChAdOx1 nCoV-19 Vaccinees after the Booster Dose
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angeli, F.; Spanevello, A.; Reboldi, G.; Visca, D.; Verdecchia, P. SARS-CoV-2 Vaccines: Lights and Shadows. Eur. J. Intern. Med. 2021, 88, 1–8. [Google Scholar] [CrossRef]
- WHO. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 May 2023).
- WHO. Health Emergency Dashboard. Available online: https://covid19.who.int/region/amro/country/br (accessed on 8 May 2023).
- World Health Organization. WHO SAGE Roadmap for Prioritizing Uses of COVID-19 Vaccines in the Context of Limited Supply: An Approach to Inform Planning and Subsequent Recommendations Based on Epidemiological Setting and Vaccine Supply Scenarios; World Health Organization: Geneva, Switzerland, 2021; pp. 1–24. [Google Scholar]
- Cerqueira-Silva, T.; Katikireddi, S.V.; de Araujo Oliveira, V.; Flores-Ortiz, R.; Júnior, J.B.; Paixão, E.S.; Robertson, C.; Penna, G.O.; Werneck, G.L.; Barreto, M.L.; et al. Vaccine Effectiveness of Heterologous CoronaVac plus BNT162b2 in Brazil. Nat. Med. 2022, 28, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Villela, D.A.; Guimarães de Noronha, T.; Bastos, L.S.; Pacheco, A.G.; Cruz, O.G.; Max Carvalho, L.; Torres Codeço, C.; Ferreira da Costa Gomes, M.; Codeço Coelho, F.; Picinini Freitas, L.; et al. Effectiveness of Mass Vaccination in Brazil against Severe COVID-19 Cases. MedRxiv 2021. [Google Scholar] [CrossRef]
- Brasil, Ministério da Saúde do Brasil. Plano Nacional de Operacionalização Da Vacinação Contra a COVID-19, 12th ed.; Ministério da Saúde Brasília (DF): Brasília, Brazil, 2022. [Google Scholar]
- Kaur, M.; Sharma, A.; Kumar, S.; Singh, G.; Barnwal, R.P. SARS-CoV-2: Insights into Its Structural Intricacies and Functional Aspects for Drug and Vaccine Development. Int. J. Biol. Macromol. 2021, 179, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Kurup, D.; Schnell, M.J. SARS-CoV-2 Vaccines—The Biggest Medical Research Project of the 21st Century. Curr. Opin. Virol. 2021, 49, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Rouet, R.; Henry, J.Y.; Johansen, M.D.; Sobti, M.; Balachandran, H.; Langley, D.B.; Walker, G.J.; Lenthall, H.; Jackson, J.; Ubiparipovic, S.; et al. Broadly Neutralizing SARS-CoV-2 Antibodies through Epitope-Based Selection from Convalescent Patients. Nat. Commun. 2023, 14, 687. [Google Scholar] [CrossRef]
- Vanderven, H.A.; Kent, S.J. The Protective Potential of Fc-Mediated Antibody Functions against Influenza Virus and Other Viral Pathogens. Immunol. Cell Biol. 2020, 98, 253–263. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Q.; Deng, C.; Li, M.; Li, L.; Liu, D.; Liu, M.; Ruan, X.; Mei, J.; Mo, R.; et al. Robust Induction of B Cell and T Cell Responses by a Third Dose of Inactivated SARS-CoV-2 Vaccine. Cell Discov. 2022, 8, 10. [Google Scholar] [CrossRef]
- Fiorino, F.; Ciabattini, A.; Sicuranza, A.; Pastore, G.; Santoni, A.; Simoncelli, M.; Polvere, J.; Galimberti, S.; Baratè, C.; Sammartano, V.; et al. The Third Dose of MRNA SARS-CoV-2 Vaccines Enhances the Spike-Specific Antibody and Memory B Cell Response in Myelofibrosis Patients. Front. Immunol. 2022, 13, 1017863. [Google Scholar] [CrossRef]
