Abstract
Rhipicephalus microplus economically impacts cattle production in tropical and subtropical countries. Application of acaricides constitutes the major control method; however, inadequate use has increased resistant tick populations, resulting in environmental and cattle product contamination. Anti-tick vaccines based on the Bm86 antigen are an environmentally friendly, safe, and economically sustainable alternative for controlling R. microplus infestations. Nevertheless, variable efficacy has been experienced against different geographic tick strains. Herein, we evaluated the efficacy of a conserved polypeptide Bm86 derived from a Mexican R. microplus strain previously characterized. Twelve cows were assigned to three experimental groups and immunized with three doses of the polypeptide Bm86 (pBm86), adjuvant/saline alone, and Bm86 antigen (control +), respectively. Specific IgG antibody levels were measured by ELISA and confirmed by Western blot. In addition, the reproductive performance of naturally infested R. microplus was also determined. The more affected parameter was the adult female tick number, with a reduction of 44% by the pBm86 compared to the controls (p < 0.05), showing a vaccine efficacy of 58%. Anti-pBm86 IgG antibodies were immunogenic and capable of recognizing the native Bm86 protein in the eggs, larvae, and guts of R. microplus. The negative correlation between antibody levels and the reduction of naturally tick-infested cattle suggested that the effect of the polypeptide Bm86 was attributed to the antibody response in immunized cattle. In conclusion, the polypeptide Bm86 showed a specific immune response in cattle and conferred protection against R. microplus in a Mexican tropical region. These findings support further experiments with this antigen to demonstrate its effectiveness as a regional vaccine.
1. Introduction
Rhipicephalus microplus is the principal vector in cattle production due to the direct skin lesions caused by the hematophagous action and indirect effect as a biological or mechanical vector of multiple diseases, such as babesiosis and anaplasmosis [1,2]. R. microplus is distributed in the tropical and subtropical countries of Africa, Australia, and Latin America (32° N, 32° S), which is the major challenge to the cattle industry, affecting milk and meat production [3]. In this context, Mexico is located between the parallels 32° N y 14° S, where the climate is predominantly humid-tropical and presents the appropriate conditions for the recurrent appearance of tick infestations [4,5]. As a result, at least 4 million cattle heads in the Mexican tropics [6] are exposed to damage, affecting livestock health and the economy of ranchers [2,7]. Furthermore, these infestations are not limited to these regions; national economic losses are estimated at around USD 573,608,076 [5].
For decades, R. microplus has been controlled by acaricides. However, inadequate and excessive use has occasioned the appearance of multi-resistant strains. The Mexican market distributes organophosphates, pyrethroids, amidines, phenylpyrazolones, IGRs, and macrocyclic lactones. Nevertheless, they have been surpassed by the genetic plasticity shown by R. microplus [8,9]. Hence, alternative approaches have been proposed, including pasture rotation, selection of resistant cattle (Bos indicus), and biological control using tick-predatory organisms, such as fungi, nematodes, bacteria, and arthropods [10,11,12,13]; most of these practices are desirable to reduce chemical residues in cattle products and the environment [14]. On the other hand, immunological control is one of the most important approaches developed since the 1990s and uses the Bm86 antigen, a glycoprotein located at the epithelial cell membrane of the R. microplus tick gut [15,16,17]. Commercial tick vaccines have been distributed as TickGARDTM (Australia), GAVACTM (Latin America), and, more recently, Bovimune IxovacTM (Mexico). These vaccines use Escherichia coli or Pichia pastoris as recombinant expression platforms that are friendly to the environment and do not contaminate milk and meat products [18,19].
On broad literature analysis, numerous tests have been performed with Bm86, and variable efficacy between 45% and 100% controlled infestations with R. microplus, R. annulatus, and R. decoloratus strains have been experienced [3,18,19,20,21] due to sequence variations (>2.8%) in the target protein among different tick strains in America [22]. These patterns also have been observed in the Bm86 protein in Mexican R. microplus strains [23]. Nevertheless, field trials with Bm86-derived vaccines have been established in Latin America and Australia as an integrated tick management program; the program controlled R. microplus infestations through reduced tick feeding, decreased reproductive capacity and egg lying, and the reduction of larvae in the field in subsequent generations [15,24].
Following the premise that the Bm86 sequence is variable, developing immunogens based on the complete protein of each local strain appears to be a viable and logical strategy. However, it is likely to be a slow undertaking with limited scope in terms of resources and future expectations. In contrast, vaccines developed from conserved regions across tick strains and potential immunogenic activity appear desirable to avoid the variability observed in the Bm86 protein [23,25,26].
Currently, different approaches are being considered for identifying and characterizing candidate tick antigens; some of these proteins are conserved across the tick species, involved in blood digestion, pathogen transmissions, and tick-host-pathogen interface, and should be accessible to the host immune system [14,27]. Therefore, vaccines based on this strategy have been designed to focus on hydrophobicity, antigenicity, cellular localization, motifs to predict regions with immunogenic potential, and epitopes recognized by B and T cells that could elicit specific humoral and cellular immune responses characterized by IgG antibodies and the complement proteins [28,29,30].
Studies of the Bm86 protein revealed that it contains antigenic epitopes, which presented within 20 different South American tick strains [31], and one of which was tested as a synthetic peptide and showed significant IgG levels and an efficacy of 81.05% in cattle against R. microplus [32]. Moreover, in silico analysis predicted conserved epitopes in the Bm86 protein from different geographical regions using a Brazilian Bm86 sequence of the R. microplus strain as a reference [26]. Additionally, some experimental vaccines based on the orthologous Bm86 have successfully controlled infestation with R. microplus, R. australis, R. annulatus, and R. decoloratus [33,34,35].
The approach used in this study is based on previous bioinformatics and molecular analysis of a polypeptide derived from the Bm86 protein, performed in a Mexican R. microplus strain. This immunogenic region consists of 178 amino acids and a 98–100% identity/similarity concerning strains of R. microplus ticks from the Americas [36]. Our findings may become valuable for developing a regional vaccine against R. microplus. Thus, this work aims to evaluate the efficacy of a conserved polypeptide derived from the Bm86 in cattle to improve and propose a new formulation against R. microplus infestations in the Mexican tropics.
2. Materials and Methods
2.1. Ticks
The susceptible “Media Joya” strain of R. microplus was obtained from laboratory colonies kept at the Tick Laboratory of the National Center of Disciplinary Research in Animal Health and Safety from the National Institute for Forestry, Agricultural and Livestock Research (CENID-SAI, INIFAP) in Jiutepec, Morelos, Mexico. This tick strain was collected originally from cattle infested in Tapalpa, Jalisco, Mexico.
