The Mechanism of bnAb Production and Its Application in Mutable Virus Broad-Spectrum Vaccines: Inspiration from HIV-1 Broad Neutralization Research
Abstract
1. Introduction
2. Broadly Neutralizing Antibody
2.1. Introduction to bnAbs
2.2. Limitations on the Production of bnAbs
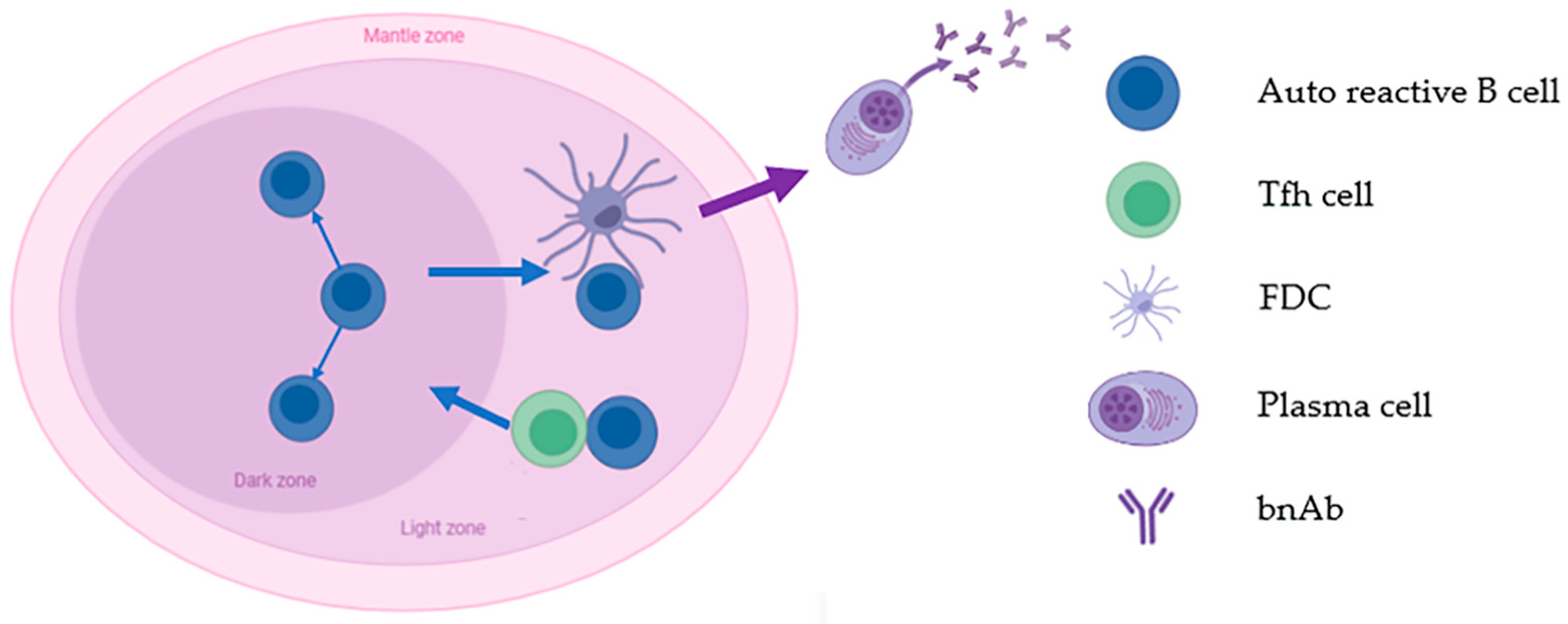
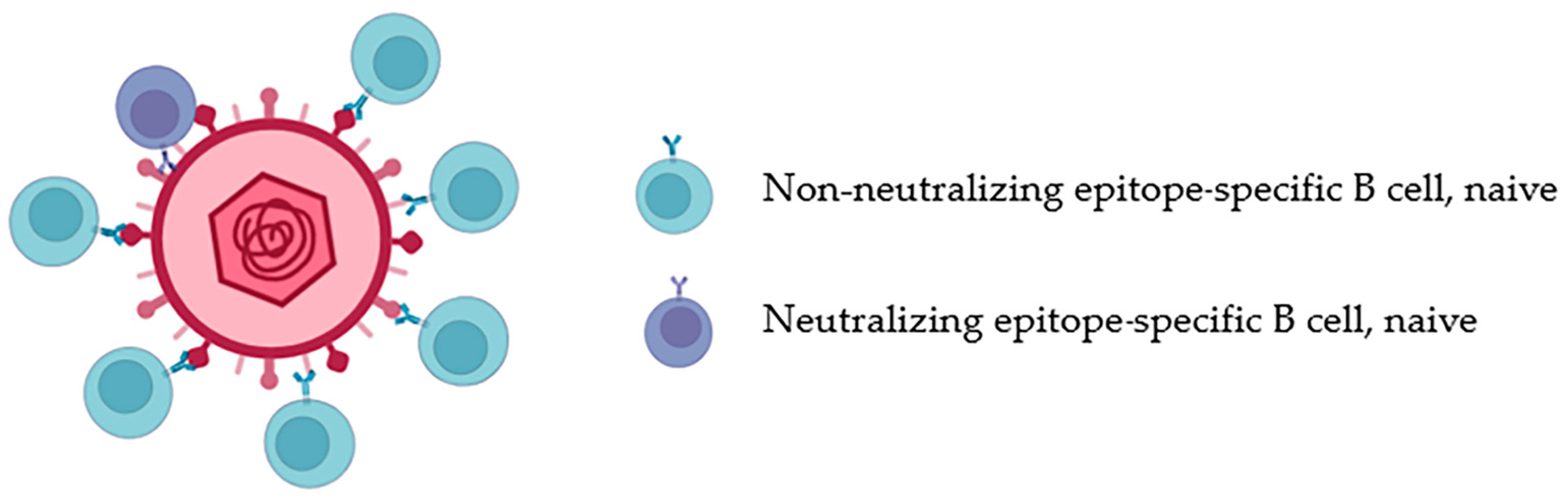
2.2.1. Lower BCR Affinity
2.2.2. Lower Frequency of Precursor B Cells
2.2.3. Less Help from Tfh Cells
2.3. Attempts to Induce bnAbs
3. Broad-Spectrum Vaccine Design to Induce bnAbs
3.1. The Need for Broad-Spectrum Vaccine Development
3.2. Strategies for Inducing bnAb Can Guide Vaccine Design
3.2.1. Role of TLR Signaling during bnAb Generation
3.2.2. Tfh- and B-Cell Interactions
3.2.3. B Cell Linage Vaccine Design
3.2.4. Prolonged Antigen Stimulation Can Enhance GC Responses
3.2.5. Multivalent Antigens Can Enhance the Broad Spectrum of Antibodies
4. Humoral Immunological Memory
5. Perspectives on Broad-Spectrum Vaccine Design
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 13 May 2023).