- Ciabattini, A.; Pastore, G.; Fiorino, F.; Polvere, J.; Lucchesi, S.; Pettini, E.; Auddino, S.; Rancan, I.; Durante, M.; Miscia, M.; et al. Evidence of SARS-CoV-2-Specific Memory B Cells Six Months After Vaccination With the BNT162b2 MRNA Vaccine. Front. Immunol. 2021, 12, 740708. [Google Scholar] [CrossRef]
- Ejemel, M.; Li, Q.; Hou, S.; Schiller, Z.A.; Tree, J.A.; Wallace, A.; Amcheslavsky, A.; Kurt Yilmaz, N.; Buttigieg, K.R.; Elmore, M.J.; et al. A Cross-Reactive Human IgA Monoclonal Antibody Blocks SARS-CoV-2 Spike-ACE2 Interaction. Nat. Commun. 2020, 11, 4198. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C. Clinical Value of Anti-SARS-CoV-2 Serum IgA Titration in Patients with COVID-19. J. Med. Virol. 2021, 93, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Varnaitė, R.; García, M.; Glans, H.; Maleki, K.T.; Sandberg, J.T.; Tynell, J.; Christ, W.; Lagerqvist, N.; Asgeirsson, H.; Ljunggren, H.-G.; et al. Expansion of SARS-CoV-2–Specific Antibody-Secreting Cells and Generation of Neutralizing Antibodies in Hospitalized COVID-19 Patients. J. Immunol. 2020, 205, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Adjobimey, T.; Meyer, J.; Sollberg, L.; Bawolt, M.; Berens, C.; Kovačević, P.; Trudić, A.; Parcina, M.; Hoerauf, A. Comparison of IgA, IgG, and Neutralizing Antibody Responses Following Immunization With Moderna, BioNTech, AstraZeneca, Sputnik-V, Johnson and Johnson, and Sinopharm’s COVID-19 Vaccines. Front. Immunol. 2022, 13, 917905. [Google Scholar] [CrossRef]
- Abu-Humaidan, A.H.A.; Ahmad, F.M.; Awajan, D.; Jarrar, R.F.; Alaridah, N. Anti-SARS-CoV-2 S-RBD IgG Formed after BNT162b2 Vaccination Can Bind C1q and Activate Complement. J. Immunol. Res. 2022, 2022, 7263740. [Google Scholar] [CrossRef]
- Kaplonek, P.; Fischinger, S.; Cizmeci, D.; Bartsch, Y.C.; Kang, J.; Burke, J.S.; Shin, S.A.; Dayal, D.; Martin, P.; Mann, C.; et al. MRNA-1273 Vaccine-Induced Antibodies Maintain Fc Effector Functions across SARS-CoV-2 Variants of Concern. Immunity 2022, 55, 355–365.e4. [Google Scholar] [CrossRef]
- Aziz, M.W.; Mukhtar, N.; Anjum, A.A.; Mushtaq, M.H.; Shahid, M.F.; Ali, M.; Shabbir, M.A.B.; Ali, M.A.; Nawaz, M.; Yaqub, T. Molecular Characterization and Selection of Indigenous SARS-CoV-2 Delta Variant for the Development of the First Inactivated SARS-CoV-2 Vaccine of Pakistan. Vaccines 2023, 11, 607. [Google Scholar] [CrossRef]
- Crystal, R.G. Adenovirus: The First Effective in Vivo Gene Delivery Vector. Hum. Gene Ther. 2014, 25, 3–11. [Google Scholar] [CrossRef]
- Mendonça, S.A.; Lorincz, R.; Boucher, P.; Curiel, D.T. Adenoviral Vector Vaccine Platforms in the SARS-CoV-2 Pandemic. NPJ Vaccines 2021, 6, 97. [Google Scholar] [CrossRef]
- Alhandod, T.A.; Rabbani, S.I.; Almuqbil, M.; Alshehri, S.; Hussain, S.A.; Alomar, N.F.; Mir, M.A.; Asdaq, S.M.B. A Systematic Review on the Safety and Efficacy of COVID-19 Vaccines Approved in Saudi Arabia. Vaccines 2023, 11, 281. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 Vaccines in Development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an Inactivated Vaccine Candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef]