2.2. Cloning, Expression, and Purification of Recombinant Polypeptide Bm86
The cloning of the experimental Bm86 fragment in Escherichia coli was done as described previously [36]. In short: the encoding Bm86 fragment was cloned using the expression vector pET101/D-TOPO® (Invitrogen, Waltham, MA, USA). The recombinant constructs were then transformed into One Shot® Top 10 E. coli cells (Invitrogen, Waltham, MA, USA) according to the standardized instructions provided by the fabricant.
For expression of the recombinant polypeptide Bm86, plasmids were transformed into E. coli BL21 Star (DE3), propagated in Luria-Bertani (LB) broth with ampicillin (50 μg/mL) and cultured at 37 °C. The recombinant synthesis was induced with 1 mM of isopropyl-beta-D-thiogalactopyranoside (IPTG) for 5 h. The cells were disrupted by sonication six times at 40 Hz on ice, centrifuged at 14,000× g for 10 min at 4 °C, and stored at −20 °C. The recombinant expression was confirmed by 15% SDS-PAGE gels stained with Coomassie Brilliant Blue. Western blot analysis was performed throughgels that were transferred to polyvinylidene difluoride (PVDF) membranes and blocked in TBS containing 0.05% of Tween-20% (TBS-T) and 5% skim milk for 1 h at room temperature and then overnight at 4 °C. An anti-His monoclonal antibody (Invitrogen) alkaline phosphatase conjugate was used 1:2000 to detect the recombinant His6x-fusion protein. After three washes with TBS-T, the color was developed using a BCIP/NBT alkaline phosphatase substrate (Millipore, Billerica, MA, USA). The recombinant proteins were purified under denatured conditions with a NI-NTA spin kit (Qiagen, Hilden, Germany) by Ni affinity chromatography according to the method recommended by the manufacturer.
2.3. Location and Cattle
The field trial was carried out in the Centre for Teaching, Research and Extension in Tropical Livestock (CEIEGT) from the National Autonomous University of Mexico (UNAM) (20°02′ N, 97°06′ W) in Martinez de la Torre, Veracruz, Mexico. The study area has a humid tropical climate with an average daily temperature of 23.4 °C, relative humidity of 85%, and annual rainfall of 1743 mm [37]. Twelve crossbred cows (Bos taurus × Bos indicus), aged 6–7 years with an average weight of 600 kg and prior exposure to R. microplus infestation, were used for the experiment. The cattle were apparently healthy and raised in an extensive grazing system on native grasses and water ad libitum. Animals had not been treated with acaricides or been pregnant or nursing calves in the preceding month. All the cows in this study were handled according to the Institutional Animal Care and Use Committee (SICUAE) of the Faculty of Veterinary Medicine and Zootechnics (FMVZ) from the UNAM and supervised by a veterinarian.
2.4. Immunization Experiment
Cattle were randomly divided into three experimental groups of four cows each. Group 1 was immunized with the polypeptide Bm86 emulsified with Montanide ISA 50 V oil adjuvant (Seppic, France). The cows of group 2 were injected with the recombinant Bm86 antigen as a positive control. Group 3 received an emulsion composed of 1 mL of phosphate-buffered saline (PBS) solution plus 1 mL of Montanide ISA 50 V per dose and were considered control. Cows in the immunized groups received three doses containing 100 μg/2 mL at days 0, 30, and 49 via subcutaneous injection. All animals were monitored for local reactions or clinical signs post-immunization.
2.5. Cattle Blood Collection
Throughout 63 days, blood samples were obtained from all 12 animals at weekly intervals. Samples were collected from the caudal vein using sterile tubes before each immunization and post-immunization period. The blood samples were centrifuged at 4000 rpm for 15 min at 25 °C, and serum was separated and stored at –20 °C until further analysis by indirect ELISA and Western blot.
2.6. ELISA Antibody Serology
Purified recombinant proteins were obtained according to the previous procedure. ELISA plates were coated overnight at 4 °C with 1 μg/well of the antigens. Subsequently, the wells were washed three times using PBS containing 0.05% of Tween-20% (PBS-T), blocked with 5% skim milk in PBS-T, and incubated for 1 h at room temperature. Following three additional washes with PBS-T, the plates were incubated with sera samples diluted 1:100 in PBS-T for 1 h at room temperature and then incubated with 1:2000 anti-bovine IgG-AP conjugate (Sigma, St. Louis, MO, USA) for 1 h at room temperature. The color reaction was developed using 3,3′,5,5′-tetramethylbenzidine (Sigma), and the optical density (OD) was measured at 405 nm. Finally, antibody levels were considered positive when the OD405 nm value was at least twice as high as the pre-immune serum and were compared using a one-way ANOVA test (p < 0.05).
2.7. Detection of Native Bm86 Protein in R. microplus Tissues by Western Blotting
A total of five ingurgitated R. microplus female ticks were collected and dissected to separate the guts using fine forceps, washed twice in PBS, and stored at –70 °C. Additionally, 100 mg of larvae and egg mass were pulverized using a frozen mortar and placed in an Eppendorf tube with 1 mL of PBS. The extracts were prepared following the method described by Popara [38]. Protein concentrations were determined by a Bradford protein assay using bovine serum albumin as a reference standard. Afterward, protein extracts were separated using 15% SDS-PAGE gels and transferred to PVDF membranes. The PVDF sheets were blocked in PBS containing 0.05% of Tween-20% (PBS-T) and 5% skim milk for 1 h at 4 °C with gentle rocking. After three washes with PBS-T, the membranes were cut into strips (~0.4 mm) and incubated with serum from one representative bovine per experimental group, collected on day 35. The serum was diluted 1:300 in PBS-T and incubated for 2 h at 4 °C. Then, strips were washed three times and incubated with anti-bovine IgG-AP conjugate (Sigma, St. Louis, MO, USA) diluted 1:2000 in PBS-T for 2 h at room temperature. The positive signal was developed using a BCIP/NBT alkaline phosphatase substrate (Millipore, Billerica, MA, USA).
2.8. Data Collection and Evaluation
The animals were kept in five paddocks fenced with barbed wire and naturally infested with R. microplus. The paddock rotation was considered; the cows roamed free in paddock 1, followed by 2,3,4, and 5, respectively (Figure 1). The cattle grazed in the paddocks for between 7 and 10 days.