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Leung, A.K. Vaccinia, Vaccination, Vaccinoiogy: Jenner, Pasteur and Their Successors; Elsevier: Amsterdam, The Netherlands, 1996; pp. 65–71. [Google Scholar]
- Lower, S.; Bernoulli, D. An attempt at a new analysis of the mortality caused by smallpox and of the advantages of inoculation to prevent it. Rev. Med. Virol. 2004, 14, 275–288. [Google Scholar]
- De Gregorio, E.; Caproni, E.; Ulmer, J.B. Vaccine adjuvants: Mode of action. Front. Immunol. 2013, 4, 214. [Google Scholar] [CrossRef] [PubMed]
- Jenner, E. Inquiry into the Causes and Effects of the Variolae Vaccinae; Sampson Low: London, UK, 1798. [Google Scholar]
- Krause, P.R.; Fleming, T.R.; Longini, I.M.; Peto, R.; Briand, S.; Heymann, D.L.; Beral, V.; Snape, M.D.; Rees, H.; Ropero, A.M.; et al. SARS-CoV-2 Variants and Vaccines. N. Engl. J. Med. 2021, 385, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Safari, I.; Elahi, E. Evolution of the SARS-CoV-2 genome and emergence of variants of concern. Arch. Virol. 2022, 167, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; An, Y.; Wu, X.; Cai, M.; Zhang, X.; Yang, C.; Tong, J.; Cui, Z.; Li, X.; Huang, W.; et al. Retrospective immunogenicity analysis of seasonal flu H3N2 vaccines recommended in the past ten years using immunized animal sera. EBioMedicine 2022, 86, 104350. [Google Scholar] [CrossRef]
- Fischer, W.; Giorgi, E.E.; Chakraborty, S.; Nguyen, K.; Bhattacharya, T.; Theiler, J.; Goloboff, P.A.; Yoon, H.; Abfalterer, W.; Foley, B.T.; et al. HIV-1 and SARS-CoV-2: Patterns in the evolution of two pandemic pathogens. Cell Host Microbe 2021, 29, 1093–1110. [Google Scholar] [CrossRef]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef]
- Haynes, B.F.; Gilbert, P.B.; McElrath, M.J.; Zolla-Pazner, S.; Tomaras, G.D.; Alam, S.M.; Evans, D.T.; Montefiori, D.C.; Karnasuta, C.; Sutthent, R.; et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012, 366, 1275–1286. [Google Scholar] [CrossRef]
- Gray, G.E.; Bekker, L.G.; Laher, F.; Malahleha, M.; Allen, M.; Moodie, Z.; Grunenberg, N.; Huang, Y.; Grove, D.; Prigmore, B.; et al. Vaccine Efficacy of ALVAC-HIV and Bivalent Subtype C gp120-MF59 in Adults. N. Engl. J. Med. 2021, 384, 1089–1100. [Google Scholar] [CrossRef]
- Whitney, J.B.; Lim, S.Y.; Osuna, C.E.; Kublin, J.L.; Chen, E.; Yoon, G.; Liu, P.T.; Abbink, P.; Borducci, E.N.; Hill, A.; et al. Prevention of SIVmac251 reservoir seeding in rhesus monkeys by early antiretroviral therapy. Nat. Commun. 2018, 9, 5429. [Google Scholar] [CrossRef] [PubMed]
- Johnson & Johnson and Global Partners. Johnson & Johnson and Global Partners Announce Results from Phase 2b Imbokodo HIV Vaccine Clinical Trial in Young Women in Sub-Saharan Africa. 2021. Available online: https://www.jnj.com/johnson-johnson-and-global-partners-announce-results-from-phase-2b-imbokodo-hiv-vaccine-clinical-trial-in-young-women-in-sub-saharan-africa (accessed on 2 February 2022).
- Sterrett, S.; Learn, G.H.; Edlefsen, P.T.; Haynes, B.F.; Hahn, B.H.; Shaw, G.M.; Bar, K.J. Low Multiplicity of HIV-1 Infection and No Vaccine Enhancement in VAX003 Injection Drug Users. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2014; Volume 1, p. ofu056. [Google Scholar]
- Montefiori, D.C.; Karnasuta, C.; Huang, Y.; Ahmed, H.; Gilbert, P.; de Souza, M.S.; McLinden, R.; Tovanabutra, S.; Laurence-Chenine, A.; Sanders-Buell, E.; et al. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J. Infect. Dis. 2012, 206, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.B.; Peterson, M.L.; Follmann, D.; Hudgens, M.G.; Francis, D.P.; Gurwith, M.; Heyward, W.L.; Jobes, D.V.; Popovic, V.; Self, S.G.; et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J. Infect. Dis. 2005, 191, 666–677. [Google Scholar] [CrossRef]
- Flynn, N.M.; Forthal, D.N.; Harro, C.D.; Judson, F.N.; Mayer, K.H.; Para, M.F.; rgp120 HIV Vaccine Study Group. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 2005, 191, 654–665. [Google Scholar] [PubMed]
- Gottardo, R.; Bailer, R.T.; Korber, B.T.; Gnanakaran, S.; Phillips, J.; Shen, X.; Tomaras, G.D.; Turk, E.; Imholte, G.; Eckler, L.; et al. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS ONE 2013, 8, e75665. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.E.; Huang, Y.; Grunenberg, N.; Laher, F.; Roux, S.; Andersen-Nissen, E.; De Rosa, S.C.; Flach, B.; Randhawa, A.K.; Jensen, R.; et al. Immune correlates of the Thai RV144 HIV vaccine regimen in South Africa. Sci. Transl. Med. 2019, 11, eaax1880. [Google Scholar] [CrossRef]
- Dommaraju, K.; Kijak, G.; Carlson, J.M.; Larsen, B.B.; Tovanabutra, S.; Geraghty, D.E.; Deng, W.; Maust, B.S.; Edlefsen, P.T.; Sanders-Buell, E.; et al. CD8 and CD4 epitope predictions in RV144: No strong evidence of a T-cell driven sieve effect in HIV-1 breakthrough sequences from trial participants. PLoS ONE 2014, 9, e111334. [Google Scholar] [CrossRef]
