Immunization with Anaplasma centrale Msp2 HVRs Is Less Effective than the Live A. centrale Vaccine against Anaplasmosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Msp2-HVR Vaccine Constructs
2.2. Animal Immunization
2.3. Immunoblotting
2.4. Infection of D. andersoni Ticks and Challenge
2.5. Animal Monitoring
2.6. DNA Extraction, Cloning, and Sequencing of msp2
2.7. Statistical Analysis
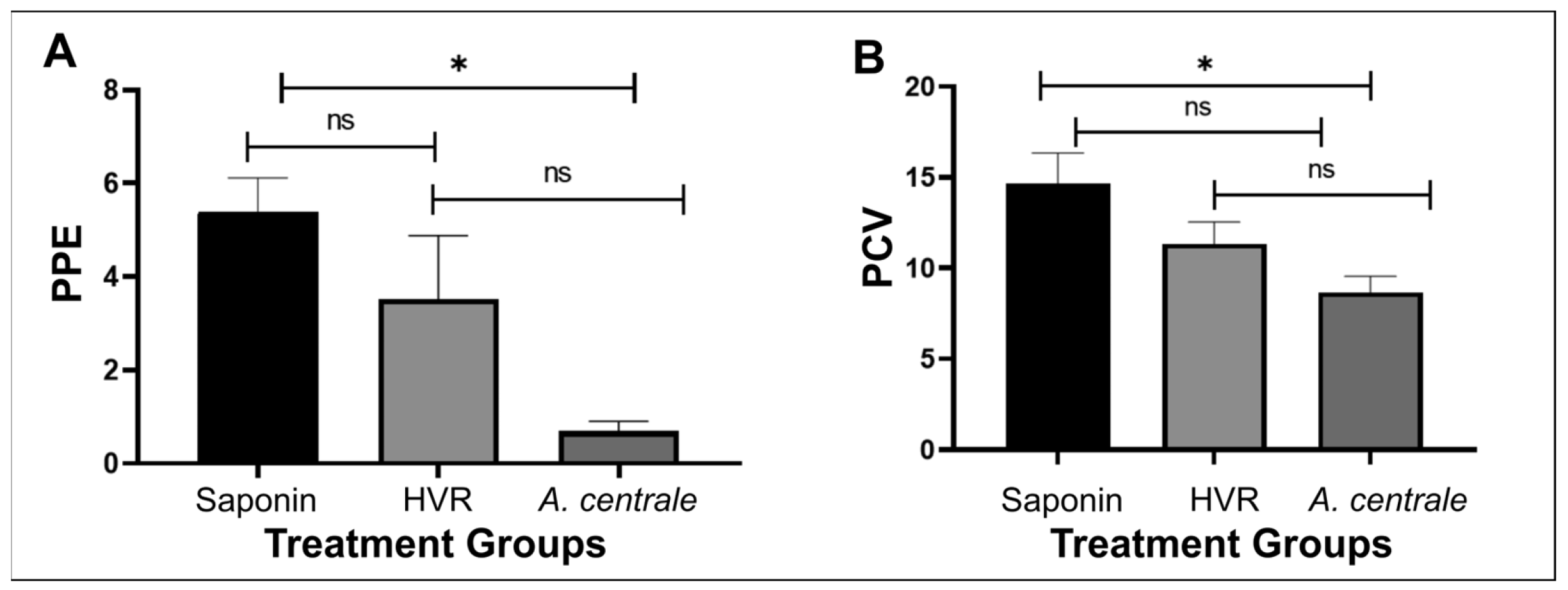
3. Results
3.1. Antibody Response
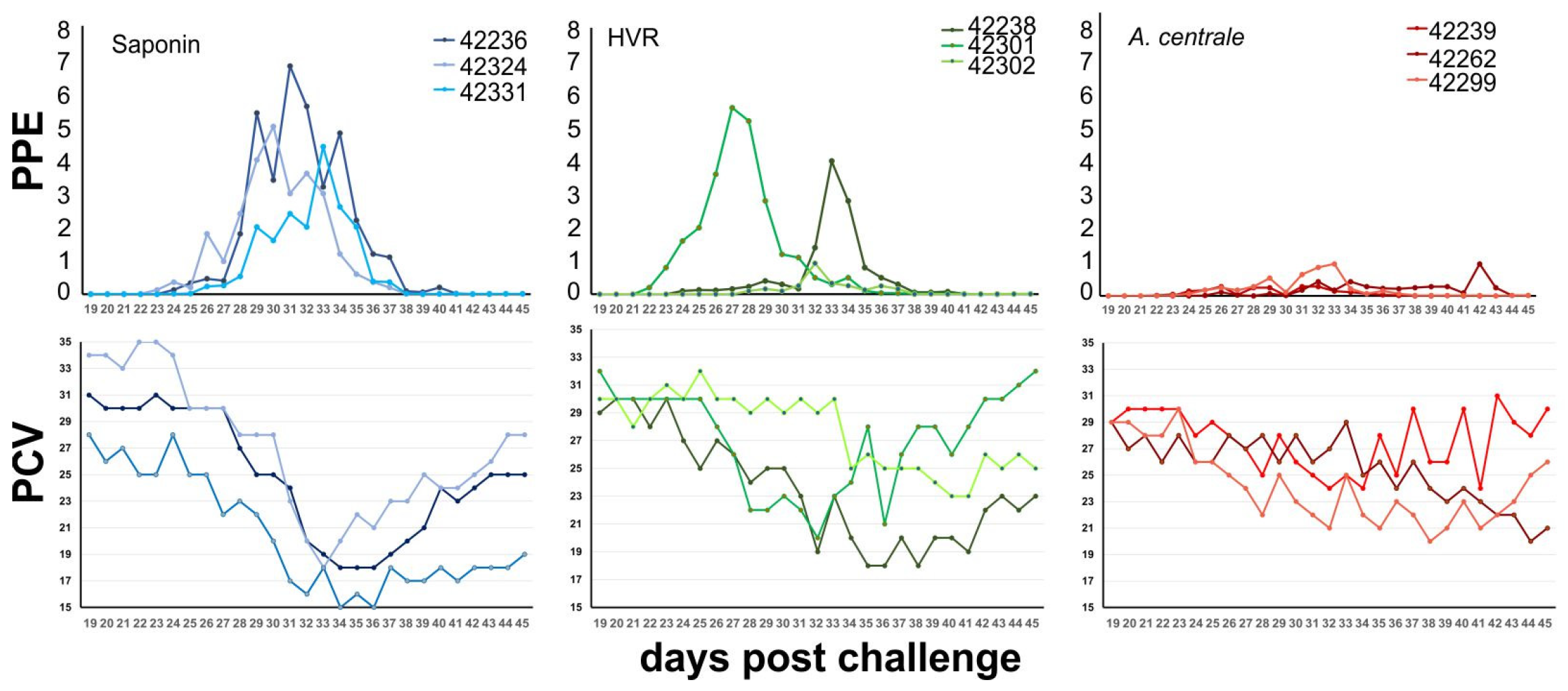
3.2. Clinical Response after Challenge
3.3. Sequence Analysis of HVR Regions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aubry, P.; Geale, D.W. A review of bovine anaplasmosis. Transbound. Emerg. Dis. 2011, 58, 1–30. [Google Scholar] [CrossRef] [PubMed]
- French, D.M.; McElwain, T.F.; McGuire, T.C.; Palmer, G.H. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect. Immun. 1998, 66, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Eid, G.; French, D.M.; Lundgren, A.M.; Barbet, A.F.; McElwain, T.F.; Palmer, G.H. Expression of major surface protein 2 antigenic variants during acute Anaplasma marginale rickettsemia. Infect. Immun. 1996, 64, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Brayton, K.A.; Knowles, D.P.; McGuire, T.C.; Palmer, G.H. Efficient use of a small genome to generate antigenic diversity in tick-borne ehrlichial pathogens. Proc. Natl. Acad. Sci. USA 2001, 98, 4130–4135. [Google Scholar] [CrossRef]
- Brayton, K.A.; Palmer, G.H.; Lundgren, A.; Yi, J.; Barbet, A.F. Antigenic variation of Anaplasma marginale msp2 occurs by combinatorial gene conversion. Mol. Microbiol. 2002, 43, 1151–1159. [Google Scholar] [CrossRef]
- Futse, J.E.; Brayton, K.A.; Knowles, D.P., Jr.; Palmer, G.H. Structural basis for segmental gene conversion in generation of Anaplasma marginale outer membrane protein variants. Mol. Microbiol. 2005, 57, 212–221. [Google Scholar] [CrossRef]
- Reinbold, J.B.; Coetzee, J.F.; Hollis, L.C.; Nickell, J.S.; Riegel, C.M.; Christopher, J.A.; Ganta, R.R. Comparison of iatrogenic transmission of Anaplasma marginale in Holstein steers via needle and needle-free injection techniques. Am. J. Vet. Res. 2010, 71, 1178–1188. [Google Scholar] [CrossRef]
