The Omicron Variant Reinfection Risk among Individuals with a Previous SARS-CoV-2 Infection within One Year in Shanghai, China: A Cross-Sectional Study
Abstract
:1. Introduction
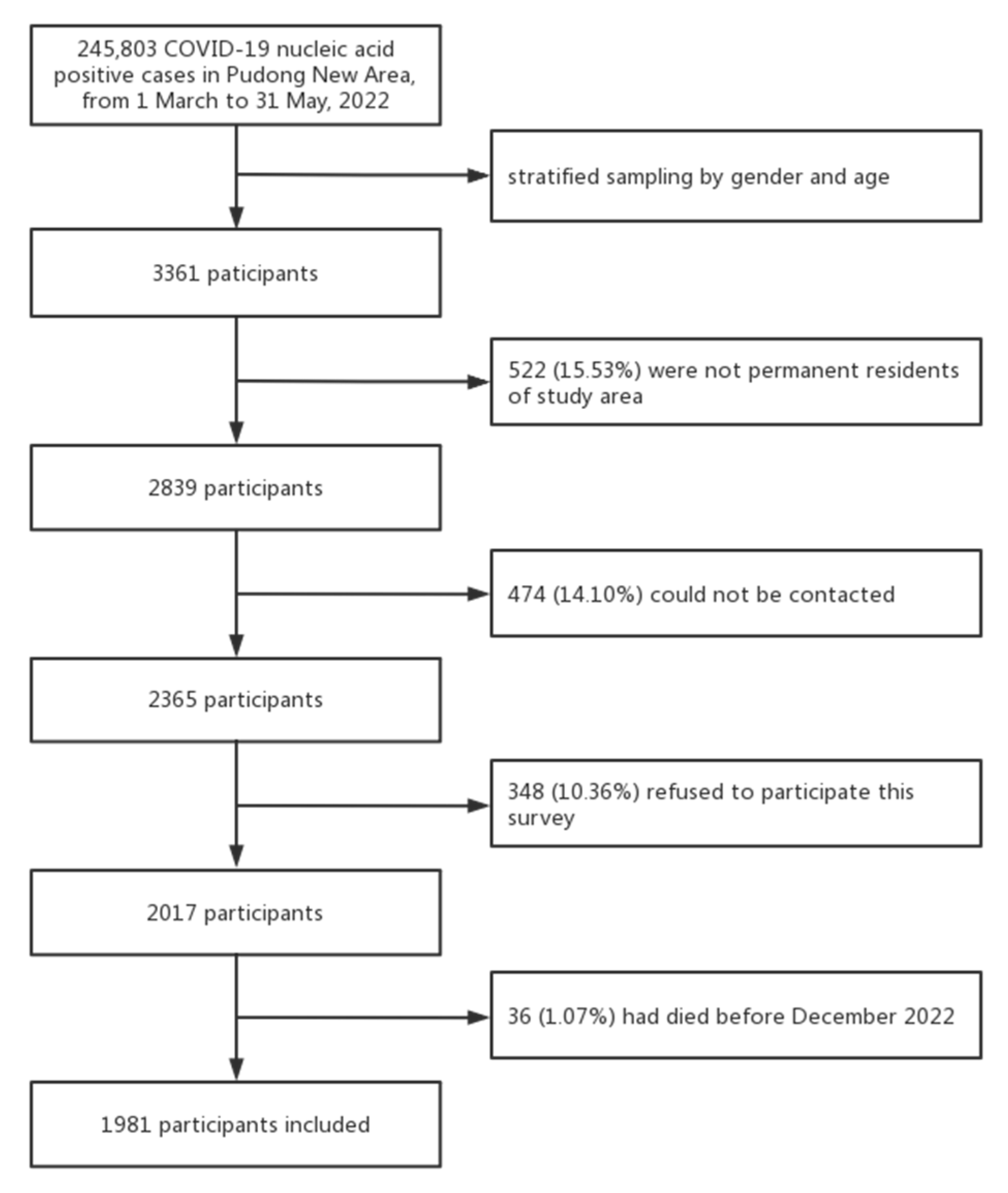
2. Materials and Methods
2.1. Study Design
2.2. Data Sources and Description
2.3. Statistical Analysis
3. Results
3.1. Basic Characteristics of Respondents