- Li, S.S.; Gilbert, P.B.; Carpp, L.N.; Pyo, C.W.; Janes, H.; Fong, Y.; Shen, X.; Neidich, S.D.; Goodman, D.; deCamp, A.; et al. Fc Gamma Receptor Polymorphisms Modulated the Vaccine Effect on HIV-1 Risk in the HVTN 505 HIV Vaccine Trial. J. Virol. 2019, 93, e02041-18. [Google Scholar] [CrossRef]
- Hammer, S.M.; Sobieszczyk, M.E.; Janes, H.; Karuna, S.T.; Mulligan, M.J.; Grove, D.; Koblin, B.A.; Buchbinder, S.P.; Keefer, M.C.; Tomaras, G.D.; et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N. Engl. J. Med. 2013, 369, 2083–2092. [Google Scholar] [CrossRef]
- Laher, F.; Salami, T.; Hornschuh, S.; Makhale, L.M.; Khunwane, M.; Andrasik, M.P.; Gray, G.E.; Van Tieu, H.; Dietrich, J.J. Willingness to use HIV prevention methods among vaccine efficacy trial participants in Soweto, South Africa: Discretion is important. BMC Public Health 2020, 20, 1669. [Google Scholar] [CrossRef]
- Barouch, D.H.; Tomaka, F.L.; Wegmann, F.; Stieh, D.J.; Alter, G.; Robb, M.L.; Michael, N.L.; Peter, L.; Nkolola, J.P.; Borducchi, E.N.; et al. Evaluation of a mosaic HIV-1 vaccine in a multicentre, randomised, double-blind, placebo-controlled, phase 1/2a clinical trial (APPROACH) and in rhesus monkeys (NHP 13-19). Lancet 2018, 392, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F.; Wiehe, K.; Acharya, P.; Saunders, K.O. Vaccine, 8th ed.; Plotkin, S.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Javaherian, K.; Langlois, A.J.; LaRosa, G.J.; Profy, A.T.; Bolognesi, D.P.; Herlihy, W.C.; Putney, S.D.; Matthews, T.J. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science 1990, 250, 1590–1593. [Google Scholar] [CrossRef]
- Schommers, P.; Gruell, H.; Abernathy, M.E.; Tran, M.K.; Dingens, A.S.; Gristick, H.B.; Barnes, C.O.; Schoofs, T.; Schlotz, M.; Vanshylla, K.; et al. Restriction of HIV-1 Escape by a Highly Broad and Potent Neutralizing Antibody. Cell 2020, 180, 471–489.e22. [Google Scholar] [CrossRef] [PubMed]
- Barin, F.; Braibant, M. HIV-1 antibodies in prevention of transmission. Curr. Opin. HIV AIDS 2019, 14, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Von Bredow, B.; Arias, J.F.; Heyer, L.N.; Moldt, B.; Le, K.; Robinson, J.E.; Zolla-Pazner, S.; Burton, D.R.; Evans, D.T. Comparison of antibody-dependent cell-mediated cytotoxicity and virus neutralization by HIV-1 Env-specific monoclonal antibodies. J. Virol. 2016, 90, 6127–6139. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, W.; Sun, M.; Li, T. Broadly neutralizing antibodies for HIV-1: Efficacies, challenges and opportunities. Emerg. Microbes Infect. 2020, 9, 194–206. [Google Scholar] [CrossRef]
- Nie, J.H. HIV-1 Membrane Antigen Engineering and Evaluation of Immunogenicity [D]; National Institute for the Control of Pharmaceutical and Biological Products: Beijing, China, 2007. [Google Scholar]
- Williams, W.B.; Wiehe, K.; Saunders, K.O.; Haynes, B.F. Strategies for induction of HIV-1 envelope-reactive broadly neutralizing antibodies. J. Int. AIDS Soc. 2021, 24 (Suppl. S7), e25831. [Google Scholar] [CrossRef]
- Mouquet, H.; Scheid, J.F.; Zoller, M.J.; Krogsgaard, M.; Ott, R.G.; Shukair, S.; Artyomov, M.N.; Pietzsch, J.; Connors, M.; Pereyra, F.; et al. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature 2010, 467, 591–595. [Google Scholar] [CrossRef]
- Haynes, B.F.; Fleming, J.; St Clair, E.W.; Katinger, H.; Stiegler, G.; Kunert, R.; Robinson, J.; Scearce, R.M.; Plonk, K.; Staats, H.F.; et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 2005, 308, 1906–1908. [Google Scholar] [CrossRef]
- Haynes, B.F.; Moody, M.A.; Verkoczy, L.; Kelsoe, G.; Alam, S.M. Antibody polyspecificity and neutralization of HIV-1: A hypothesis. Hum. Antibodies 2005, 14, 59–67. [Google Scholar] [CrossRef]
- Haynes, B.F.; Kelsoe, G.; Harrison, S.C.; Kepler, T.B. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat. Biotechnol. 2012, 30, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Ho, J.; Malaspina, A.; Wang, W.; DiPoto, A.C.; O’Shea, M.A.; Roby, G.; Kottilil, S.; Arthos, J.; Proschan, M.A.; et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 2008, 205, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Kardava, L.; Moir, S.; Shah, N.; Wang, W.; Wilson, R.; Buckner, C.M.; Santich, B.H.; Kim, L.J.; Spurlin, E.E.; Nelson, A.K.; et al. Abnormal B cell memory subsets dominate HIV-specific responses in infected individuals. J. Clin. Investig. 2014, 124, 3252–3262. [Google Scholar] [CrossRef] [PubMed]
- Boliar, S.; Murphy, M.K.; Tran, T.C.; Carnathan, D.G.; Armstrong, W.S.; Silvestri, G.; Derdeyn, C.A. B-lymphocyte dysfunction in chronic HIV-1 infection does not prevent cross-clade neutralization breadth. J. Virol. 2012, 86, 8031–8040. [Google Scholar] [CrossRef]
- Klein, F.; Diskin, R.; Scheid, J.F.; Gaebler, C.; Mouquet, H.; Georgiev, I.S.; Pancera, M.; Zhou, T.; Incesu, R.B.; Fu, B.Z.; et al. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell 2013, 153, 126–138. [Google Scholar] [CrossRef]