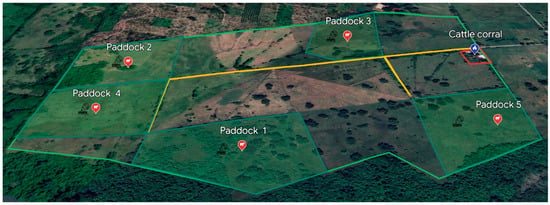
Figure 1.
Spatial distribution of cattle in paddocks at CEIEGT-UNAM (Rancho “El Clarín”), Veracruz, Mexico.
To assess the vaccination effects on tick biology, adult female ticks (4.5–8 mm) were collected weekly on one side of the animal and multiplying by two to obtain the total number of adult ticks per animal [39]. All the ticks collected were counted and individually weighted and placed in tick chambers maintained at 27 °C and 80% relative humidity to allow oviposition and larvae hatching. The vaccine efficacy was measured based on reduced number of adult female ticks, tick weight, oviposition, and egg fertility. The percent reduction was calculated with respect to the control group using the standardized formula [34,40].
2.9. Statistical Analysis
The statistic comparison of tick number, tick weight, oviposition, and egg fertility for each group was analyzed using a one-way ANOVA test. A least significant difference (LSD) test was applied to verify the differences between vaccinated and control cattle, and data with p < 0.05 were considered statistically significant. The results are presented as average ± standard deviation (SD). Additionally, a correlation analysis was performed using Spearman’s test to compare the number of ticks collected after feeding with the antibody levels over time in each cow. These statistical procedures were carried out using StatGraphics® software (version 19.1.3).
3. Results
3.1. Characterization and Production of Recombinant Polypeptide Bm86
The sequence for the Bm86 fragment encodes 178 amino acids and was derived from the R. microplus “Media Joya” tick strain. The immunogenic region selected was expressed in E. coli as an inclusion body, solubilized using 8 M urea, and purified by His-affinity chromatography. After induction, expression levels of the polypeptide Bm86 reached approximately 10% of total cellular proteins. The molecular size was approximately 26 kDa, based on visualization on SDS-PAGE gels and Western blot using an anti-His monoclonal antibody recognized the His6x-fusion protein on the C-terminal of the pBm86 (Figure 2). The purified recombinant polypeptide Bm86 was successfully obtained, and the sample purity was analyzed and estimated to be 90% pure.
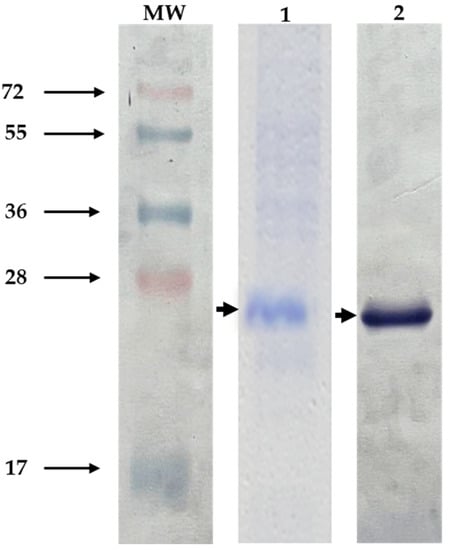
Figure 2.
Recombinant polypeptide Bm86 expression. Protein samples containing the recombinant pBm86 were analyzed by 15% SDS-PAGE gels stained with Coomassie Blue and Western blotting. (1) induced E. coli cultures with IPTG expressing the recombinant polypeptide Bm86. (2) Western blot probed with anti-His monoclonal antibody (1:2000). Arrows indicate the size of the recombinant Bm86. MW, molecular weight.
3.2. Effect of Immunization on Biological Parameters of R. microplus Ticks
Table 1 shows the effect on the biological parameters of engorged adult female R. microplus ticks collected from the cattle immunized. The immunization of cattle with the polypeptide Bm86 and Bm86 antigen reduced the number of ticks engorged on the animals by 44% and 48%, respectively, compared to the control group (p < 0.05). Additionally, several ticks collected from both experimental groups were visibly damaged and showed effects such as physical damage, dry appearance, a dark-red coloration, and, in some cases, death was observed (Figure 3). The tick weight was not affected; the oviposition was reduced by 16% for the polypeptide Bm86 and 14% for the Bm86 antigen compared to the controls. Furthermore, egg fertility was moderately reduced by 11% and 5% for the polypeptide and Bm86, respectively. The overall efficacy of the polypeptide Bm86 was 58%, compared to 57% for the Bm86 antigen, against R. microplus infestations.

Table 1.
Efficacy of the polypeptide Bm86 against natural infestations by Rhipicephalus microplus ticks.
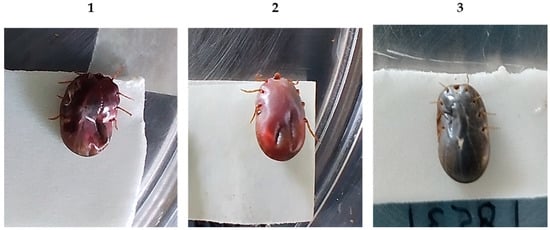
Figure 3.
Ticks were collected from experimental groups on day 56. The damaged tick engorged on (1) polypeptide Bm86 and (2) Bm86 showed a characteristic dark-red coloration. (3) Ticks collected from controls.
3.3. Humoral Immune Response
The humoral immune response of cattle immunized with the polypeptide Bm86 and the formulation containing the Bm86 antigen are shown in Figure 4. The IgG levels in the polypeptide Bm86-immunized animals increased after the first immunization and decreased after the second immunization, achieving at least 2.40 OD units compared to the control group. Cattle immunized with the Bm86 antigen generated a slightly humoral immune response one week after the second booster. However, a significant decline occurred after the third booster obtained, at most, 1.90 OD IgG levels. These results showed that antibody levels were atypical in all animals tested and only were significantly different (p < 0.05) on some days of the experiment.
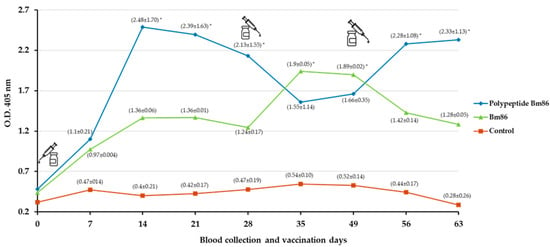
Figure 4.
Antibody response in immunized cattle. Bovine serum antibody levels to recombinant antigens were determined by indirect ELISA. Antibody levels in immunized cattle were expressed as the OD405 nm value for the 1:100 serum dilution and compared between immunized and control cattle using a one-way ANOVA test (* p < 0.05; N = 4). The time of the three immunization shots is indicated.
