Comparison of Humoral Antibody Responses and Seroconversion Rates between Two Homologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination in Patients Undergoing Maintenance Hemodialysis
Abstract
1. Introduction
2. Methods
2.1. Study Approval and Patients
2.2. Study Procedure, Primary Endpoints, and Serological Assays
2.3. Statistics and Analyses
3. Results
3.1. Baseline Characteristics of Study Population
3.2. Humoral Antibody Responses Induced by ChAdOx1 and mRNA-1273 Vaccination
3.3. Seroconversion Rates between ChAdOx1 and mRNA-1273 Vaccination
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, J.L.; Grint, D.J.; Strongman, H.; Eggo, R.M.; Peppa, M.; Minassian, C.; Mansfield, K.E.; Rentsch, C.T.; Douglas, I.J.; Mathur, R.; et al. UK prevalence of underlying conditions which increase the risk of severe COVID-19 disease: A point prevalence study using electronic health records. BMC Public Health 2021, 21, 484. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Jit, M.; Warren-Gash, C.; Guthrie, B.; Wang, H.H.X.; Mercer, S.W.; Sanderson, C.; McKee, M.; Troeger, C.; Ong, K.L.; et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: A modelling study. Lancet Glob. Health 2020, 8, e1003–e1017. [Google Scholar] [CrossRef] [PubMed]
- Flythe, J.E.; Assimon, M.M.; Tugman, M.J.; Chang, E.H.; Gupta, S.; Shah, J.; Sosa, M.A.; Renaghan, A.D.; Melamed, M.L.; Wilson, F.P.; et al. Characteristics and Outcomes of Individuals With Pre-existing Kidney Disease and COVID-19 Admitted to Intensive Care Units in the United States. Am. J. Kidney Dis. 2021, 77, 190–203. [Google Scholar] [CrossRef]
- Ng, J.H.; Hirsch, J.S.; Wanchoo, R.; Sachdeva, M.; Sakhiya, V.; Hong, S.; Jhaveri, K.D.; Fishbane, S.; Northwell COVID-19 Research Consortium and the Northwell Nephrology COVID-19 Research Consortium. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020, 98, 1530–1539. [Google Scholar] [CrossRef]