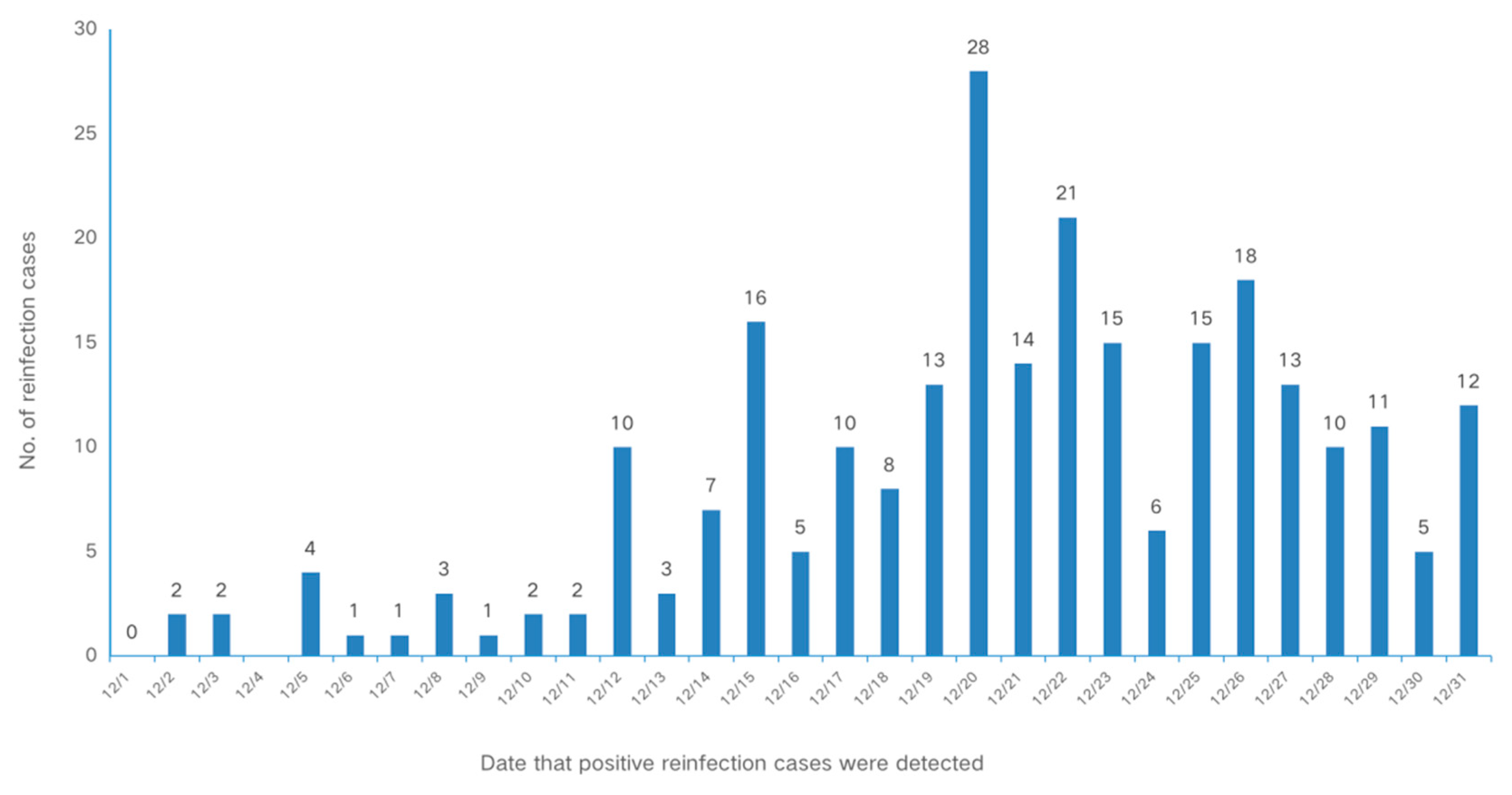
3.2. Reinfection Rate of SARS-CoV-2 among Different Populations
3.3. Factors That Influenced Reinfection
4. Discussion
4.1. Main Findings
4.2. Reinfection Rate of Omicron
4.3. Reinfection Rates among Different Groups
4.4. Hybrid Immunity against Omicron
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int/ (accessed on 23 February 2023).