3.4. Western Blot Detection of Native Bm86 Protein in Tick Tissues
Western blot revealed that sera collected at day 35 from cattle immunized with the 2 antigens recognize the native Bm86 protein compared to the control group (Figure 5). The IgG antibodies produced from one representative cow per group recognized tick proteins derived from engorged R. microplus female guts. Furthermore, the antibodies also recognized the native protein in larvae and eggs. The analysis revealed an affinity for a protein of ~72 kDa, similar to the expected molecular weight of the Bm86 protein from the R. microplus ticks. In contrast, the native Bm86 protein was not recognized by the serum of the control animal. However, we detected other bands with different molecular weights in tick tissues; possibly, these antibodies were produced before the immunization trial due to previous tick exposure.
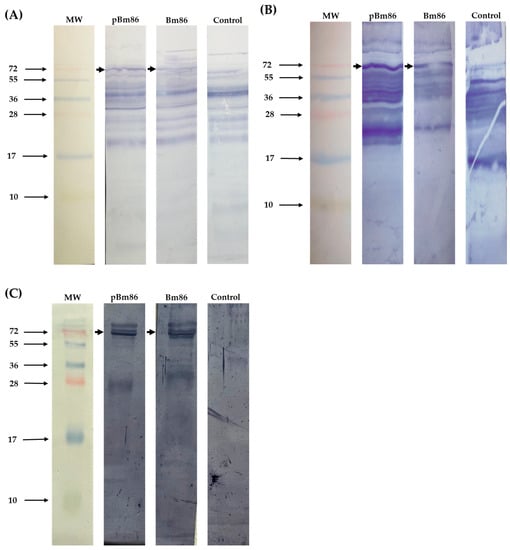
Figure 5.
Western blot of R. microplus tick tissues probed with anti-Bm86 IgG antibodies. 10 µg of (A) egg, (B) larvae, and (C) guts sections were loaded per well in 15% SDS-PAGE gels and transferred to PVDF membranes. Sera from one representative cow per group were used and developed with an anti-bovine IgG-AP conjugate. Native Bm86 protein is indicated with arrows. MW, molecular weight.
3.5. Correlation Analysis
The correlation analysis between the IgG antibody level at day 30, just after the first immunization, and the reduction of tick infestation until the end of the study (day 63) showed a negative correlation for ticks fed on polypeptide Bm86-immunized cattle (r = −0.93, p = 0.01), suggesting that the reduction of ticks collected after feeding was the result of the protective antibodies elicited in immunized cattle. However, no correlation was observed in ticks fed on animals immunized with the Bm86 antigen (r = −0.64, p = 0.09) (Figure 6).
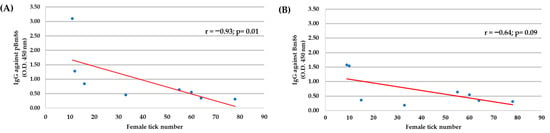
Figure 6.
The Spearman’s Rho correlation analyses (p < 0.05) were conducted to correlate the IgG antibody levels in immunized cattle with the adult female tick number collected during the experimental trial in individual cattle (N = 8). (A) polypeptide Bm86, and (B) Bm86. The linear correlation coefficients (r) and p-value are shown.
4. Discussion
Developing improved tick vaccines requires the characterization of new candidate tick-protective antigens and the design of more effective and cheaper anti-tick vaccine formulations. Different approaches are currently used in bioinformatics, such as immunoinformatics, based on the application of computational tools to study the molecules of the immune system. These are able to guide the design of experiments to answer important questions in immunobiology and vaccinology [41]. Following this strategy, this study is derived from in silico analysis of antigenic and immunogenic epitopes on the Bm86 protein to develop a conserved recombinant polypeptide using E. coli as a heterologous host for protein expression. This system provides an efficient and economically viable method to rapidly produce high quantities of recombinant protein, making it a cost-effective platform for developing immunogens. In contrast, other systems—such as yeast, insect, and mammalian cells— have outstanding protein production capabilities but nevertheless involve expensive development, steep learning curves, and significant standardization costs [42,43,44].
Herein, the polypeptide Bm86 evaluated in cattle against R. microplus ticks under field conditions confirmed the immunogenic potential to elicit a humoral immune response. The more affected parameter was the adult female tick number, with a reduction of 44% by the polypeptide Bm86 and 48% by the Bm86 antigen compared to the control group (p < 0.05). These findings were within the range reported in previous experiments [19,33,34,45,46]. Interestingly, both experimental groups did not significantly reduce tick weight, oviposition, and egg fertility compared to controls. Although the results achieved by the polypeptide Bm86 were higher in reducing oviposition (16%) and egg fertility (11%), these results contradict other research where reduction of the oviposition has an important effect due to interactions between anti-Bm86 antibodies and R. microplus ovary proteins [32,47,48,49]. This finding may be explained by the amount of protective anti-Bm86 antibodies elicited by cattle, sequence divergence, or possibly by recombinant protein folding. Nevertheless, the overall efficacy obtained with the polypeptide Bm86 (58%) and Bm86 antigen (57%) were similar to previous cattle field trials carried out in Argentina, Brazil, Colombia, Cuba, Mexico, and Venezuela, reaching 51–91% efficacy [19,45,46,50].
On the other hand, similar research using synthetic peptides derived from the Bm86 protein reported vaccine efficacy ranging from 70% to 81% [25,32]. In the same way, using P0-Bm86 protein conjugates has demonstrated great immunogenic capacity and obtained 84% vaccine efficacy in a controlled pen trial [48].
Both experimental groups demonstrated an immune response based on concentrations of anti-Bm86 antibody levels. Cattle immunized with the polypeptide Bm86 showed higher anti-pBm86 levels after the first immunization and decreased after the second immunization. However, remained significantly different (p < 0.05) compared to the control group, which has been commonly found in previous studies with the Bm86 vaccine [25,32,48,51]. Contrary to the Bm86 antigen results, antibody levels were low in immunized cattle, possibly for different reasons, such as the health condition and the age of the animals used in the experiment [40]. According to Mughini-Gras [52], studies with Leptospira spp. vaccines have shown a negative correlation between the age of cows and post-vaccination antibody titers, suggesting that immunosenescence increases in adult cattle over time, resulting in a reduced response to vaccination. Our results agree, since the average age of the cows used was 6.5 years; this probably revealed that older cattle have a weak organism affecting the immune response. It is known that CD4+ cells produce IL-2, a cytokine that induces the differentiation of B cells into antibody-secreting plasma cells. However, with aging, the capacity of CD4+ cells to produce IL-2 gradually decreases, resulting in limited activation of B cells [53]. Consequently, plasma cells are compromised, and the anti-Bm86 antibody production is diminished and shows moderated affinity for the antigen.