- Betjes, M.G. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 2013, 9, 255–265. [Google Scholar] [CrossRef]
- Meijers, R.W.; Litjens, N.H.; de Wit, E.A.; Langerak, A.W.; van der Spek, A.; Baan, C.C.; Weimar, W.; Betjes, M.G. Uremia causes premature ageing of the T cell compartment in end-stage renal disease patients. Immun. Ageing 2012, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Pahl, M.V.; Gollapudi, S.; Sepassi, L.; Gollapudi, P.; Elahimehr, R.; Vaziri, N.D. Effect of end-stage renal disease on B-lymphocyte subpopulations, IL-7, BAFF and BAFF receptor expression. Nephrol. Dial. Transplant. 2010, 25, 205–212. [Google Scholar] [CrossRef]
- Soni, R.; Horowitz, B.; Unruh, M. Immunization in end-stage renal disease: Opportunity to improve outcomes. Semin. Dial. 2013, 26, 416–426. [Google Scholar] [CrossRef]
- Janus, N.; Vacher, L.V.; Karie, S.; Ledneva, E.; Deray, G. Vaccination and chronic kidney disease. Nephrol. Dial. Transplant. 2008, 23, 800–807. [Google Scholar] [CrossRef]
- Johnson, D.W.; Fleming, S.J. The use of vaccines in renal failure. Clin. Pharmacokinet. 1992, 22, 434–446. [Google Scholar] [CrossRef]
- Fabrizi, F.; Martin, P. Hepatitis B vaccine and dialysis: Current issues. Int. J. Artif. Organs 2001, 24, 683–694. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; K Aley, P.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.P.K.; Shrotri, M.; Kampmann, B. Keeping track of the SARS-CoV-2 vaccine pipeline. Nat. Rev. Immunol. 2020, 20, 650. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Lee, T.H.; Tian, Y.C.; Lee, C.C.; Fan, P.C.; Chang, C.H. Immunogenicity Rates After SARS-CoV-2 Vaccination in People With End-stage Kidney Disease: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2131749. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Chang, J.H.; Chiou, J.F.; Hung, C.S.; Liu, M.C.; Chang, H.W.; Hong, S.Y.; Wang, C.Y.; Lin, Y.L.; Hsieh, Y.C.; Chung, C.L.; et al. Humoral Immunogenicity and Reactogenicity of the Standard ChAdOx1 nCoV-19 Vaccination in Taiwan. Vaccines 2022, 10, 312. [Google Scholar] [CrossRef]