- Mesin, L.; Ersching, J.; Victora, G.D. Germinal Center B-Cell Dynamics. Immunity 2016, 45, 471–482. [Google Scholar] [CrossRef]
- Liao, H.X.; Lynch, R.; Zhou, T.; Gao, F.; Alam, S.M.; Boyd, S.D.; Fire, A.Z.; Roskin, K.M.; Schramm, C.A.; Zhang, Z.; et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 2013, 496, 469–476. [Google Scholar] [CrossRef]
- Andrabi, R.; Voss, J.E.; Liang, C.H.; Briney, B.; McCoy, L.E.; Wu, C.Y.; Wong, C.H.; Poignard, P.; Burton, D.R. Identification of Common Features in Prototype Broadly Neutralizing Antibodies to HIV Envelope V2 Apex to Facilitate Vaccine Design. Immunity 2015, 43, 959–973. [Google Scholar] [CrossRef]
- Jardine, J.; Julien, J.P.; Menis, S.; Ota, T.; Kalyuzhniy, O.; McGuire, A.; Sok, D.; Huang, P.S.; MacPherson, S.; Jones, M.; et al. Rational HIV immunogen design to target specific germline B-cell receptors. Science 2013, 340, 711–716. [Google Scholar] [CrossRef]
- Dal Porto, J.M.; Haberman, A.M.; Shlomchik, M.J.; Kelsoe, G. Antigen drives very low affinity B cells to become plasmacytes and enter germinal centers. J. Immunol. 1998, 161, 5373–5381. [Google Scholar] [CrossRef]
- Steichen, J.M.; Lin, Y.C.; Havenar-Daughton, C.; Pecetta, S.; Ozorowski, G.; Willis, J.R.; Toy, L.; Sok, D.; Liguori, A.; Kratochvil, S.; et al. A generalized HIV vaccine design strategy for priming of broadly neutralizing antibody responses. Science 2019, 366, eaax4380. [Google Scholar] [CrossRef]
- Jardine, J.G.; Kulp, D.W.; Havenar-Daughton, C.; Sarkar, A.; Briney, B.; Sok, D.; Sesterhenn, F.; Ereño-Orbea, J.; Kalyuzhniy, O.; Deresa, I.; et al. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science 2016, 351, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Havenar-Daughton, C.; Sarkar, A.; Kulp, D.W.; Toy, L.; Hu, X.; Deresa, I.; Kalyuzhniy, O.; Kaushik, K.; Upadhyay, A.A.; Menis, S.; et al. The human naive B cell repertoire contains distinct subclasses for a germline-targeting HIV-1 vaccine immunogen. Sci. Transl. Med. 2018, 10, eaat0381. [Google Scholar] [CrossRef]
- Cao, L.; Diedrich, J.K.; Kulp, D.W.; Pauthner, M.; He, L.; Park, S.R.; Sok, D.; Su, C.Y.; Delahunty, C.M.; Menis, S.; et al. Global site-specific N-glycosylation analysis of HIV envelope glycoprotein. Nat. Commun. 2017, 8, 14954. [Google Scholar] [CrossRef]
- Doores, K.J.; Bonomelli, C.; Harvey, D.J.; Vasiljevic, S.; Dwek, R.A.; Burton, D.R.; Crispin, M.; Scanlan, C.N. Envelope glycans of immunodeficiency virions are almost entirely oligomannose antigens. Proc. Natl. Acad. Sci. USA 2010, 107, 13800–13805. [Google Scholar] [CrossRef]
- Steichen, J.M.; Kulp, D.W.; Tokatlian, T.; Escolano, A.; Dosenovic, P.; Stanfield, R.L.; McCoy, L.E.; Ozorowski, G.; Hu, X.; Kalyuzhniy, O.; et al. HIV Vaccine Design to Target Germline Precursors of Glycan-Dependent Broadly Neutralizing Antibodies. Immunity 2016, 45, 483–496. [Google Scholar] [CrossRef]
- Crotty, S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014, 41, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Victora, G.D.; Mesin, L. Clonal and cellular dynamics in germinal centers. Curr. Opin. Immunol. 2014, 28, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Ritzau-Jost, J.; Hutloff, A. T-Cell/B-Cell Interactions in the Establishment of Protective Immunity. Vaccines 2021, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Dal Porto, J.M.; Haberman, A.M.; Kelsoe, G.; Shlomchik, M.J. Very low affinity B cells form germinal centers, become memory B cells, and participate in secondary immune responses when higher affinity competition is reduced. J. Exp. Med. 2002, 195, 1215–1221. [Google Scholar] [CrossRef]
- Shih, T.A.; Meffre, E.; Roederer, M.; Nussenzweig, M.C. Role of BCR affinity in T-cell dependent antibody responses in vivo. Nat. Immunol. 2002, 3, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Schwickert, T.A.; Victora, G.D.; Fooksman, D.R.; Kamphorst, A.O.; Mugnier, M.R.; Gitlin, A.D.; Dustin, M.L.; Nussenzweig, M.C. A dynamic T-cell-limited checkpoint regulates affinity-dependent B-cell entry into the germinal center. J. Exp. Med. 2011, 208, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Locci, M.; Havenar-Daughton, C.; Landais, E.; Wu, J.; Kroenke, M.A.; Arlehamn, C.L.; Su, L.F.; Cubas, R.; Davis, M.M.; Sette, A.; et al. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 2013, 39, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Moody, M.A.; Pedroza-Pacheco, I.; Vandergrift, N.A.; Chui, C.; Lloyd, K.E.; Parks, R.; Soderberg, K.A.; Ogbe, A.T.; Cohen, M.S.; Liao, H.X.; et al. Immune perturbations in HIV-1–infected individuals who make broadly neutralizing antibodies. Sci. Immunol. 2016, 1, aag0851. [Google Scholar] [CrossRef]
- Havenar-Daughton, C.; Carnathan, D.G.; Torrents de la Peña, A.; Pauthner, M.; Briney, B.; Reiss, S.M.; Wood, J.S.; Kaushik, K.; van Gils, M.J.; Rosales, S.L.; et al. Direct probing of germinal center responses reveals immunological features and bottlenecks for neutralizing Antibody Responses to HIV Env Trimer. Cell Rep. 2016, 17, 2195–2209. [Google Scholar] [CrossRef]