Moreover, studies in mice demonstrated that antibodies generated in old mice are less protective than those produced in young mice. In addition, IgG1 plasma cells elicit high-affinity antibodies in young mice, different from aged mice that predominantly produced IgM plasma cells [54]. Another study investigated the relationship between age and leukocyte population during the pre-calving period in cows, finding a lower number of B cells in older cows, which affected the role of antigen-presenting cells, production of antibodies, and the development of memory B cells. Our results are similar to previous research where the number of lymphocytes decreased due to aging, particularly in cows older than 1.5 years. Shihab [55] attributed this to the generation of free radicals that affect the composition of cell membranes and compromise lymphocyte survival.
A negative correlation was found between the number of engorged female ticks and the level of anti-pBm86 IgG antibodies in cattle; this suggests that protective antibodies were directed against tick protein epitopes in the recombinant polypeptide, occasioning a reduced number of adult female ticks collected. These results agree with those reported previously [33,45,48,49] and underline the anti-tick vaccine’s effect on controlling cattle tick infestations. On the other hand, the Bm86-immunized cattle showed low antibody levels and no significant correlation, possibly due to using older cattle in the study.
The Western blot analysis indicates that both antigens are immunogenic and induced protective antibodies; anti-IgG antibodies recognized the native Bm86 protein present in the eggs, larvae, and guts of R. microplus, whereas pre-immune sera recognized other native proteins due to the presence of natural antibodies in cattle. Furthermore, the recognition of anti-pBm86 antibodies to larvae and midgut proteins was strongly detected, suggesting a higher expression, similar to those tick antigens previously tested against R. microplus tick infestations [39,45,49,56]. In addition, some ticks collected on vaccinated cattle showed marked changes from their standard gray/green color to a characteristic dark-red color, indicating gut damage. The atrophy of guts observed is the same as previous vaccinated cattle studies where ingesting blood containing anti-tick antibodies affected multiple biological processes [40,57,58,59,60] and commonly reduced the biotic potential, resulting in a progressive reduction of tick populations.
Although the conditions present in this study were a field trial in a free-grazing system and with previous exposure to R. microplus tick infestations, the results obtained with the polypeptide Bm86 were slightly better than other reports [45,50], probably due to the conserved epitopes considered in the polypeptide design, which are effective to elicit antibody levels and demonstrate immunogenic potential in cattle against R. microplus [36]. However, the results reflected the fact that increased age and physiological status of the animals affected the response to the vaccination without forgetting that environmental factors, such as stress and condition, could also be involved in cow-to-cow variation in response to vaccination, as suggested by de la Fuente [61].
Several authors currently follow reverse vaccinology as a better method to produce tick antigens because it offers advantages such as the speed identification and characterization of novel potential tick antigens [28]. Others focus their anti-tick vaccine research on combining antigens (cocktail anti-tick vaccines) or selecting protective peptides that could improve the efficacies of individual vaccines [62,63,64]. Those approaches are important to optimize the efficacy of vaccines, similar to what was done in this study with the polypeptide Bm86 derived from a Mexican R. microplus strain. In addition, it is essential to note the need to test immunogens against tick infestations under field conditions because vaccination trials are the main component required to determine if a tick antigen is immunoprotective and effective in reducing tick populations.
Finally, immunological control emerges as a financially feasible and ecologically favorable alternative, with high potential in developing integrated tick management options involving the rational use of acaricides, cultural practices, and/or biological control agents. This all-inclusive strategy aims to reduce the excessive use of acaricides, environmental contamination, and animal/human health risks, following the One Health context [65].
5. Conclusions
This study reports the first vaccination trial using a polypeptide Bm86 derived from a Mexican R. microplus strain against natural tick infestations. The results allow us to conclude that the polypeptide Bm86 can induce a specific immune response in cattle and confer protection against R. microplus in a Mexican tropical region. These findings highlight the importance of considering an integrated management approach for tick control and support further experiments with this antigen to demonstrate its effectiveness as a regional vaccine.
Author Contributions
R.C.: methodology, formal analysis, writing—original draft preparation. M.Á.A.-D.: visualization, resources, supervision. M.M.-V.: conceptualization, investigation, supervision. E.C.-S.: visualization, validation, methodology. R.H.-O.: conceptualization, methodology, investigation. R.L.-Q.: funding acquisition, project administration, supervision, writing–review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by CONACYT, project number 316097.
Institutional Review Board Statement
The study was approved by the Internal Sub-Committee for Care and Use of Experimental Animals (SICUAE.MC-2022/4-4) from the Faculty of Veterinary Medicine of the National Autonomous University of Mexico (FMVZ-UNAM).
Informed Consent Statement
Not applicable.
Data Availability Statement
The database and the statistical analyses are available upon reasonable request.