- Wu, J.; Nie, J.; Zhang, L.; Song, H.; An, Y.; Liang, Z.; Yang, J.; Ding, R.; Liu, S.; Li, Q.; et al. The antigenicity of SARS-CoV-2 delta variants aggregated 10 high-frequency mutations in RBD has not changed sufficiently to replace the current vaccine strain. Signal Transduct. Target. Ther. 2022, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Tracking SARS-CoV-2 Variants. 2023. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants (accessed on 16 March 2023).
- World Health Organization. Classification of omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. 2021. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed on 23 February 2023).
- Breathnach, A.S.; Duncan, C.J.A.; Bouzidi, K.E.; Hanrath, A.T.; Payne, B.A.I.; Randell, P.A.; Habibi, M.S.; Riley, P.A.; Planche, T.D.; Busby, J.S.; et al. Prior COVID-19 protects against reinfection, even in the absence of detectable antibodies. J. Infect. 2021, 83, 237–279. [Google Scholar] [CrossRef]
- Abo-Leyah, H.; Gallant, S.; Cassidy, D.; Giam, Y.H.; Killick, J.; Marshall, B.; Hay, G.; Snowdon, C.; Hothersall, E.J.; Pembridge, T.; et al. The protective effect of SARS-CoV-2 antibodies in scottish healthcare workers. ERJ Open Res. 2021, 7, 18. [Google Scholar] [CrossRef]
- To, K.K.; Hung, I.F.; Ip, J.D.; Chu, A.W.; Chan, W.M.; Tam, A.R.; Fong, C.H.; Yuan, S.; Tsoi, H.W.; Ng, A.C.; et al. Coronavirus disease 2019 (COVID-19) re-infection by a phylogenetically distinct severe acute respiratory syndrome coronavirus 2 strain confirmed by whole genome sequencing. Clin. Infect. Dis. 2021, 73, e2946–e2951. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Ayoub, H.H.; Coyle, P.; Tang, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Hasan, M.R.; Al-Kanaani, Z.; Al-Kuwari, E.; et al. Protection of omicron sub-lineage infection against reinfection with another omicron sub-lineage. Nat. Commun. 2022, 13, 4675. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- Deng, L.; Li, P.; Zhang, X.; Jiang, Q.; Turner, D.; Zhou, C.; Gao, Y.; Qian, F.; Zhang, C.; Lu, H.; et al. Risk of SARS-CoV-2 reinfection: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 20763. [Google Scholar] [CrossRef]
- Özüdoğru, O.; Bahçe, Y.G.; Acer, Ö. SARS CoV-2 reinfection rate is higher in the omicron variant than in the alpha and delta variants. Ir. J. Med. Sci. 2022, 192, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.; Nassereldine, H.; Sorensen, R.J.D.; Amlag, J.O.; Bisignano, C.; Byrne, S.; Castro, E.; Coberly, K.; Collins, J.K.; Dalos, J.; et al. Past SARS-CoV-2 infection protection against re-infection: A systematic review and meta-analysis. Lancet 2023, 401, 833–842. [Google Scholar] [CrossRef]
- Lai, S.; Ruktanonchai, N.W.; Zhou, L.; Prosper, O.; Luo, W.; Floyd, J.R.; Wesolowski, A.; Santillana, M.; Zhang, C.; Du, X.; et al. Effect of non-pharmaceutical interventions to contain COVID-19 in China. Nature 2020, 585, 410–413. [Google Scholar] [CrossRef]
- Baker, M.G.; Wilson, N.; Blakely, T. Elimination could be the optimal response strategy for COVID-19 and other emerging pandemic diseases. BMJ 2020, 371, m4907. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zheng, C.; Wang, L.; Geng, M.; Chen, H.; Zhou, S.; Ran, L.; Li, Z.; Zhang, Y.; Feng, Z.; et al. Interpretation of the protocol for prevention and control of COVID-19 in China (Edition 8). China CDC Wkly. 2021, 3, 527–530. [Google Scholar] [CrossRef]