- Roark, R.S.; Li, H.; Williams, W.B.; Chug, H.; Mason, R.D.; Gorman, J.; Wang, S.; Lee, F.H.; Rando, J.; Bonsignori, M.; et al. Recapitulation of HIV-1 Env-antibody coevolution in macaques leading to neutralization breadth. Science 2021, 371, eabd2638. [Google Scholar] [CrossRef]
- Mu, Z.; Haynes, B.F.; Cain, D.W. Strategies for eliciting multiple lineages of broadly neutralizing antibodies to HIV by vaccination. Curr. Opin. Virol. 2021, 51, 172–178. [Google Scholar] [CrossRef]
- Saunders, K.O.; Wiehe, K.; Tian, M.; Acharya, P.; Bradley, T.; Alam, S.M.; Go, E.P.; Scearce, R.; Sutherland, L.; Henderson, R.; et al. Targeted selection of HIV-specific antibody mutations by engineering B-cell maturation. Science 2019, 366, eaay7199. [Google Scholar] [CrossRef]
- Jardine, J.G.; Ota, T.; Sok, D.; Pauthner, M.; Kulp, D.W.; Kalyuzhniy, O.; Skog, P.D.; Thinnes, T.C.; Bhullar, D.; Briney, B.; et al. HIV-1 VACCINES. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science 2015, 349, 156–161. [Google Scholar] [CrossRef]
- McGuire, A.T.; Hoot, S.; Dreyer, A.M.; Lippy, A.; Stuart, A.; Cohen, K.W.; Jardine, J.; Menis, S.; Scheid, J.F.; West, A.P.; et al. Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. J. Exp. Med. 2013, 210, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Stamatatos, L.; Pancera, M.; McGuire, A.T. Germline-targeting immunogens. Immunol. Rev. 2017, 275, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Wiehe, K.; Bradley, T.; Meyerhoff, R.R.; Hart, C.; Williams, W.B.; Easterhoff, D.; Faison, W.J.; Kepler, T.B.; Saunders, K.O.; Alam, S.M.; et al. Functional Relevance of Improbable Antibody Mutations for HIV Broadly Neutralizing Antibody Development. Cell Host Microbe 2018, 23, 759–765. [Google Scholar] [CrossRef]
- Madan-Lala, R.; Pradhan, P.; Roy, K. Combinatorial Delivery of Dual and Triple TLR Agonists via Polymeric Pathogen-like Particles Synergistically Enhances Innate and Adaptive Immune Responses. Sci. Rep. 2017, 7, 2530. [Google Scholar] [CrossRef] [PubMed]
- Desbien, A.L.; Dubois Cauwelaert, N.; Reed, S.J.; Bailor, H.R.; Liang, H.; Carter, D.; Duthie, M.S.; Fox, C.B.; Reed, S.G.; Orr, M.T. IL-18 and Subcapsular Lymph Node Macrophages are Essential for Enhanced B-Cell Responses with TLR4 Agonist Adjuvants. J. Immunol. 2016, 197, 4351–4359. [Google Scholar] [CrossRef]
- Ugolini, M.; Gerhard, J.; Burkert, S.; Jensen, K.J.; Georg, P.; Ebner, F.; Volkers, S.M.; Thada, S.; Dietert, K.; Bauer, L.; et al. Recognition of microbial viability via TLR8 drives TFHcell differentiation and vaccine responses. Nat. Immunol. 2018, 19, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Rookhuizen, D.C.; DeFranco, A.L. Toll-like receptor 9 signaling acts on multiple elements of the germinal center to enhance antibody responses. Proc. Natl. Acad. Sci. USA 2014, 111, E3224–E3233. [Google Scholar] [CrossRef]
- Kasturi, S.P.; Skountzou, I.; Albrecht, R.A.; Koutsonanos, D.; Hua, T.; Nakaya, H.I.; Ravindran, R.; Stewart, S.; Alam, M.; Kwissa, M.; et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011, 470, 543–547. [Google Scholar] [CrossRef]
- Baumjohann, D.; Preite, S.; Reboldi, A.; Ronchi, F.; Ansel, K.M.; Lanzavecchia, A.; Sallusto, F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 2013, 38, 596–605. [Google Scholar] [CrossRef]
- Tian, M.; McGovern, K.; Cheng, H.L.; Waddicor, P.; Rieble, L.; Dao, M.; Chen, Y.; Kimble, M.T.; Cantor, E.; Manfredonia, N.; et al. Conditional antibody expression to avoid central B cell deletion in humanized HIV-1 vaccine mouse models. Proc. Natl. Acad. Sci. USA 2020, 117, 7929–7940. [Google Scholar] [CrossRef]
- Verkoczy, L. Humanized Immunoglobulin Mice: Models for HIV Vaccine Testing and Studying the Broadly Neutralizing Antibody Problem. Adv. Immunol. 2017, 134, 235–352. [Google Scholar]
- Finzi, A. Exposing HIV-1 Env: Implications for therapeutic strategies. Clinical and investigative medicine. Med. Clin. Exp. 2019, 42, E2–E6. [Google Scholar]
- Han, C.; Johnson, J.; Dong, R.; Kandula, R.; Kort, A.; Wong, M.; Yang, T.; Breheny, P.J.; Brown, G.D.; Haim, H. Key Positions of HIV-1 Env and Signatures of Vaccine Efficacy Show Gradual Reduction of Population Founder Effects at the Clade and Regional Levels. mBio 2020, 11, e00126-20. [Google Scholar] [CrossRef]
- Liu, C.; Ginn, H.M.; Dejnirattisai, W.; Supasa, P.; Wang, B.; Tuekprakhon, A.; Nutalai, R.; Zhou, D.; Mentzer, A.J.; Zhao, Y.; et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell 2021, 184, 4220–4236.e13. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Lam, E.C.; St Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2372–2383.e9. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Nie, J.; Wu, J.; Zhang, L.; Ding, R.; Wang, H.; Zhang, Y.; Li, T.; Liu, S.; Zhang, M.; et al. SARS-CoV-2 501Y.V2 variants lack higher infectivity but do have immune escape. Cell 2021, 184, 2362–2371.e9. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 1997, 388, 394–397. [Google Scholar] [CrossRef]