Acknowledgments
The authors thank the students and academics of Rancho “El Clarín” (CEIEGT-UNAM) and Nancy Mendoza-Martínez for technical assistance. Raymundo Coate was supported by the CONACYT fellowship, Mexico, and CEMAI-CONACYT for writing draft preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marques, R.; Krüger, R.F.; Peterson, A.T.; de Melo, L.F.; Vicenzi, N.; Jiménez-García, D. Climate change implications for the distribution of the babesiosis and anaplasmosis tick vector, Rhipicephalus (Boophilus) microplus. Veter Res. 2020, 51, 81. [Google Scholar] [CrossRef]
- Rojas Martínez, C.; Loza Rubio, E.; Rodríguez Camarillo, S.D.; Figueroa Millán, J.V.; Aguilar Romero, F.; Lagunes Quintanilla, R.E.; Morales Álvarez, J.F.; Santillán Flores, M.A.; Socci Escatell, G.A.; Álvarez Martínez, J.A. Background and perspectives of certain priority diseases affecting cattle farming in Mexico. Rev. Mex. Cienc. Pecu. 2021, 12, 111–148. [Google Scholar] [CrossRef]
- de la Fuente, J.; Almazán, C.; Canales, M.; de la Lastra, J.M.P.; Kocan, K.M.; Willadsen, P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim. Health Res. Rev. 2007, 8, 23–28. [Google Scholar] [CrossRef]
- Alonso-Díaz, M.A.; Fernández-Salas, A. Entomopathogenic Fungi for Tick Control in Cattle Livestock From Mexico. Front. Fungal Biol. 2021, 2, 657694. [Google Scholar] [CrossRef]
- Rodríguez-Vivas, R.I.; Grisi, L.; de León, A.A.P.; Silva Villela, H.; Torres-Acosta, J.F.d.J.; Fragoso Sánchez, H.; Romero Salas, D.; Rosario Cruz, R.; Saldierna, F.; García Carrasco, D. Potential economic impact assessment for cattle parasites in Mexico. Review. Rev. Mex. Cienc. Pecu. 2017, 8, 61. [Google Scholar] [CrossRef]
- Aguilar-Domínguez, M.; Sánchez-Montes, S.; Esteve-Gassent, M.D.; Barrientos-Salcedo, C.; de León, A.P.; Romero-Salas, D. Genetic structure analysis of Amblyomma mixtum populations in Veracruz State, Mexico. Ticks Tick-Borne Dis. 2019, 10, 86–92. [Google Scholar] [CrossRef]
- Higa, L.O.S.; Barradas Piña, F.T.; Rodrigues, V.S.; Garcia, M.V.; Salas, D.R.; Miller, R.J.; de Leon, A.P.; Barros, J.C.; Andreotti, R. Evidence of acaricide resistance in different life stages of Amblyomma mixtum and Rhipicephalus microplus (Acari: Ixodidae) collected from the same farm in the state of Veracruz, Mexico. Prev. Vet. Med. 2020, 174, 104837. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Zaman, M.A.; Colwell, D.D.; Gilleard, J.; Iqbal, Z. Acaricide resistance in cattle ticks and approaches to its management: The state of play. Veter Parasitol. 2014, 203, 6–20. [Google Scholar] [CrossRef]
- Torrents, J.; Sarli, M.; Rossner, M.V.; Toffaletti, J.R.; Morel, N.; Martínez, N.C.; Webster, A.; Mangold, A.J.; Guglielmone, A.A.; Nava, S. Resistance of the cattle tick Rhipicephalus (Boophilus) microplus to ivermectin in Argentina. Res. Veter Sci. 2020, 132, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Díaz, M.A.; Jiménez-Ruíz, M.; Fernández-Salas, A. First Evidence of the Tickicide Effect of Entomopathogenic Fungi Isolated from Mexican Cattle Farms Against Amblyomma mixtum. J. Parasitol. 2022, 108, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Goolsby, J.A.; Mays, D.T.; Schuster, G.L.; Kashefi, J.; Smith, L.; Amalin, D.; Cruz, M.; Racelis, A. Rationale for Classical Biological Control of Cattle Fever Ticks and Proposed Methods for Field Collection of Natural Enemies. Subtrop. Agric. Environ. 2015, 66, 7–15. [Google Scholar]
- Samish, M.; Ginsberg, H.; Glazer, I. Anti-tick biological control agents: Assessment and future perspectives. In Ticks; Bowman, A.S., Nuttall, P.A., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 447–469. ISBN 978-0-511-55180-2. [Google Scholar]
- Showler, A.T.; Saelao, P. Integrative Alternative Tactics for Ixodid Control. Insects 2022, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, B.; Han, Q. Understanding Tick Biology and Its Implications in Anti-tick and Transmission Blocking Vaccines Against Tick-Borne Pathogens. Front. Veter Sci. 2020, 7, 319. [Google Scholar] [CrossRef]
- Rodríguez-Mallon, A. The Bm86 Discovery: A Revolution in the Development of Anti-Tick Vaccines. Pathogens 2023, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Rand, K.N.; Moore, T.; Sriskantha, A.; Spring, K.; Tellam, R.; Willadsen, P.; Cobon, G.S. Cloning and expression of a protective antigen from the cattle tick Boophilus microplus. Proc. Natl. Acad. Sci. USA 1989, 86, 9657–9661. [Google Scholar] [CrossRef]
- Willadsen, P.; Riding, G.A.; McKenna, R.V.; Kemp, D.H.; Tellam, R.L.; Nielsen, J.N.; Lahnstein, J.; Cobon, G.S. Identification of a protective antigen from Boophilus microplus. J. Immunol. 1989, 143, 1346–1351. [Google Scholar] [CrossRef]
- Tabor, A.E. A Review of Australian Tick Vaccine Research. Vaccines 2021, 9, 1030. [Google Scholar] [CrossRef]
- Canales, M.; Enríquez, A.; Ramos, E.; Cabrera, D.; Dandie, H.; Soto, A.; Falcón, V.; Rodríguez, M.; de la Fuente, J. Large-scale production in Pichia pastoris of the recombinant vaccine GavacTM against cattle tick. Vaccine 1997, 15, 414–422. [Google Scholar] [CrossRef]
- Willadsen, P.; Bird, P.; Cobon, G.S.; Hungerford, J. Commercialisation of a recombinant vaccine against Boophilus microplus. Parasitology 1995, 110, S43–S50. [Google Scholar] [CrossRef]
- Tabor, E.A. The Enigma of Identifying New Cattle Tick Vaccine Antigens. In Ticks and Tick-Borne Pathogens; Abubakar, M.K., Perera, P., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-765-8. [Google Scholar]
- García-García, J.C.; González, I.L.; González, D.M.; Izquierdo, G.B.; de la Fuente, J. Sequence variations in the Boophilus microplus Bm86 locus and implications for immunoprotection in cattle vaccinated with this antigen. Biochem. Soc. Trans. 1999, 28, A201. [Google Scholar] [CrossRef]