- Li, Z.; Liu, F.; Cui, J.; Peng, Z.; Chang, Z.; Lai, S.; Chen, Q.; Wang, L.; Gao, G.F.; Feng, Z. Comprehensive large-scale nucleic acid-testing strategies support China’s sustained containment of COVID-19. Nat. Med. 2021, 27, 740–742. [Google Scholar] [CrossRef]
- Chen, Q.; Rodewald, L.; Lai, S.; Gao, G.F. Rapid and sustained containment of COVID-19 is achievable and worthwhile: Implications for pandemic response. BMJ 2021, 375, e066169. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Q.; Feng, L.; Rodewald, L.; Xia, Y.; Yu, H.; Zhang, R.; An, Z.; Yin, W.; Chen, W.; et al. Active case finding with case management: The key to tackling the COVID-19 pandemic. Lancet 2020, 396, 63–70. [Google Scholar] [CrossRef]
- Chen, Z.; Deng, X.; Fang, L.; Sun, K.; Wu, Y.; Che, T.; Zou, J.; Cai, J.; Liu, H.; Wang, Y.; et al. Epidemiological characteristics and transmission dynamics of the outbreak caused by the SARS-CoV-2 Omicron variant in Shanghai, China: A descriptive study. Lancet Reg. Health West. Pac. 2022, 29, 100592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, W.; Chen, S. Shanghai’s life-saving efforts against the current omicron wave of the COVID-19 pandemic. Lancet 2022, 399, 2011–2012. [Google Scholar] [CrossRef] [PubMed]
- Carazo, S.; Skowronski, D.M.; Brisson, M.; Sauvageau, C.; Brousseau, N.; Gilca, R.; Ouakki, M.; Barkati, S.; Fafard, J.; Talbot, D.; et al. Estimated protection of prior SARS-CoV-2 infection against reinfection with the omicron variant among messenger rna-vaccinated and nonvaccinated individuals in Quebec, Canada. JAMA Netw. Open 2022, 5, e2236670. [Google Scholar] [CrossRef]
- Bastard, J.; Taisne, B.; Figoni, J.; Mailles, A.; Durand, J.; Fayad, M.; Josset, L.; Maisa, A.; van der Werf, S.; du Châtelet, I.P.; et al. Impact of the omicron variant on SARS-CoV-2 reinfections in France, March 2021 to February 2022. Eurosurveillance 2022, 27, 2200247. [Google Scholar] [CrossRef]
- Pulliam, J.R.C.; Van Schalkwyk, C.; Govender, N.; Von Gottberg, A.; Cohen, C.; Groome, M.J.; Dushoff, J.; Mlisana, K.; Moultrie, H. Increased risk of SARS-CoV-2 reinfection associated with emergence of omicron in South Africa. Science 2022, 376, eabn4947. [Google Scholar] [CrossRef]
- Flacco, M.E.; Martellucci, C.A.; Baccolini, V.; De Vito, C.; Renzi, E.; Villari, P.; Manzoli, L. Risk of reinfection and disease after SARS-CoV-2 primary infection: Meta-analysis. Eur. J. Clin. Investig. 2022, 52, e13845. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.B.; Foster, C.; Rawlinson, W.; Tedla, N.; Bull, R.A. Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: Implications for immune escape and transmission. Rev. Med. Virol. 2022, 32, e2381. [Google Scholar] [CrossRef] [PubMed]
- Sacco, C.; Petrone, D.; Del Manso, M.; Mateo-Urdiales, A.; Fabiani, M.; Bressi, M.; Bella, A.; Pezzotti, P.; Rota, M.C.; Riccardo, F.; et al. Risk and protective factors for SARS-CoV-2 reinfections, surveillance data, Italy, August 2021 to March 2022. Eurosurveillance 2022, 27, 2200372. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Hasan, M.R.; Ayoub, H.H.; Altarawneh, H.N.; Al-Kanaani, Z.; et al. Protection against Reinfection with the Omicron BA.2.75 Subvariant. N. Engl. J. Med. 2023, 388, 665–667. [Google Scholar] [CrossRef]