- Wei, Y.; Jia, K.M.; Zhao, S.; Hung, C.T.; Mok, C.K.P.; Poon, P.K.M.; Man Leung, E.Y.; Wang, M.H.; Yam, C.H.K.; Chow, T.Y.; et al. Estimation of Vaccine Effectiveness of CoronaVac and BNT162b2 Against Severe Outcomes Over Time Among Patients With SARS-CoV-2 Omicron. JAMA Netw. Open 2023, 6, e2254777. [Google Scholar] [CrossRef]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.-G.; Lévy, J.-P.; Meulien, P. Induction of Virus-specific Cytotoxic T Lymphocytes in Vivo by Liposome-entrapped MRNA. Eur. J. Immunol. 1993, 23, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Teresa Vietri, M.; D’Elia, G.; Caliendo, G.; Passariello, L.; Albanese, L.; Maria Molinari, A.; Francesco Angelillo, I. Antibody Levels after BNT162b2 Vaccine Booster and SARS-CoV-2 Omicron Infection. Vaccine 2022, 40, 5726–5731. [Google Scholar] [CrossRef] [PubMed]
- Shrotri, M.; Fragaszy, E.; Nguyen, V.; Navaratnam, A.M.D.; Geismar, C.; Beale, S.; Kovar, J.; Byrne, T.E.; Fong, W.L.E.; Patel, P.; et al. Spike-Antibody Responses to COVID-19 Vaccination by Demographic and Clinical Factors in a Prospective Community Cohort Study. Nat. Commun. 2022, 13, 5780. [Google Scholar] [CrossRef]
- Ward, H.; Whitaker, M.; Flower, B.; Tang, S.N.; Atchison, C.; Darzi, A.; Donnelly, C.A.; Cann, A.; Diggle, P.J.; Ashby, D.; et al. Population Antibody Responses Following COVID-19 Vaccination in 212,102 Individuals. Nat. Commun. 2022, 13, 907. [Google Scholar] [CrossRef]
- Filardi, B.A.; Monteiro, V.S.; Schwartzmann, P.V.; do Prado Martins, V.; Zucca, L.E.R.; Baiocchi, G.C.; Malik, A.A.; Silva, J.; Hahn, A.M.; Chen, N.F.; et al. Age-Dependent Impairment in Antibody Responses Elicited by a Homologous CoronaVac Booster Dose. Sci. Transl. Med. 2023, 15, eade6023. [Google Scholar] [CrossRef]
- Anjos, D.; Fiaccadori, F.S.; do Prado Servian, C.; da Fonseca, S.G.; Guilarde, A.O.; Borges, M.A.S.B.; Franco, F.C.; Ribeiro, B.M.; Souza, M. SARS-CoV-2 Loads in Urine, Sera and Stool Specimens in Association with Clinical Features of COVID-19 Patients. J. Clin. Virol. Plus 2022, 2, 100059. [Google Scholar] [CrossRef] [PubMed]
- RT-PCR SARS-CoV-2 Coronavirus Detection. Available online: https://www.idtdna.com/pages/landing/coronavirus-research-reagents/cdc-assays (accessed on 8 May 2023).
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A Serological Assay to Detect SARS-CoV-2 Seroconversion in Humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef]
- Cunha, L.E.R.; Stolet, A.A.; Strauch, M.A.; Pereira, V.A.R.; Dumard, C.H.; Gomes, A.M.O.; Monteiro, F.L.; Higa, L.M.; Souza, P.N.C.; Fonseca, J.G.; et al. Polyclonal F(Ab’)2 Fragments of Equine Antibodies Raised against the Spike Protein Neutralize SARS-CoV-2 Variants with High Potency. iScience 2021, 24, 103315. [Google Scholar] [CrossRef]
- Andreata-Santos, R.; Machado, R.R.G.; Alves, R.P.D.S.; Sales, N.S.; Soares, C.P.; Rodrigues, K.B.; Silva, M.O.; Favaro, M.T.D.P.; Rodrigues-Jesus, M.J.; Yamamoto, M.M.; et al. Validation of Serological Methods for COVID-19 and Retrospective Screening of Health Employees and Visitors to the São Paulo University Hospital, Brazil. Front. Cell. Infect. Microbiol. 2022, 12, 677. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.R.; Ruiz, C.M.R.; Machado, R.R.G.; Magawa, J.Y.; Daher, I.P.; Urbanski, A.H.; Schmitz, G.J.H.; Arcuri, H.A.; Ferreira, M.A.; Sasahara, G.L.; et al. Immunodominant Antibody Responses Directed to SARS-CoV-2 Hotspot Mutation Sites and Risk of Immune Escape. Front. Immunol. 2023, 13, 1010105. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, G.X.; Sasahara, G.L.; Magawa, J.Y.; Nunes, J.P.S.; Bruno, F.R.; Kuramoto, A.C.; Almeida, R.R.; Ferreira, M.A.; Scagion, G.P.; Candido, É.D.; et al. Reduced T Cell and Antibody Responses to Inactivated Coronavirus Vaccine Among Individuals Above 55 Years Old. Front. Immunol. 2022, 13, 812126. [Google Scholar] [CrossRef]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and Cellular Immune Memory to Four COVID-19 Vaccines. Cell 2022, 185, 2434–2451.e17. [Google Scholar] [CrossRef]
- Pegu, A.; O’Connell, S.E.; Schmidt, S.D.; O’Dell, S.; Talana, C.A.; Lai, L.; Albert, J.; Anderson, E.; Bennett, H.; Corbett, K.S.; et al. Durability of MRNA-1273 Vaccine-Induced Antibodies against SARS-CoV-2 Variants. Science 2021, 373, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S. Antibody Persistence through 6 Months after the Second Dose of MRNA-1273 Vaccine for COVID-19. N. Engl. J. Med. 2021, 384, 2257–2259. [Google Scholar] [CrossRef]
- Chenchula, S.; Karunakaran, P.; Sharma, S.; Chavan, M. Current Evidence on Efficacy of COVID-19 Booster Dose Vaccination against the Omicron Variant: A Systematic Review. J. Med. Virol. 2022, 94, 2969–2976. [Google Scholar] [CrossRef]
- Chiu, N.C.; Chi, H.; Tu, Y.K.; Huang, Y.N.; Tai, Y.L.; Weng, S.L.; Chang, L.; Huang, D.T.N.; Huang, F.Y.; Lin, C.Y. To Mix or Not to Mix? A Rapid Systematic Review of Heterologous Prime–Boost COVID-19 Vaccination. Expert Rev. Vaccines 2021, 20, 1211–1220. [Google Scholar] [CrossRef]
- Çağlayan, D.; Süner, A.F.; Şiyve, N.; Güzel, I.; Irmak, Ç.; Işik, E.; Appak, Ö.; Çelik, M.; Öztürk, G.; Alp Çavuş, S.; et al. An Analysis of Antibody Response Following the Second Dose of CoronaVac and Humoral Response after Booster Dose with BNT162b2 or CoronaVac among Healthcare Workers in Turkey. J. Med. Virol. 2022, 94, 2212–2221. [Google Scholar] [CrossRef]
- Zuo, F.; Abolhassani, H.; Du, L.; Piralla, A.; Bertoglio, F.; de Campos-Mata, L.; Wan, H.; Schubert, M.; Cassaniti, I.; Wang, Y.; et al. Heterologous Immunization with Inactivated Vaccine Followed by MRNA-Booster Elicits Strong Immunity against SARS-CoV-2 Omicron Variant. Nat. Commun. 2022, 13, 2670. [Google Scholar] [CrossRef] [PubMed]
- Costa Clemens, S.A.; Weckx, L.; Clemens, R.; Almeida Mendes, A.V.; Ramos Souza, A.; Silveira, M.B.V.; da Guarda, S.N.F.; de Nobrega, M.M.; de Moraes Pinto, M.I.; Gonzalez, I.G.S.; et al. Heterologous versus Homologous COVID-19 Booster Vaccination in Previous Recipients of Two Doses of CoronaVac COVID-19 Vaccine in Brazil (RHH-001): A Phase 4, Non-Inferiority, Single Blind, Randomised Study. Lancet 2022, 399, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.d.C.R.; Vasconcelos, G.S.; de Melo, A.C.L.; Matsui, T.C.; Caetano, L.F.; de Carvalho Araújo, F.M.; Fonseca, M.H.G. Influence of Age, Gender, Previous SARS-CoV-2 Infection, and Pre-Existing Diseases in Antibody Response after COVID-19 Vaccination: A Review. Mol. Immunol. 2023, 156, 148–155. [Google Scholar] [CrossRef]
- Falahi, S.; Kenarkoohi, A. Sex and Gender Differences in the Outcome of Patients with COVID-19. J. Med. Virol. 2021, 93, 151–152. [Google Scholar] [CrossRef]