- Martínez-Arzate, S.G.; Sánchez-Bermúdez, J.C.; Sotelo-Gómez, S.; Diaz-Albiter, H.M.; Hegazy-Hassan, W.; Tenorio-Borroto, E.; Barbabosa-Pliego, A.; Vázquez-Chagoyán, J.C. Genetic diversity of Bm86 sequences in Rhipicephalus (Boophilus) microplus ticks from Mexico: Analysis of haplotype distribution patterns. BMC Genet 2019, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Kasaija, P.D.; Contreras, M.; Kirunda, H.; Nanteza, A.; Kabi, F.; Mugerwa, S.; de la Fuente, J. Inspiring Anti-Tick Vaccine Research, Development and Deployment in Tropical Africa for the Control of Cattle Ticks: Review and Insights. Vaccines 2022, 11, 99. [Google Scholar] [CrossRef]
- Patarroyo, S.J.H.; de Sousa Neves, E.; Fidelis, C.F.; Tafur-Gómez, G.A.; de Araujo, L.; Vargas, M.I.; Sossai, S.; Prates-Patarroyo, P.A. Bovine immunisation with a recombinant peptide derived from synthetic SBm7462® (Bm86 epitope construct) immunogen for Rhipicephalus microplus control. Ticks Tick-Borne Dis. 2020, 11, 101461. [Google Scholar] [CrossRef] [PubMed]
- Blecha, I.M.Z.; Csordas, B.G.; Aguirre, A.A.R.; Cunha, R.C.; Garcia, M.V.; Andreotti, R. Analysis of Bm86 conserved epitopes: Is a global vaccine against Cattle Tick Rhipicephalus microplus possible? Rev. Bras. Parasitol. Vet. 2018, 27, 267–279. [Google Scholar] [CrossRef]
- Parthasarathi, B.C.; Kumar, B.; Nagar, G.; Manjunathachar, H.V.; de la Fuente, J.; Ghosh, S. Analysis of Genetic Diversity in Indian Isolates of Rhipicephalus microplus Based on Bm86 Gene Sequence. Vaccines 2021, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Muhanguzi, D.; Ndekezi, C.; Nkamwesiga, J.; Kalayou, S.; Ochwo, S.; Vuyani, M.; Kimuda, M.P. Anti-Tick Vaccines: Current Advances and Future Prospects. In Vaccine Design: Methods and Protocols, Volume 2. Vaccines for Veterinary Diseases; Springer: New York, NY, USA, 2022; pp. 253–268. ISBN 978-1-07-161887-5. [Google Scholar]
- de la Fuente, J.; Contreras, M. Vaccinomics: A future avenue for vaccine development against emerging pathogens. Expert Rev. Vaccines 2021, 20, 1561–1569. [Google Scholar] [CrossRef]
- De La Fuente, J.; Merino, O. Vaccinomics, the new road to tick vaccines. Vaccine 2013, 31, 5923–5929. [Google Scholar] [CrossRef]
- Peconick, A.P.; Sossai, S.; Girão, F.A.; Rodrigues, M.Q.R.B.; Souza e Silva, C.H.; Guzman, Q.F.; Patarroyo, V.A.M.; Vargas, M.I.; Patarroyo, J.H. Synthetic vaccine (SBm7462) against the cattle tick Rhipicephalus (Boophilus) microplus: Preservation of immunogenic determinants in different strains from South America. Exp. Parasitol. 2008, 119, 37–43. [Google Scholar] [CrossRef]
- Patarroyo, J.H.; Portela, R.W.; De Castro, R.O.; Couto Pimentel, J.; Guzman, F.; Patarroyo, M.E.; Vargas, M.I.; Prates, A.A.; Dias Mendes, M.A. Immunization of cattle with synthetic peptides derived from the Boophilus microplus gut protein (Bm86). Veter Immunol. Immunopathol. 2002, 88, 163–172. [Google Scholar] [CrossRef]
- Hüe, T.; Petermann, J.; Bonnefond, R.; Mermoud, I.; Rantoen, D.; Vuocolo, T. Experimental efficacy of a vaccine against Rhipicephalus australis. Exp. Appl. Acarol. 2017, 73, 245–256. [Google Scholar] [CrossRef]
- Canales, M.; Almazán, C.; Naranjo, V.; Jongejan, F.; de la Fuente, J. Vaccination with recombinant Boophilus annulatus Bm86 ortholog protein, Ba86, protects cattle against B. annulatus and B. microplus infestations. BMC Biotechnol. 2009, 9, 29. [Google Scholar] [CrossRef]
- Kopp, N.; Diaz, D.; Amacker, M.; Odongo, D.O.; Beier, K.; Nitsch, C.; Bishop, R.P.; Daubenberger, C.A. Identification of a synthetic peptide inducing cross-reactive antibodies binding to Rhipicephalus (Boophilus) decoloratus, Rhipicephalus (Boophilus) microplus, Hyalomma anatolicum anatolicum and Rhipicephalus appendiculatus BM86 homologues. Vaccine 2009, 28, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Espinoza, U.; Gómez-Romero, N.; Hernández-Ortiz, R.; Castro-Saines, E.; Lagunes-Quintanilla, R. Characterization of an immunogenic region of the Bm86 antigen as an improved vaccine against the Rhipicephalus microplus tick. Mexjbiotechnol 2021, 6, 35–49. [Google Scholar] [CrossRef]
- National, Institute of Statistics, Geography and Informatics (INEGI). Anuario Estadístico y Geográfico de Veracruz de Ignacio de la Llave; INEGI: Mexico City, Mexico, 2017; p. 1225.
- Popara, M.; Villar, M.; Mateos-Hernández, L.; de Mera, I.G.F.; Marina, A.; del Valle, M.; Almazán, C.; Domingos, A.; de la Fuente, J. Lesser protein degradation machinery correlates with higher Bm86 tick vaccine efficacy in Rhipicephalus annulatus when compared to Rhipicephalus microplus. Vaccine 2013, 31, 4728–4735. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Massardj, C.L.; Ramo, N.F.; Labartail, V.; De la Fuente, J. Effect of vaccination with a recombinant Bm86 antigen preparation on natural infestations of Boophilus microplus in grazing dairy and beef pure and cross-bred cattle in Brazil. Vaccine 1995, 13, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Almazán, C.; Lagunes, R.; Villar, M.; Canales, M.; Rosario-Cruz, R.; Jongejan, F.; de la Fuente, J. Identification and characterization of Rhipicephalus (Boophilus) microplus candidate protective antigens for the control of cattle tick infestations. Parasitol. Res. 2010, 106, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Merino-Charrez, J.O.; Gómez-Romero, N.; Barrera-Molina, I.; Lagunes-Quintanilla, R. Análisis in silico del gen subolesina como posible vacuna contra garrapatas Rhipicephalus microplus. Ecosist. Recur. Agropec. 2019, 6, 129–136. [Google Scholar] [CrossRef]
- Thomas, S. (Ed.) Vaccine Design: Methods and Protocols, Volume 2: Vaccines for Veterinary Diseases (Methods in Molecular Biology, 1404); Springer: New York, NY, USA, 2016; Volume 1404, ISBN 978-1-4939-3388-4. [Google Scholar]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef]