- Alhaddad, F.; Abdulkareem, A.; Alsharrah, D.; Alkandari, A.; Bin-Hasan, S.; Al-Ahmad, M.; Al Hashemi, H.; Alghounaim, M. Incidence of SARS-CoV-2 reinfection in a paediatric cohort in Kuwait. BMJ Open 2022, 12, e056371. [Google Scholar] [CrossRef]
- Almadhi, M.; Alsayyad, A.S.; Conroy, R.; Atkin, S.; Awadhi, A.A.; Al-Tawfiq, J.A.; AlQahtani, M. Epidemiological assessment of SARS-CoV-2 reinfection. Int. J. Infect. Dis. 2022, 123, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.H.; Friis, N.U.; Bager, P.; Stegger, M.; Fonager, J.; Fomsgaard, A.; Gram, M.A.; Christiansen, L.E.; Ethelberg, S.; Legarth, R.; et al. Risk of reinfection, vaccine protection, and severity of infection with the BA.5 omicron subvariant: A nation-wide population-based study in Denmark. Lancet Infect. Dis. 2023, 23, 167–176. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Shen, X.R.; Geng, R.; Xie, N.; Han, J.F.; Zhang, Q.-M.; Shi, Z.-L.; Zhou, P. A 1-year longitudinal study on COVID-19 convalescents reveals persistence of anti-SARS-CoV-2 humoral and cellular immunity. Emerg. Microbes Infect. 2022, 11, 902–913. [Google Scholar] [CrossRef]
- Gargouri, S.; Souissi, A.; Abid, N.; Chtourou, A.; Feki-Berrajah, L.; Karray, R.; Kossentini, H.; Ben Ayed, I.; Abdelmoula, F.; Chakroun, O.; et al. Evidence of SARS-CoV-2 symptomatic reinfection in four healthcare professionals from the same hospital despite the presence of antibodies. Int. J. Infect. Dis. 2022, 117, 146–154. [Google Scholar] [CrossRef]
- Dhumal, S.; Patil, A.; More, A.; Kamtalwar, S.; Joshi, A.; Gokarn, A.; Mirgh, S.; Thatikonda, P.; Bhat, P.; Murthy, V.; et al. SARS-CoV-2 reinfection after previous infection and vaccine breakthrough infection through the second wave of pandemic in India: An observational study. Int. J. Infect. Dis. 2022, 118, 95–103. [Google Scholar] [CrossRef]
- Russell, R.S. Hybrid immunity against severe acute respiratory syndrome coronavirus 2. Viral Immunol. 2022, 35, 391. [Google Scholar] [CrossRef]
- Andreano, E.; Paciello, I.; Piccini, G.; Manganaro, N.; Pileri, P.; Hyseni, I.; Leonardi, M.; Pantano, E.; Abbiento, V.; Benincasa, L.; et al. Hybrid immunity improves B cells and antibodies against SARS-CoV-2 variants. Nature 2021, 600, 530–535. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Sharma, A.R.; Dhama, K.; Agoramoorthy, G.; Chakraborty, C. Hybrid immunity against COVID-19 in different countries with a special emphasis on the Indian scenario during the omicron period. Int. Immunopharmacol. 2022, 108, 108766. [Google Scholar] [CrossRef] [PubMed]
- Halfmann, P.J.; Kuroda, M.; Maemura, T.; Chiba, S.; Armbrust, T.; Wright, R.; Balaram, A.; Florek, K.R.; Bateman, A.C.; Kawaoka, Y. Efficacy of vaccination and previous infection against the Omicron BA.1 variant in syrian hamsters. Cell Rep. 2022, 39, 110688. [Google Scholar] [CrossRef]
- Al-Otaiby, M.; Krissaane, I.; Al Seraihi, A.; Alshenaifi, J.; Qahtani, M.H.; Aljeri, T.; Zaatari, E.; Hassanain, M.; Algwizani, A.; Albarrag, A.; et al. SARS-CoV-2 reinfection rate and outcomes in Saudi Arabia: A national retrospective study. Int. J. Infect. Dis. 2022, 122, 758–766. [Google Scholar] [CrossRef]