- Fujigaki, H.; Yamamoto, Y.; Koseki, T.; Banno, S.; Ando, T.; Ito, H.; Fujita, T.; Naruse, H.; Hata, T.; Moriyama, S.; et al. Antibody Responses to BNT162b2 Vaccination in Japan: Monitoring Vaccine Efficacy by Measuring IgG Antibodies against the Receptor-Binding Domain of SARS-CoV-2. Microbiol. Spectr. 2022, 10, e01181-21. [Google Scholar] [CrossRef]
- Bayram, A.; Demirbakan, H.; Günel Karadeniz, P.; Erdoğan, M.; Koçer, I. Quantitation of Antibodies against SARS-CoV-2 Spike Protein after Two Doses of CoronaVac in Healthcare Workers. J. Med. Virol. 2021, 93, 5560–5567. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. Personalized Vaccinology: A Review. Vaccine 2018, 36, 5350–5357. [Google Scholar] [CrossRef]
- Dietz, L.L.; Juhl, A.K.; Søgaard, O.S.; Reekie, J.; Nielsen, H.; Johansen, I.S.; Benfield, T.; Wiese, L.; Stærke, N.B.; Jensen, T.Ø.; et al. Impact of Age and Comorbidities on SARS-CoV-2 Vaccine-Induced T Cell Immunity. Commun. Med. 2023, 3, 58. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Landin, A.M.; Blomberg, B.B. Age Effects on B Cells and Humoral Immunity in Humans. Ageing Res. Rev. 2011, 10, 330–335. [Google Scholar] [CrossRef]
- Cancro, M.P.; Hao, Y.; Scholz, J.L.; Riley, R.L.; Frasca, D.; Dunn-Walters, D.K.; Blomberg, B.B. B Cells and Aging: Molecules and Mechanisms. Trends Immunol. 2009, 30, 313–318. [Google Scholar] [CrossRef]
- Sugiyama, A.; Kurisu, A.; Nagashima, S.; Hando, K.; Saipova, K.; Akhmedova, S.; Abe, K.; Imada, H.; Hussain, M.R.A.; Ouoba, S.; et al. Seroepidemiological Study of Factors Affecting Anti-Spike IgG Antibody Titers after a Two-Dose MRNA COVID-19 Vaccination in 3744 Healthy Japanese Volunteers. Sci. Rep. 2022, 12, 16294. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, C.; Wang, J.; Liu, Y. The Factors Correlated with COVID-19 Vaccination Coverage in Chinese Hypertensive Patients Managed by Community General Practitioner. Hum. Vaccines Immunother. 2023, 19, 2197839. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of Comorbidities and Its Effects in Coronavirus Disease 2019 Patients: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Chung, H.; He, S.; Nasreen, S.; Sundaram, M.E.; Buchan, S.A.; Wilson, S.E.; Chen, B.; Calzavara, A.; Fell, D.B.; Austin, P.C.; et al. Effectiveness of BNT162b2 and MRNA-1273 COVID-19 Vaccines against Symptomatic SARS-CoV-2 Infection and Severe COVID-19 Outcomes in Ontario, Canada: Test Negative Design Study. BMJ 2021, 374, n1943. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Dall’Olmo, L.; della Rocca, F.; Barbaro, F.; Cosma, C.; Basso, D.; Cattelan, A.; Cianci, V.; Plebani, M. Antibody Response to First and Second Dose of BNT162b2 in a Cohort of Characterized Healthcare Workers. Clin. Chim. Acta 2021, 519, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.X.; Durrant, L.G.; Chok, Y.L.; Lai, O.M. Susceptibility to SARS-CoV-2 Omicron Following ChAdOx1 NCoV-19 and BNT162b2 versus CoronaVac Vaccination. iScience 2022, 25, 105379. [Google Scholar] [CrossRef] [PubMed]





| ChAdOx1 nCoV-19 (n = 33) | BNT162b2 (n = 27) | CoronaVac (n = 18) | p Value (ChAdOx1 nCoV-19 vs. BNT162b2) | p Value (ChAdOx1 nCoV-19 vs. CoronaVac) | p Value (BNT162b2 vs. CoronaVac) | |
|---|---|---|---|---|---|---|
| Sex. No (%) | ||||||
| Women (n = 49) | 23 (69.7) | 11 (40.7) | 15 (83.3) | 0.0363 * | 0.3359 | 0.0061 ** |
| Men (n = 29) | 10 (30.3) | 16 (59.3) | 3 (16.7) | |||
| Age group. No (%) | ||||||