- Möller, G. One nonspecific signal triggers b lymphocytes. Transpl. Rev. 1975, 23, 126–137. [Google Scholar]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef]
- Kasturi, S.P.; Rasheed, M.A.U.; Havenar-Daughton, C.; Pham, M.; Legere, T.; Sher, Z.J.; Kovalenkov, Y.; Gumber, S.; Huang, J.Y.; Gottardo, R.; et al. 3 M-052, a synthetic TLR-7/8 agonist, induces durable HIV-1 envelope-specific plasma cells and humoral immunity in nonhuman primates. Sci. Immunol. 2020, 5, eabb1025. [Google Scholar] [CrossRef]
- Anokhin, V.V.; Bakhteeva, L.B.; Khasanova, G.R.; Khaiboullina, S.F.; Martynova, E.V.; Tillett, R.L.; Schlauch, K.A.; Lombardi, V.C.; Rizvanov, A.A. Previously Unidentified Single Nucleotide Polymorphisms in HIV/AIDS Cases Associate with Clinical Parameters and Disease Progression. BioMed Res. Int. 2016, 2016, 2742648. [Google Scholar] [CrossRef]
- Cingöz, O.; Goff, S.P. HIV-1 Is a Poor Inducer of Innate Immune Responses. mBio 2019, 10, e02834-18. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Zhang, Z.; Liu, H.; Tian, M.; Zhu, X.; Zhang, Z.; Wang, W.; Zhou, X.; Zhang, F.; Ge, Q.; et al. B Cells Are the Dominant Antigen-Presenting Cells that Activate Naive CD4+ T Cells upon Immunization with a Virus-Derived Nanoparticle Antigen. Immunity 2018, 49, 695–708.e4. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Peng, Y.; Lin, L.; Pan, X.; Fang, M.; Zhao, Y.; Bao, K.; Li, R.; Han, J.; Chen, J.; et al. A pathogen-like antigen-based vaccine confers immune protection against SARS-CoV-2 in non-human primates. Cell Rep. Med. 2021, 2, 100448. [Google Scholar] [CrossRef]
- Bannard, O.; McGowan, S.J.; Ersching, J.; Ishido, S.; Victora, G.D.; Shin, J.S.; Cyster, J.G. Ubiquitin-mediated fluctuations in MHC class II facilitate efficient germinal center B-cell responses. J. Exp. Med. 2016, 213, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Nojima, T.; Kuraoka, M.; Kelsoe, G. Germinal center entry not selection of B cells is controlled by peptide-MHCII complex density. Nat. Commun. 2018, 9, 928. [Google Scholar] [CrossRef]
- Roskin, K.M.; Jackson, K.J.L.; Lee, J.Y.; Hoh, R.A.; Joshi, S.A.; Hwang, K.K.; Bonsignori, M.; Pedroza-Pacheco, I.; Liao, H.X.; Moody, M.A.; et al. Aberrant B cell repertoire selection associated with HIV neutralizing antibody breadth. Nat. Immunol. 2020, 21, 199–209. [Google Scholar] [CrossRef]
- Petrovas, C.; Yamamoto, T.; Gerner, M.Y.; Boswell, K.L.; Wloka, K.; Smith, E.C.; Ambrozak, D.R.; Sandler, N.G.; Timmer, K.J.; Sun, X.; et al. CD4 T follicular helper cell dynamics during SIV infection. J. Clin. Investig. 2012, 122, 3281–3294. [Google Scholar] [CrossRef]
- Chowdhury, A.; Del Rio Estrada, P.M.; Tharp, G.K.; Trible, R.P.; Amara, R.R.; Chahroudi, A.; Reyes-Teran, G.; Bosinger, S.E.; Silvestri, G. Decreased T Follicular Regulatory Cell/T Follicular Helper Cell (Tfh) in Simian Immunodeficiency Virus-Infected Rhesus Macaques May Contribute to Accumulation of Tfh in Chronic Infection. J. Immunol. 2015, 195, 3237–3247. [Google Scholar] [CrossRef]
- Blackburn, M.J.; Zhong-Min, M.; Caccuri, F.; McKinnon, K.; Schifanella, L.; Guan, Y.; Gorini, G.; Venzon, D.; Fenizia, C.; Binello, N.; et al. Regulatory and Helper Follicular T Cells and Antibody Avidity to Simian Immunodeficiency Virus Glycoprotein 120. J. Immunol. 2015, 195, 3227–3236. [Google Scholar] [CrossRef]
- Victora, G.D.; Nussenzweig, M.C. Germinal centers. Annu. Rev. Immunol. 2012, 30, 429–457. [Google Scholar] [CrossRef]
- Preite, S.; Cannons, J.L.; Radtke, A.J.; Vujkovic-Cvijin, I.; Gomez-Rodriguez, J.; Volpi, S.; Huang, B.; Cheng, J.; Collins, N.; Reilley, J.; et al. Hyperactivated PI3Kδ promotes self and commensal reactivity at the expense of optimal humoral immunity. Nat. Immunol. 2018, 19, 986–1000. [Google Scholar] [CrossRef]
- Riteau, N.; Radtke, A.J.; Shenderov, K.; Mittereder, L.; Oland, S.D.; Hieny, S.; Jankovic, D.; Sher, A. Water-in-Oil-Only Adjuvants Selectively Promote T Follicular Helper Cell Polarization through a Type I IFN and IL-6-Dependent Pathway. J. Immunol. 2016, 197, 3884–3893. [Google Scholar] [CrossRef] [PubMed]
- Obst, R.; van Santen, H.M.; Mathis, D.; Benoist, C. Antigen persistence is required throughout the expansion phase of a CD4+ T-cell response. J. Exp. Med. 2005, 201, 1555–1565. [Google Scholar] [CrossRef]
- Espeseth, A.S.; Cejas, P.J.; Citron, M.P.; Wang, D.; DiStefano, D.J.; Callahan, C.; Donnell, G.O.; Galli, J.D.; Swoyer, R.; Touch, S.; et al. Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection. NPJ Vaccines 2020, 5, 16. [Google Scholar] [CrossRef]