- Diani, S.; Leonardi, E.; Cavezzi, A.; Ferrari, S.; Iacono, O.; Limoli, A.; Bouslenko, Z.; Natalini, D.; Conti, S.; Mantovani, M.; et al. SARS-CoV-2-the role of natural immunity: A narrative review. J. Clin. Med. 2022, 11, 6272. [Google Scholar] [CrossRef] [PubMed]
- Lutrick, K.; Rivers, P.; Yoo, Y.M.; Grant, L.; Hollister, J.; Jovel, K.; Khan, S.; Lowe, A.; Baccam, Z.; Hanson, H.; et al. Interim estimate of vaccine effectiveness of BNT162b2 (Pfizer-BioNTech) vaccine in preventing SARS-CoV-2 infection among adolescents aged 12–17 years—Arizona, July-December 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1761–1765. [Google Scholar] [CrossRef]
- Tan, C.Y.; Chiew, C.J.; Pang, D.; Lee, V.J.; Ong, B.; Lye, D.C.; Tan, K.B. Protective immunity of SARS-CoV-2 infection and vaccines against medically attended symptomatic omicron BA.4, BA.5, and XBB reinfections in Singapore: A national cohort study. Lancet Infect. Dis. 2023. [Google Scholar] [CrossRef]
- Wu, S.; Li, Y.; Mishra, S.; Bodner, K.; Baral, S.; Kwong, J.C.; Wei, X. Effect of the incremental protection of previous infection against omicron infection among individuals with a hybrid of infection- and vaccine-induced immunity: A population-based cohort study in Canada. Int. J. Infect. Dis. 2023, 127, 69–76. [Google Scholar] [CrossRef]
- Medić, S.; Anastassopoulou, C.; Lozanov-Crvenković, Z.; Vuković, V.; Dragnić, N.; Petrović, V.; Ristić, M.; Pustahija, T.; Gojković, Z.; Tsakris, A.; et al. Risk and severity of SARS-CoV-2 reinfections during 2020–2022 in Vojvodina, Serbia: A population-level observational study. Lancet Reg. Health Eur. 2022, 20, 100453. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Coyle, P.; Al-Kanaani, Z.; et al. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N. Engl. J. Med. 2022, 387, 21–34. [Google Scholar] [CrossRef] [PubMed]
| Sex | Age Group | COVID-19 Infections from March to May 2022 N = 245,803 | No. of People with COVID-19 Sampled N = 3361 | No. of People with COVID-19 Who Responded N = 1981 | Response Rate (%) |
|---|---|---|---|---|---|
| Male | 0–9 | 4453 (1.81%) | 53 (1.58%) | 40 (2.02%) | 65.57 |
| 10–19 | 5653 (2.3%) | 76 (2.26%) | 38 (1.92%) | 49.35 | |
| 20–29 | 22,151 (9.01%) | 333 (9.91%) | 153 (7.72%) | 50.50 | |
| 30–39 | 30,411 (12.37%) | 405 (12.05%) | 238 (12.01%) | 57.21 | |
| 40–49 | 24,528 (9.98%) | 365 (10.86%) | 199 (10.05%) | 59.40 | |
| 50–59 | 28,536 (11.61%) | 376 (11.19%) | 229 (11.56%) | 58.72 | |
| 60–69 | 14,337 (5.83%) | 189 (5.62%) | 106 (5.35%) | 54.08 | |
| 70–79 | 7038 (2.86%) | 96 (2.86%) | 59 (2.98%) | 61.46 | |
| 80+ | 3310 (1.35%) | 55 (1.64%) | 34 (1.72%) | 75.56 | |
| Female | 0–9 | 3826 (1.56%) | 49 (1.46%) | 33 (1.67%) | 63.46 |
| 10–19 | 3946 (1.61%) | 53 (1.58%) | 29 (1.46%) | 53.70 | |
| 20–29 | 12,485 (5.08%) | 182 (5.42%) | 95 (4.80%) | 55.56 | |
| 30–39 | 20,122 (8.19%) | 244 (7.26%) | 175 (8.83%) | 63.64 | |
| 40–49 | 18,182 (7.4%) | 232 (6.90%) | 165 (8.33%) | 66.27 | |
| 50–59 | 20,647 (8.4%) | 300 (8.93%) | 174 (8.78%) | 61.70 | |
| 60–69 | 13,721 (5.58%) | 170 (5.06%) | 113 (5.70%) | 60.11 | |