- Reinbold, J.B.; Coetzee, J.F.; Sirigireddy, K.R.; Ganta, R.R. Detection of Anaplasma marginale and A. phagocytophilum in bovine peripheral blood samples by duplex real-time reverse transcriptase PCR assay. J. Clin. Microbiol. 2010, 48, 2424–2432. [Google Scholar] [CrossRef]
- Toillion, A.R.; Reppert, E.J.; Amachawadi, R.G.; Olson, K.C.; Coetzee, J.F.; Kang, Q.; Reif, K.E. Effect of Protracted Free-Choice Chlortetracycline-Medicated Mineral for Anaplasmosis Control on Escherichia coli Chlortetracycline Resistance Profile from Pastured Beef Cattle. Microorganisms 2021, 9, 2495. [Google Scholar] [CrossRef]
- George, J.E.; Pound, J.M.; Davey, R.B. Chemical control of ticks on cattle and the resistance of these parasites to acaricides. Parasitology 2004, 129 (Suppl. S1), S353–S366. [Google Scholar] [CrossRef]
- Edvantoro, B.B.; Naidu, R.; Megharaj, M.; Singleton, I. Changes in microbial properties associated with long-term arsenic and DDT contaminated soils at disused cattle dip sites. Ecotoxicol. Environ. Saf. 2003, 55, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Tebele, N.; McGuire, T.C.; Palmer, G.H. Induction of protective immunity by using Anaplasma marginale initial body membranes. Infect. Immun. 1991, 59, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.M.; Brayton, K.A.; Brown, W.C.; Norimine, J.; Munske, G.R.; Davitt, C.M.; Palmer, G.H. Composition of the surface proteome of Anaplasma marginale and its role in protective immunity induced by outer membrane immunization. Infect. Immun. 2008, 19, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.C.; Shkap, V.; Zhu, D.; McGuire, T.C.; Tuo, W.; McElwain, T.F.; Palmer, G.H. CD4(+) T-lymphocyte and immunoglobulin G2 responses in calves immunized with Anaplasma marginale outer membranes and protected against homologous challenge. Infect. Immun. 1998, 66, 5406–5413. [Google Scholar] [CrossRef]
- Hove, P.; Madesh, S.; Nair, A.; Jaworski, D.; Liu, H.; Ferm, J.; Kleinhenz, M.D.; Highland, M.A.; Curtis, A.K.; Coetzee, J.F.; et al. Targeted mutagenesis in Anaplasma marginale to define virulence and vaccine development against bovine anaplasmosis. PLoS Pathog. 2022, 18, e1010540. [Google Scholar] [CrossRef]
- Salinas-Estrella, E.; Amaro-Estrada, I.; Cobaxin-Cardenas, M.E.; Preciado de la Torre, J.F.; Rodriguez, S.D. Bovine Anaplasmosis: Will there ever be an almighty effective vaccine? Front. Vet. Sci. 2022, 9, 946545. [Google Scholar] [CrossRef]