- Eggink, D.; Goff, P.H.; Palese, P. Guiding the immune response against influenza virus hemagglutinin toward the conserved stalk domain by hyperglycosylation of the globular head domain. J. Virol. 2014, 88, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Impagliazzo, A.; Milder, F.; Kuipers, H.; Wang, D.; DiStefano, D.J.; Callahan, C.; Donnell, G.O.; Galli, J.D.; Swoyer, R.; Touch, S.; et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 2015, 349, 1301–1306. [Google Scholar] [CrossRef]
- Krammer, F. The Quest for a Universal Flu Vaccine: Headless HA 2.0. Cell Host Microbe 2015, 18, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Yassine, H.M.; Boyington, J.C.; McTamney, P.M.; Wei, C.J.; Kanekiyo, M.; Kong, W.P.; Gallagher, J.R.; Wang, L.; Zhang, Y.; Joyce, M.G.; et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015, 21, 1065–1070. [Google Scholar] [CrossRef]
- Bonsignori, M.; Scott, E.; Wiehe, K.; Easterhoff, D.; Alam, S.M.; Hwang, K.K.; Cooper, M.; Xia, S.M.; Zhang, R.; Montefiori, D.C.; et al. Inference of the HIV-1 VRC01 Antibody Lineage Unmutated Common Ancestor Reveals Alternative Pathways to Overcome a Key Glycan Barrier. Immunity 2018, 49, 1162–1174. [Google Scholar] [CrossRef]
- Umotoy, J.; Bagaya, B.S.; Joyce, C.; Schiffner, T.; Menis, S.; Saye-Francisco, K.L.; Biddle, T.; Mohan, S.; Vollbrecht, T.; Kalyuzhniy, O.; et al. Rapid and Focused Maturation of a VRC01-Class HIV Broadly Neutralizing Antibody Lineage Involves Both Binding and Accommodation of the N276-Glycan. Immunity 2019, 51, 141–154. [Google Scholar] [CrossRef]
- Havenar-Daughton, C.; Carnathan, D.G.; Boopathy, A.V.; Upadhyay, A.A.; Murrell, B.; Reiss, S.M.; Enemuo, C.A.; Gebru, E.H.; Choe, Y.; Dhadvai, P.; et al. Rapid Germinal Center and Antibody Responses in Non-human Primates after a Single Nanoparticle Vaccine Immunization. Cell Rep. 2019, 29, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- IAVI. First-in-Human Clinical Trial Confirms Novel HIV Vaccine Approach Developed by IAVI and Scripps Research. 2021. Available online: https://www.iavi.org/news-resources/press-releases/2021/first-in-human-clinical-trial-confirms-novel-hiv-vaccine-approach-developed-by-iavi-and-scripps-research (accessed on 5 June 2021).
- Gaya, M.; Barral, P.; Burbage, M.; Aggarwal, S.; Montaner, B.; Warren Navia, A.; Aid, M.; Tsui, C.; Maldonado, P.; Nair, U.; et al. Initiation of Antiviral B-Cell Immunity Relies on Innate Signals from Spatially Positioned NKT Cells. Cell 2018, 172, 517–533.e20. [Google Scholar] [CrossRef] [PubMed]
- Tam, H.H.; Melo, M.B.; Kang, M.; Pelet, J.M.; Ruda, V.M.; Foley, M.H.; Hu, J.K.; Kumari, S.; Crampton, J.; Baldeon, A.D.; et al. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc. Natl. Acad. Sci. USA 2016, 113, E6639–E6648. [Google Scholar] [CrossRef] [PubMed]
- Pauthner, M.; Havenar-Daughton, C.; Sok, D.; Nkolola, J.P.; Bastidas, R.; Boopathy, A.V.; Carnathan, D.G.; Chandrashekar, A.; Cirelli, K.M.; Cottrell, C.A.; et al. Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches. Immunity 2017, 46, 1073–1088.e6. [Google Scholar] [CrossRef]
- Hu, J.K.; Crampton, J.C.; Cupo, A.; Ketas, T.; van Gils, M.J.; Sliepen, K.; de Taeye, S.W.; Sok, D.; Ozorowski, G.; Deresa, I.; et al. Murine Antibody Responses to Cleaved Soluble HIV-1 Envelope Trimers Are Highly Restricted in Specificity. J. Virol. 2015, 89, 10383–10398. [Google Scholar] [CrossRef] [PubMed]
- Szmuness, W.; Stevens, C.E.; Harley, E.J.; Zang, E.A.; Oleszko, W.R.; William, D.C.; Sadovsky, R.; Morrison, J.M.; Kellner, A. Hepatitis B vaccine: Demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N. Engl. J. Med. 1980, 303, 833–841. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef]
- Kuter, B.J.; Garland, S.M.; Giuliano, A.R.; Stanley, M.A. Current and future vaccine clinical research with the licenced 2-, 4-, and 9-valent VLP HPV vaccines: What’s ongoing, what’s needed? Prev. Med. 2021, 144, 106321. [Google Scholar] [CrossRef]
- Kim, J.; Vasan, S.; Kim, J.H.; Ake, J.A. Current approaches to HIV vaccine development: A narrative review. J. Int. AIDS Soc. 2021, 24 (Suppl. S7), e25793. [Google Scholar] [CrossRef]
- Shi, J.; Wang, G.; Zheng, J.; Verma, A.K.; Guan, X.; Malisheni, M.M.; Geng, Q.; Li, F.; Perlman, S.; Du, L. Effective vaccination strategy using SARS-CoV-2 spike cocktail against Omicron and other variants of concern. NPJ Vaccines 2022, 7, 169. [Google Scholar] [CrossRef]
- Kato, Y.; Abbott, R.K.; Freeman, B.L.; Haupt, S.; Groschel, B.; Silva, M.; Menis, S.; Irvine, D.J.; Schief, W.R.; Crotty, S. Multifaceted Effects of Antigen Valency on B-Cell Response Composition and Differentiation In Vivo. Immunity 2020, 53, 548–563. [Google Scholar] [CrossRef]
- Shinnakasu, R.; Inoue, T.; Kometani, K.; Moriyama, S.; Adachi, Y.; Nakayama, M.; Takahashi, Y.; Fukuyama, H.; Okada, T.; Kurosaki, T. Regulated selection of germinal-center cells into the memory B cell compartment. Nat. Immunol. 2016, 17, 861–869. [Google Scholar] [CrossRef]