| 70–79 | 7245 (2.95%) | 103 (3.06%) | 60 (3.03%) | 60.61 | |
| 80+ | 5212 (2.12%) | 80 (2.38%) | 41 (2.07%) | 57.75 |
| Characteristics | No. of Respondents | Proportions (%) | No. of Reinfections | Adjusted Reinfection Rate (95% CI) | p |
|---|---|---|---|---|---|
| Total | 1981 | 100.0 | 260 | 13.12 (11.64–14.61) | |
| Sex | |||||
| Male | 1115 | 56.3 | 161 | 14.69 (12.59–16.79) | 0.025 |
| Female | 866 | 43.7 | 99 | 11.19 (9.11–13.26) | |
| Age | |||||
| 0–9 | 73 | 3.7 | 2 | 2.74 (0–6.48) | 0.000 |
| 10–19 | 67 | 3.4 | 6 | 8.96 (2.12–15.79) | |
| 20–29 | 248 | 12.5 | 33 | 13.31 (9.08–17.53) | |
| 30–39 | 413 | 20.8 | 87 | 21.07 (17.13–25.00) | |
| 40–49 | 364 | 18.4 | 47 | 12.91 (9.47–16.36) | |
| 50–59 | 403 | 20.3 | 42 | 10.42 (7.44–13.41) | |
| 60–69 | 219 | 11.1 | 22 | 10.05 (6.06–14.03) | |
| 70–79 | 119 | 6.0 | 10 | 8.40 (3.42–13.39) | |
| 80+ | 75 | 3.8 | 11 | 14.67 (6.66–22.67) | |
| Vaccination | |||||
| Incomplete | 579 | 29.2 | 88 | 15.20 (12.27–18.12) | 0.022 |
| Complete | 632 | 31.9 | 84 | 13.29 (10.64–15.94) | |
| Booster | 770 | 38.9 | 88 | 11.43 (9.18–13.68) |
| Characteristics | No. of Reinfections | Adjusted Odds Ratio (95% CI) | p |
|---|---|---|---|
| Total | 260 | ||
| Sex | |||
| Male | 161 | REF | 0.0245 |
| Female | 99 | 0.732 (0.557–0.961) | |
| Age | |||
| 0–9 | 2 | 0.163 (0.035–0.764) | 0.0213 |
| 10–19 | 6 | 0.664 (0.226–1.949) | 0.4565 |
| 20–29 | 33 | 1.086 (0.509–2.317) | 0.8316 |
| 30–39 | 87 | 2.034 (0.997–4.148) | 0.0510 |
| 40–49 | 47 | 1.156 (0.551–2.425) | 0.7006 |
| 50–59 | 42 | 0.903 (0.429–1.903) | 0.7888 |
| 60–69 | 22 | 0.823 (0.371–1.823) | 0.6305 |
| 70–79 | 10 | 0.643 (0.256–1.616) | 0.3477 |
| 80+ | 11 | REF | |
| Vaccination | |||
| Incomplete | 88 | REF | |
| Complete | 84 | 0.741 (0.528–1.042) | 0.0849 |
| Booster | 88 | 0.579 (0.412–0.813) | 0.0016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, C.; Zhang, G.; Zhang, A.; Xin, H.; Wu, K.; Li, Z.; Jia, Y.; Hao, L.; Xue, C.; Wang, Y.; et al. The Omicron Variant Reinfection Risk among Individuals with a Previous SARS-CoV-2 Infection within One Year in Shanghai, China: A Cross-Sectional Study. Vaccines 2023, 11, 1146. https://doi.org/10.3390/vaccines11071146
Ye C, Zhang G, Zhang A, Xin H, Wu K, Li Z, Jia Y, Hao L, Xue C, Wang Y, et al. The Omicron Variant Reinfection Risk among Individuals with a Previous SARS-CoV-2 Infection within One Year in Shanghai, China: A Cross-Sectional Study. Vaccines. 2023; 11(7):1146. https://doi.org/10.3390/vaccines11071146
Chicago/Turabian StyleYe, Chuchu, Ge Zhang, Anran Zhang, Hualei Xin, Kang Wu, Zhongjie Li, Yilin Jia, Lipeng Hao, Caoyi Xue, Yuanping Wang, and et al. 2023. "The Omicron Variant Reinfection Risk among Individuals with a Previous SARS-CoV-2 Infection within One Year in Shanghai, China: A Cross-Sectional Study" Vaccines 11, no. 7: 1146. https://doi.org/10.3390/vaccines11071146
APA StyleYe, C., Zhang, G., Zhang, A., Xin, H., Wu, K., Li, Z., Jia, Y., Hao, L., Xue, C., Wang, Y., Xu, H., Zhu, W., & Zhou, Y. (2023). The Omicron Variant Reinfection Risk among Individuals with a Previous SARS-CoV-2 Infection within One Year in Shanghai, China: A Cross-Sectional Study. Vaccines, 11(7), 1146. https://doi.org/10.3390/vaccines11071146