- Abdala, A.A.; Pipano, E.; Aguirre, D.H.; Gaido, A.B.; Zurbriggen, M.A.; Mangold, A.J.; Guglielmone, A.A. Frozen and fresh Anaplasma centrale vaccines in the protection of cattle against Anaplasma marginale infection. Rev. Elev. Med. Vet. Pays Trop. 1990, 43, 155–158. [Google Scholar] [CrossRef]
- Pipano, E. Live vaccines against hemoparasitic diseases in livestock. Vet. Parasitol. 1995, 57, 213–231. [Google Scholar] [CrossRef]
- Palmer, G.H.; McElwain, T.F. Molecular basis for vaccine development against anaplasmosis and babesiosis. Vet. Parasitol. 1995, 57, 233–253. [Google Scholar] [CrossRef]
- Futse, J.E.; Brayton, K.A.; Dark, M.J.; Knowles, D.P., Jr.; Palmer, G.H. Superinfection as a driver of genomic diversification in antigenically variant pathogens. Proc. Natl. Acad. Sci. USA 2008, 105, 2123–2127. [Google Scholar] [CrossRef]
- Herndon, D.R.; Palmer, G.H.; Shkap, V.; Knowles, D.P., Jr.; Brayton, K.A. Complete genome sequence of Anaplasma marginale subsp. centrale. J. Bacteriol. 2010, 192, 379–380. [Google Scholar] [CrossRef] [PubMed]
- Dark, M.J.; Herndon, D.R.; Kappmeyer, L.S.; Gonzales, M.P.; Nordeen, E.; Palmer, G.H.; Knowles, D.P., Jr.; Brayton, K.A. Conservation in the face of diversity: Multistrain analysis of an intracellular bacterium. BMC Genom. 2009, 10, 16. [Google Scholar] [CrossRef]
- Brayton, K.A.; Kappmeyer, L.S.; Herndon, D.R.; Dark, M.J.; Tibbals, D.L.; Palmer, G.H.; McGuire, T.C.; Knowles, D.P., Jr. Complete genome sequencing of Anaplasma marginale reveals that the surface is skewed to two superfamilies of outer membrane proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Knowles, D.; Torioni de Echaide, S.; Palmer, G.; McGuire, T.; Stiller, D.; McElwain, T. Antibody against an Anaplasma marginale MSP5 epitope common to tick and erythrocyte stages identifies persistently infected cattle. J. Clin. Microbiol. 1996, 34, 2225–2230. [Google Scholar] [CrossRef] [PubMed]
- Graca, T.; Silva, M.G.; Kostyukova, A.S.; Palmer, G.H. Structural Basis for Recombinatorial Permissiveness in the Generation of Anaplasma marginale Msp2 Antigenic Variants. Infect. Immun. 2016, 84, 2740–2747. [Google Scholar] [CrossRef]
- Noh, S.M.; Turse, J.E.; Brown, W.C.; Norimine, J.; Palmer, G.H. Linkage between Anaplasma marginale outer membrane proteins enhances immunogenicity but is not required for protection from challenge. Clin. Vaccine Immunol. 2013, 20, 651–656. [Google Scholar] [CrossRef]