| 18–30 | 20 (60.6) | 15 (55.5) | 4 (22.2) | 0.7944 | 0.0176 * | 0.0347 * |
| 31–50 | 5 (15.2) | 4 (14.8) | 11 (61.1) | >0.9999 | 0.0013 ** | 0.0029 ** |
| >50 | 8 (24.2) | 8 (29.7) | 3 (16.7) | 0.7711 | 0.7255 | 0.4824 |
| Median age (minimum and maximum) | 26 (21–65) | 30 (19–59) | 40 (22–54) | |||
| Collection times (mean ± SD) | ||||||
| 1 month post-second dose (days) | 101.3 ± 4.11 | 113.8 ± 16.6 | - | |||
| 4–6 months post-second dose (days) | 205.0 ± 3.5 | 213.8 ± 21.3 | 234.1 ± 9.9 | |||
| 1 month post-third dose (days) | 296.9 ± 22.0 | 259.3 ± 31.5 | 310.7 ± 7.8 | |||
| Comorbidities. No (%) | ||||||
| Diabetes | 2 (6.06) | 3 (11.1) | 0 | 0.6494 | 0.5341 | 0.2636 |
| Hypertension | 1 (3.03) | 2 (7.4) | 2 (11.1) | 0.5834 | 0.2816 | >0.9999 |
| Autoimmune diseases | 1 (3.03) | 2 (7.4) | 3 (16.6) | 0.5834 | 0.1200 | 0.3751 |
| Respiratory diseases | 1 (3.03) | 1 (5.6) | 2 (11.1) | >0.9999 | 0.2816 | 0.5548 |
| Others | 2 (6.06) | 2 (3.7) | 2 (11.1) | >0.9999 | 0.6070 | >0.9999 |
| No comorbidities | 27 (81.8) | 8 (29.6) | 10 (55.5) | 0.0148 * | 0.0620 | 0.7459 |
| Individuals by Collection Period. No (% in Relation to the Total) | Pre-Vaccine | 1 Month Post-Second Dose | 4–6 Months Post-Second Dose | 1 Month Post-Third Dose |
|---|---|---|---|---|
| ChAdOx1 nCoV-19 (n = 33) | 32 (96.9) | 31 (94) | 30 (91) | 26 (78.8) |
| Women (n = 23) | 21 (65.6) | 20 (64.5) | 19 (63.3) | 16 (61.5) |
| Men (n = 10) | 11 (34.4) | 11 (35.5) | 11 (36.7) | 10 (38.5) |
| BNT162b2 (n= 27) | 7 (25.9) | 26 (96.2) | 21 (77.7) | 20 (74.1) |
| Women (n = 11) | 2 (28.6) | 11 (42.3) | 10 (47.6) | 9 (45) |
| Men (n = 16) | 5 (71.4) | 15 (57.7) | 11 (52.4) | 11 (55) |
| CoronaVac (n= 18) | 1 (5.6) | - | 17 (94.4) | 10 (55.5) |
| Women (n = 15) | - | - | 14 (82.4) | 9 (90) |
| Men (n = 3) | - | - | 3 (17.6) | 1 (10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masson, L.C.; Servian, C.d.P.; Jardim, V.H.; dos Anjos, D.; Dorta, M.L.; Batalha-Carvalho, J.V.; Moro, A.M.; Romão, P.R.T.; Souza, M.; Fiaccadori, F.S.; et al. Heterologous Booster with BNT162b2 Induced High Specific Antibody Levels in CoronaVac Vaccinees. Vaccines 2023, 11, 1183. https://doi.org/10.3390/vaccines11071183
Masson LC, Servian CdP, Jardim VH, dos Anjos D, Dorta ML, Batalha-Carvalho JV, Moro AM, Romão PRT, Souza M, Fiaccadori FS, et al. Heterologous Booster with BNT162b2 Induced High Specific Antibody Levels in CoronaVac Vaccinees. Vaccines. 2023; 11(7):1183. https://doi.org/10.3390/vaccines11071183
Chicago/Turabian StyleMasson, Letícia Carrijo, Carolina do Prado Servian, Vitor Hugo Jardim, Déborah dos Anjos, Miriam Leandro Dorta, João Victor Batalha-Carvalho, Ana Maria Moro, Pedro Roosevelt Torres Romão, Menira Souza, Fabiola Souza Fiaccadori, and et al. 2023. "Heterologous Booster with BNT162b2 Induced High Specific Antibody Levels in CoronaVac Vaccinees" Vaccines 11, no. 7: 1183. https://doi.org/10.3390/vaccines11071183
APA StyleMasson, L. C., Servian, C. d. P., Jardim, V. H., dos Anjos, D., Dorta, M. L., Batalha-Carvalho, J. V., Moro, A. M., Romão, P. R. T., Souza, M., Fiaccadori, F. S., & Fonseca, S. G. (2023). Heterologous Booster with BNT162b2 Induced High Specific Antibody Levels in CoronaVac Vaccinees. Vaccines, 11(7), 1183. https://doi.org/10.3390/vaccines11071183