- Demonbreun, A.R.; Sancilio, A.; Velez, M.E.; Ryan, D.T.; Pesce, L.; Saber, R.; Vaught, L.A.; Reiser, N.L.; Hsieh, R.R.; Richard, T.; et al. COVID-19 mRNA Vaccination Generates Greater Immunoglobulin G Levels in Women Compared to Men. J. Infect. Dis. 2021, 224, 793–797. [Google Scholar] [CrossRef]
- Buchwinkler, L.; Solagna, C.A.; Messner, J.; Pirklbauer, M.; Rudnicki, M.; Mayer, G.; Kerschbaum, J. Antibody Response to mRNA Vaccines against SARS-CoV-2 with Chronic Kidney Disease, Hemodialysis, and after Kidney Transplantation. J. Clin. Med. 2021, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Carr, E.J.; Wu, M.; Harvey, R.; Wall, E.C.; Kelly, G.; Hussain, S.; Howell, M.; Kassiotis, G.; Swanton, C.; Gandhi, S.; et al. Neutralising antibodies after COVID-19 vaccination in UK haemodialysis patients. Lancet 2021, 398, 1038–1041. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Gleeson, S.; Clarke, C.L.; Thomson, T.; Edwards, H.; Spensley, K.; Mortimer, P.; McIntyre, S.; Cox, A.; Pickard, G.; et al. Comparison of immunogenicity and clinical effectiveness between BNT162b2 and ChAdOx1 SARS-CoV-2 vaccines in people with end-stage kidney disease receiving haemodialysis: A prospective, observational cohort study. Lancet Reg. Health Eur. 2022, 21, 100478. [Google Scholar] [CrossRef] [PubMed]
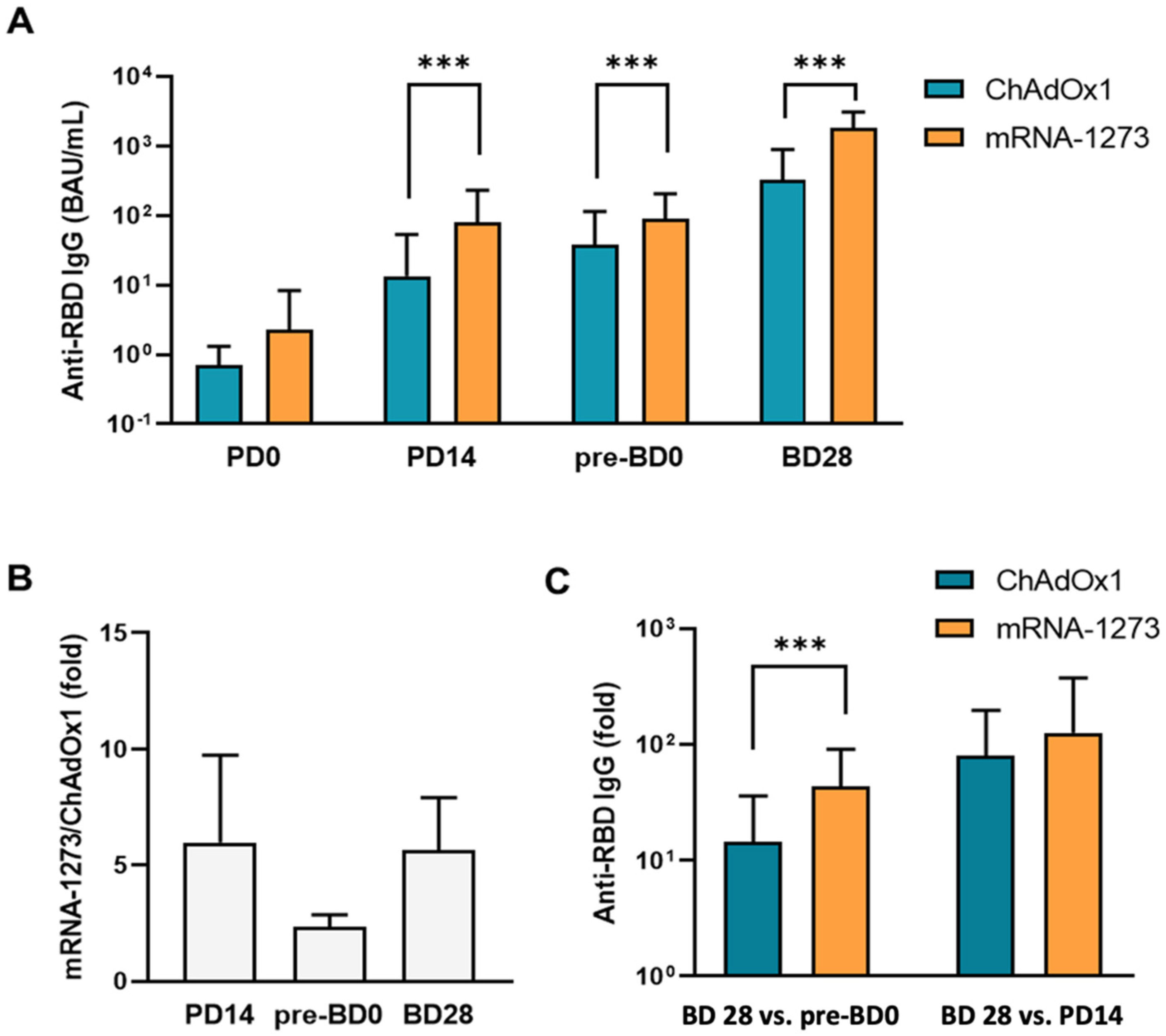
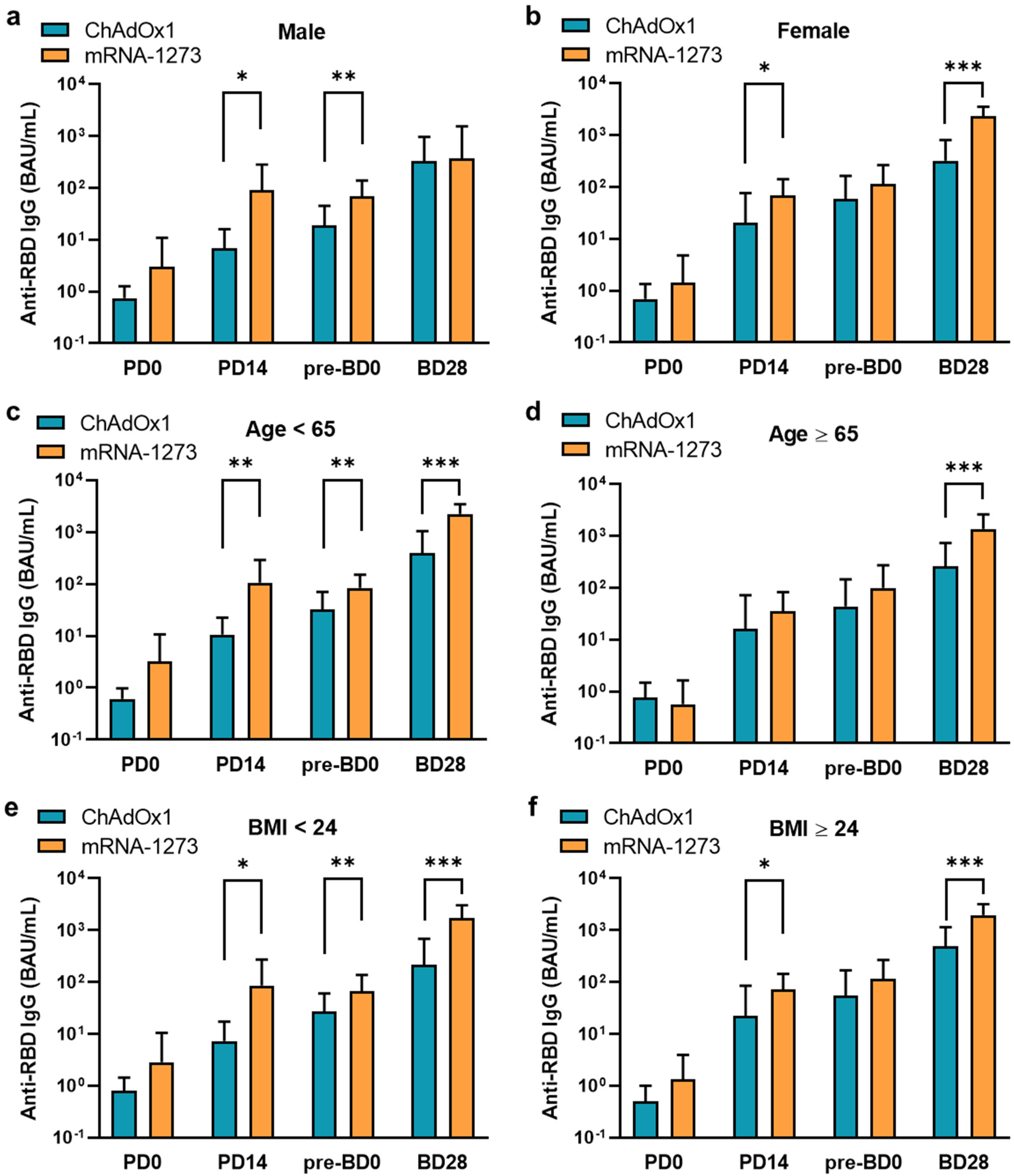
| Variable | ChAdOx1 (n = 55) | mRNA-1273 (n = 48) | p Value | ||
|---|---|---|---|---|---|
| Age, mean (SD) | 64.24 | (11.66) | 63.67 | (12.52) | 0.8116 |
| Sex, n(%) | 0.9267 | ||||