- Vieira Gomes, A.; Souza Carmo, T.; Silva Carvalho, L.; Mendonça Bahia, F.; Parachin, N. Comparison of Yeasts as Hosts for Recombinant Protein Production. Microorganisms 2018, 6, 38. [Google Scholar] [CrossRef]
- Mendoza-Martínez, N.; Alonso-Díaz, M.A.; Merino, O.; Fernández-Salas, A.; Lagunes-Quintanilla, R. Protective efficacy of the peptide Subolesin antigen against the cattle tick Rhipicephalus microplus under natural infestation. Veter Parasitol. 2021, 299, 109577. [Google Scholar] [CrossRef]
- Suarez, M.; Rubi, J.; Pérez, D.; Cordova, V.; Salazar, Y.; Vielma, A.; Barrios, F.; Gil, C.A.; Segura, N.; Carrillo, Y.; et al. High impact and effectiveness of GavacTM vaccine in the national program for control of bovine ticks Rhipicephalus microplus in Venezuela. Livest. Sci. 2016, 187, 48–52. [Google Scholar] [CrossRef]
- Rodríguez-Valle, M.R.; Montero, C.; Machado, H.; Joglar, M. The evaluation of yeast derivatives as adjuvants for the immune response to the Bm86 antigen in cattle. BMC Biotechnol. 2001, 1, 2. [Google Scholar] [CrossRef]
- Rodríguez-Mallón, A.; Javier González, L.; Encinosa Guzmán, P.E.; Bechara, G.H.; Sanches, G.S.; Pousa, S.; Cabrera, G.; Cabrales, A.; Garay, H.; Mejías, R.; et al. Functional and Mass Spectrometric Evaluation of an Anti-Tick Antigen Based on the P0 Peptide Conjugated to Bm86 Protein. Pathogens 2020, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Lagunes-Quintanilla, R.; Valdez-Espinoza, U.M.; Hernández-Ortiz, R.; Castro-Saines, E.; Merino, O.; Mendoza-Martínez, N. Experimental vaccination in rabbits using the peptide RmS-17 antigen reduces the performance of a Mexican Rhipicephalus microplus tick strain. Ticks Tick-Borne Dis. 2022, 13, 102044. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.C.; de León, A.A.P.; Leite, F.P.L.; Pinto, L.S.; Santos Júnior, A.G.; Andreotti, R. Bovine immunoprotection against Rhipicephalus (Boophilus) microplus with recombinant Bm86-Campo Grande antigen. Rev. Bras. Parasitol. Veter 2012, 21, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, B.C.; Kumar, B.; Bhure, S.K.; Sharma, A.K.; Manisha; Nagar, G.; Kumar, S.; Nandi, A.; Manjunathachar, H.V.; Chigure, G.M.; et al. Co-Immunization Efficacy of Recombinant Antigens against Rhipicephalus microplus and Hyalomma anatolicum Tick Infestations. Pathogens 2023, 12, 433. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Bonfanti, L.; Natale, A.; Comin, A.; Ferronato, A.; La Greca, E.; Patregnani, T.; Lucchese, L.; Marangon, S. Application of an integrated outbreak management plan for the control of leptospirosis in dairy cattle herds. Epidemiol. Infect. 2014, 142, 1172–1181. [Google Scholar] [CrossRef]
- Giese, M. Elderly Immunology. In Introduction to Molecular Vaccinology; Springer International Publishing: Cham, Switzerland, 2016; pp. 111–121. ISBN 978-3-319-25830-0. [Google Scholar]
- Frasca, D.; Blomberg, B.B. Effects of aging on B cell function. Curr. Opin. Immunol. 2009, 21, 425–430. [Google Scholar] [CrossRef]
- Shihab, H. Effect of Aging on some Blood Parameters of Local Cows. J. Tikrit Univ. Agri. Sci. Vol 2018, 18, 28–33. [Google Scholar]
- Jackson, L.A.; Opdebeeck, J.P. Humoral immune responses of Hereford cattle vaccinated with midgut antigens of the cattle tick, Boophilus microplus. Parasite Immunol. 1990, 12, 141–151. [Google Scholar] [CrossRef]
- Antunes, S.; Merino, O.; Mosqueda, J.; Moreno-Cid, J.A.; Bell-Sakyi, L.; Fragkoudis, R.; Weisheit, S.; Pérez De La Lastra, J.M.; Alberdi, P.; Domingos, A.; et al. Tick capillary feeding for the study of proteins involved in tick-pathogen interactions as potential antigens for the control of tick infestation and pathogen infection. Parasites Vectors 2014, 7, 42. [Google Scholar] [CrossRef]
- Lagunes, R.; Domínguez-García, D.; Quiroz, H.; Martínez-Velázquez, M.; Rosario-Cruz, R. Potential effects on Rhipicephalus microplus tick larvae fed on calves immunized with a Subolesin peptide predicted by epitope analysis. Trop Biomed 2016, 33, 726–738. [Google Scholar]
- Willadsen, P.; Kemp, D.H. Vaccination with ‘concealed’ antigens for tick control. Parasitol. Today 1988, 4, 196–198. [Google Scholar] [CrossRef]
- Willadsen, P.; McKenna, R.V.; Riding, G.A. Isolation from the cattle tick, Boophilus microplus, of antigenic material capable of eliciting a protective immunological response in the bovine host. Int. J. Parasitol. 1988, 18, 183–189. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Rodrigues, M.; Redondo, M.; Montero, C.; García-García, J.C.; Méndez, L.; Serrano, E.; Valdés, M.; Enríquez, A.; Canales, M.; et al. Field studies and cost-effectiveness analysis of vaccination with Gavac against the cattle tick Boophilus microplus. Vaccine 1998, 16, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Ndawula, C.; Tabor, A.E. Cocktail Anti-Tick Vaccines: The Unforeseen Constraints and Approaches Toward Enhanced Efficacies. Vaccines 2020, 8, 457. [Google Scholar] [CrossRef]
- Imamura, S.; Konnai, S.; da Silva Vaz, I.J.; Yamada, S.; Nakajima, C.; Ito, Y.; Tajima, T.; Yasuda, J.; Simuunza, M.; Onuma, M.; et al. Effects of anti-tick cocktail vaccine against Rhipicephalus appendiculatus. Jpn. J. Veter Res. 2008, 56, 85–98. [Google Scholar] [CrossRef]
- Imamura, S.; Namangala, B.; Tajima, T.; Tembo, M.E.; Yasuda, J.; Ohashi, K.; Onuma, M. Two serine protease inhibitors (serpins) that induce a bovine protective immune response against Rhipicephalus appendiculatus ticks. Vaccine 2006, 24, 2230–2237. [Google Scholar] [CrossRef]
- Tiffin, H.S.; Rajotte, E.G.; Sakamoto, J.M.; Machtinger, E.T. Tick Control in a Connected World: Challenges, Solutions, and Public Policy from a United States Border Perspective. Tropical Med 2022, 7, 388. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).