- Weisel, F.J.; Zuccarino-Catania, G.V.; Chikina, M.; Shlomchik, M.J. A Temporal Switch in the Germinal Center Determines Differential Output of Memory B and Plasma Cells. Immunity 2016, 44, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; Palm, A.E.; Krammer, F.; Wilson, P.C. From original antigenic sin to the universal influenza virus vaccine. Trends Immunol. 2018, 39, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, C.P.; Le Sage, V.; Bolton, M.J.; Eilola, T.; Jones, J.E.; Kormuth, K.A.; Nturibi, E.; Balmaseda, A.; Gordon, A.; Lakdawala, S.S.; et al. Original antigenic sin priming of influenza virus hemagglutinin stalk antibodies. Proc. Natl. Acad. Sci. USA 2020, 117, 17221–17227. [Google Scholar] [CrossRef]
- Huang, K.Y.; Rijal, P.; Schimanski, L.; Powell, T.J.; Lin, T.Y.; McCauley, J.W.; Daniels, R.S.; Townsend, A.R. Focused antibody response to influenza linked to antigenic drift. J. Clin. Investig. 2015, 125, 2631–2645. [Google Scholar] [CrossRef] [PubMed]
- Francis, T. On the doctrine of original antigenic sin. Proc. Am. Philos. Soc. 1960, 104, 572–578. [Google Scholar]
- Angeletti, D.; Yewdell, J.W. Understanding and manipulating viral immunity: Antibody immunodominance enters center stage. Trends Immunol. 2018, 39, 549–561. [Google Scholar] [CrossRef]
- Andrews, S.F.; Huang, Y.; Kaur, K.; Popova, L.I.; Ho, I.Y.; Pauli, N.T.; Henry Dunand, C.J.; Taylor, W.M.; Lim, S.; Huang, M.; et al. Immune history profoundly affects broadly protective B cell responses to influenza. Sci. Transl. Med. 2015, 7, 316ra192. [Google Scholar] [CrossRef]
- Gray, L.T.; Raczy, M.M.; Briquez, P.S.; Marchell, T.M.; Alpar, A.T.; Wallace, R.P.; Volpatti, L.R.; Sasso, M.S.; Cao, S.; Nguyen, M.; et al. Generation of potent cellular and humoral immunity against SARS-CoV-2 antigens via conjugation to a polymeric glyco-adjuvant. Biomaterials 2021, 278, 121159. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Hou, L.; Yu, X.; Cheng, H.; Chen, J.; Zheng, Q.; Hou, J. Pattern-Recognition Receptor Agonist-Containing Immunopotentiator CVC1302 Boosts High-Affinity Long-Lasting Humoral Immunity. Front. Immunol. 2021, 12, 697292. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; van der Most, R.; Lodaya, R.N.; Coccia, M.; Lofano, G. “World in motion”—Emulsion adjuvants rising to meet the pandemic challenges. NPJ Vaccines 2021, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, B.; Caserto, J.S.; Shariati, K.; Cao, P.; Pan, Y.; Xu, Q.; Ma, M. Sustained Delivery of SARS-CoV-2 RBD Subunit Vaccine Using a High Affinity Injectable Hydrogel Scaffold. Adv. Healthc. Mater. 2021, 11, e2101714. [Google Scholar] [CrossRef] [PubMed]

| Trial | Start | End | Vaccine | Location | Efficacy | References |
|---|---|---|---|---|---|---|
| VAX003 (NCT00006327) | 1999 | 2000 | Bivalent CRF_01AE/B gp120 in alum | Thailand | No efficacy | [16,17] |
| VAX004 (NCT00002441) | 1999 | 2000 | Bivalent clade B gp120 in alum | United States, Europe | No efficacy | [18,19] |
| RV144 (NCT00223080) | 2005 | 2009 | ALVAC with gag/pro/Env; bivalent CRF_01AE/B gp120 in alum | Thailand | Estimated 60% vaccine efficacy at 12 months; 42-month efficacy, 31.2% | [20,21,22] |
| HVTN 505 (NCT00865566) | 2009 | 2017 | DNAs with clade B gag/pol/nef and DNAs with clade A, B, C Envs; adenovirus type 5 with gag/pol and clade A, B, C Envs | United States | No efficacy | [23,24] |
| HVTN 702 Uhambo (NCT02968849) | 2016 | 2021 | ALVAC-C with gag/pol/Env; bivalent gp120s in MF59 | South Africa | No efficacy | [25] |
| HVTN 705 Imbokodo (NCT03060629) | 2017 | 2021 | Ad26, 4 valent T cell mosaic genes, boost with clade C gp140 Env | Sub-Saharan Africa | No efficacy | [26] |
| HVTN 706 Mosaico (NCT03964415) | 2019 | On going (est. 2024) | Ad26, 4 valent T cell mosaic genes, boost with clade C gp140 Env+B cell mosaic gp140 Env | United States, Spain, Central/South America | Ongoing | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhou, Z. The Mechanism of bnAb Production and Its Application in Mutable Virus Broad-Spectrum Vaccines: Inspiration from HIV-1 Broad Neutralization Research. Vaccines 2023, 11, 1143. https://doi.org/10.3390/vaccines11071143
Zhang X, Zhou Z. The Mechanism of bnAb Production and Its Application in Mutable Virus Broad-Spectrum Vaccines: Inspiration from HIV-1 Broad Neutralization Research. Vaccines. 2023; 11(7):1143. https://doi.org/10.3390/vaccines11071143
Chicago/Turabian StyleZhang, Xinyu, and Zehua Zhou. 2023. "The Mechanism of bnAb Production and Its Application in Mutable Virus Broad-Spectrum Vaccines: Inspiration from HIV-1 Broad Neutralization Research" Vaccines 11, no. 7: 1143. https://doi.org/10.3390/vaccines11071143
APA StyleZhang, X., & Zhou, Z. (2023). The Mechanism of bnAb Production and Its Application in Mutable Virus Broad-Spectrum Vaccines: Inspiration from HIV-1 Broad Neutralization Research. Vaccines, 11(7), 1143. https://doi.org/10.3390/vaccines11071143