| Female | 28 | (50.91) | 24 | (50.00) | |
| Male | 27 | (49.09) | 24 | (50.00) | |
| BMI, mean (SD) | 24.05 | (4.23) | 23.71 | (2.99) | 0.6357 |
| DM | 0.2015 | ||||
| No | 24 | (43.64) | 27 | (56.25) | |
| Yes | 31 | (56.36) | 21 | (43.75) | |
| Hypertension | 0.1253 | ||||
| No | 14 | (25.45) | 19 | (39.58) | |
| Yes | 41 | (74.55) | 29 | (60.42) | |
| WBC, median (IQR) | 5800 | (1960) | 5600 | (2870) | 0.4691 |
| Hb, median (IQR) | 10.50 | (1.20) | 10.55 | (1.20) | 0.3508 |
| Time of dialysis history (year), median (IQR) | 4.37 | (5.2) | 5.54 | (5.46) | 0.1810 |
| PB interval (week), median (IQR) | 10.71 | (3.85) | 11.50 | (0.35) | 0.4839 |
| Time of serological tests after a boost dose (week), Median (IQR) | 4.00 | (0) | 4.00 | (0.50) | 0.7803 |
| Threshold of SCR | PD14 | Pre-BD0 | BD28 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ChAdOx1 | mRNA-1273 | p Value | ChAdOx1 | mRNA-1273 | p Value | ChAdOx1 | mRNA-1273 | p Value | |
| ≥7.1 BAU/mL | 17/55 (30.91%) | 27/35 (77.14%) | <0.0001 | 38/52 (73.08%) | 38/41 (92.68%) | 0.0151 | 53/55 (96.36%) | 47/48 (97.92%) | >0.999 |
| ≥17 BAU/mL | 10/55 (18.18%) | 24/35 (68.57%) | <0.0001 | 28/52 (53.85%) | 33/41 (80.49%) | 0.0073 | 51/55 (92.73%) | 47/48 (97.92%) | 0.3686 |
| ≥20.2% | 1/55 (1.82%) | 7/35 (20.00%) | 0.0051 | 2/52 (3.85%) | 12/41 (29.27%) | 0.0007 | 29/55 (52.73%) | 46/48 (95.83%) | <0.0001 |
| Variable | Threshold of SCR | |||
|---|---|---|---|---|
| ≥17 BAU/mL | ≥20.2% | |||
| OR (CI 95%) | * p Value | OR (95% CI) | * p Value | |
| Vaccine type (mRNA-1273 vs. ChAdOx1) | 3.86(0.39–38.41) | 0.2488 | 30.88(6.04–157.96) | <0.0001 |
| Age (<65 vs. ≥65) | 3.72(0.38–37.01) | 0.2617 | 2.03(0.68–6.10) | 0.2059 |
| Gender (female vs. male) | 4.76(0.47–47.81) | 0.1849 | 1.15(0.37–3.58) | 0.8052 |
| BMI (≥24 vs. <24) | 2.80(0.27–28.84) | 0.3868 | 4.35(1.32–14.37) | 0.0160 |