- Macmillan, H.; Norimine, J.; Brayton, K.A.; Palmer, G.H.; Brown, W.C. Physical linkage of naturally complexed bacterial outer membrane proteins enhances immunogenicity. Infect. Immun. 2007, 76, 1223–1229. [Google Scholar] [CrossRef]
- Barigye, R.; Garcia-Ortiz, M.A.; Rojas Ramirez, E.E.; Rodriguez, S.D. Identification of IgG2-specific antigens in Mexican Anaplasma marginale strains. Ann. N. Y Acad. Sci. 2004, 1026, 84–94. [Google Scholar] [CrossRef]
- Ducken, D.R.; Brown, W.C.; Alperin, D.C.; Brayton, K.A.; Reif, K.E.; Turse, J.E.; Palmer, G.H.; Noh, S.M. Subdominant Outer Membrane Antigens in Anaplasma marginale: Conservation, Antigenicity, and Protective Capacity Using Recombinant Protein. PLoS ONE 2015, 10, e0129309. [Google Scholar] [CrossRef]
- Albarrak, S.M.; Brown, W.C.; Noh, S.M.; Reif, K.E.; Scoles, G.A.; Turse, J.E.; Norimine, J.; Ueti, M.W.; Palmer, G.H. Subdominant antigens in bacterial vaccines: AM779 is subdominant in the Anaplasma marginale outer membrane vaccine but does not associate with protective immunity. PLoS ONE 2012, 7, e46372. [Google Scholar] [CrossRef]
- Palmer, G.H.; Barbet, A.F.; Cantor, G.H.; McGuire, T.C. Immunization of cattle with the MSP-1 surface protein complex induces protection against a structurally variant Anaplasma marginale isolate. Infect. Immun. 1989, 57, 3666–3669. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.H.; Barbet, A.F.; Davis, W.C.; McGuire, T.C. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science 1986, 231, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.H.; Oberle, S.M.; Barbet, A.F.; Goff, W.L.; Davis, W.C.; McGuire, T.C. Immunization of cattle with a 36-kilodalton surface protein induces protection against homologous and heterologous Anaplasma marginale challenge. Infect. Immun. 1988, 56, 1526–1531. [Google Scholar] [CrossRef] [PubMed]


| Locus-Specific Primers | ||
|---|---|---|
| ψ name | Primer Name | Primer Sequence * |
| AcA1 AcF | A1, F-Fx attB2 | GGGGACAAGTTTGTACAAAAAAGCAGGCTATCAGGCAGTAGAGGCTAAT |
| AcA1 | A1-Rx attB2 | GGGGACCACTTTGTACAAGAAAGCTGGGTACTTCACGCTGATGGTGGC |
| AcB1 | B1-Fx attB1 | GGGGACAAGTTTGTACAAAAAAGCAGGCTATAAAGCAGTAGAAGGTGTT |
| AcB1 AcAF | B1, AF-Rx attB2 | GGGGACCACTTTGTACAAGAAAGCTGGGTACTGCAAGCTGATGGTGTT |