| Vaccine reactogenicity # (Yes vs. No) | 0.67(0.09–4.89) | 0.6901 | 3.44(0.99–11.93) | 0.0519 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, S.-H.; Sue, Y.-M.; Kao, C.-C.; Chang, H.-W.; Lin, Y.-C.; Hung, C.-S.; Hsieh, Y.-C.; Hong, S.-Y.; Chung, C.-L.; Chang, J.-H.; et al. Comparison of Humoral Antibody Responses and Seroconversion Rates between Two Homologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination in Patients Undergoing Maintenance Hemodialysis. Vaccines 2023, 11, 1161. https://doi.org/10.3390/vaccines11071161
Hsiao S-H, Sue Y-M, Kao C-C, Chang H-W, Lin Y-C, Hung C-S, Hsieh Y-C, Hong S-Y, Chung C-L, Chang J-H, et al. Comparison of Humoral Antibody Responses and Seroconversion Rates between Two Homologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination in Patients Undergoing Maintenance Hemodialysis. Vaccines. 2023; 11(7):1161. https://doi.org/10.3390/vaccines11071161
Chicago/Turabian StyleHsiao, Shih-Hsin, Yuh-Mou Sue, Chih-Chin Kao, Hui-Wen Chang, Yen-Chung Lin, Ching-Sheng Hung, Yi-Chen Hsieh, Shiao-Ya Hong, Chi-Li Chung, Jer-Hwa Chang, and et al. 2023. "Comparison of Humoral Antibody Responses and Seroconversion Rates between Two Homologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination in Patients Undergoing Maintenance Hemodialysis" Vaccines 11, no. 7: 1161. https://doi.org/10.3390/vaccines11071161
APA StyleHsiao, S.-H., Sue, Y.-M., Kao, C.-C., Chang, H.-W., Lin, Y.-C., Hung, C.-S., Hsieh, Y.-C., Hong, S.-Y., Chung, C.-L., Chang, J.-H., Su, Y.-S., Liu, M.-C., Lai, K. S.-L., Chien, K.-L., Wang, J. C.-C., Cheng, C.-Y., & Fang, T.-C. (2023). Comparison of Humoral Antibody Responses and Seroconversion Rates between Two Homologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination in Patients Undergoing Maintenance Hemodialysis. Vaccines, 11(7), 1161. https://doi.org/10.3390/vaccines11071161