| AcAF AcG2 AcC | AF, G2, C-Fx attB1 | GGGGACAAGTTTGTACAAAAAAGCAGGCTATACTGCAGTAGAGGCTGCC |
| AcG2 | G2-Rx attB2 | GGGGACCACTTTGTACAAGAAAGCTGGGTACTGCACGTCGATGGTGGG |
| AcA22 | A22-Fx attB1 | GGGGACAAGTTTGTACAAAAAAGCAGGCTATACTGCAGTAGAGGCTGCT |
| A22-Rx attB2 | GGGGACCACTTTGTACAAGAAAGCTGGGTACTTCACGCTGATGGTGCT | |
| AcF AcC | F, C-Rx attB2 | GGGGACCACTTTGTACAAGAAAGCTGGGTATGACACACTGATGGTAGC |
| Number Clones from Each Animal | ||||
|---|---|---|---|---|
| Saponin | HVR | A. centrale | Variant Name | Type of Variant |
| 20, 22, 17 *,a | 14, 12, 12 *,b | 13, 7, 15 *,c | V1 | Simple |
| 0, 0, 1 | 1, 1, 0 | 0, 0, 1 | V2 | Complex |
| 6, 1, 0 | 1, 2, 3 | 0, 0, 1 | V3 | Complex |
| 0, 3, 0 | ND # | 1, 0, 1 | V4 | Complex |
| 1, 1, 0 | ND | ND | V5 | Simple |
| 0, 0, 2 | ND | ND | V6 | Simple |
| 0, 0, 1 | ND | ND | V7 | Complex |
| ND | 1, 0, 0 | ND | V8 | Simple |
| ND | 0, 4, 0 | ND | V9 | Simple |
| ND | ND | 1, 0, 0 | V10 | Simple |
| ND | ND | 0, 0, 1 | V11 | Simple |
| 1, 0, 2 | 4, 2, 0 | 2, 0, 1 | ψ 1 | A. marginale StM |
| 2, 1, 3 | 1, 0, 0 | 3, 0, 2 | ψ 2 | A. marginale StM |
| 0, 2, 2 | 8, 8, 2 | 2, 0, 1 | ψ G 11 | A. marginale StM |
| 0, 0, 1 | 0, 1, 9 | 8, 17, 7 | ψ E6 F7 | A. marginale StM |
| 0, 0, 1 | 0, 0, 4 | 0, 1, 0 | ψ 9H 1 | A. marginale StM |
| ND | ND | 0, 5, 0 | ψ A22 | A. centrale |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falghoush, A.; Ku, P.-S.; Brayton, K.A. Immunization with Anaplasma centrale Msp2 HVRs Is Less Effective than the Live A. centrale Vaccine against Anaplasmosis. Vaccines 2023, 11, 1544. https://doi.org/10.3390/vaccines11101544
Falghoush A, Ku P-S, Brayton KA. Immunization with Anaplasma centrale Msp2 HVRs Is Less Effective than the Live A. centrale Vaccine against Anaplasmosis. Vaccines. 2023; 11(10):1544. https://doi.org/10.3390/vaccines11101544
Chicago/Turabian StyleFalghoush, Azeza, Pei-Shin Ku, and Kelly A. Brayton. 2023. "Immunization with Anaplasma centrale Msp2 HVRs Is Less Effective than the Live A. centrale Vaccine against Anaplasmosis" Vaccines 11, no. 10: 1544. https://doi.org/10.3390/vaccines11101544
APA StyleFalghoush, A., Ku, P.-S., & Brayton, K. A. (2023). Immunization with Anaplasma centrale Msp2 HVRs Is Less Effective than the Live A. centrale Vaccine against Anaplasmosis. Vaccines, 11(10), 1544. https://doi.org/10.3390/vaccines11101544






