The Humoral Response to SARS-CoV-2 Vaccine in Hemodialysis Patients Is Correlated with Nutritional Status
Abstract
1. Introduction
2. Methods
2.1. Study Population and Design
2.2. Nutritional Status Assessments
2.3. Antibody Response Assessment
2.4. Statistical Analyses
3. Results
3.1. Patients’ Characteristics
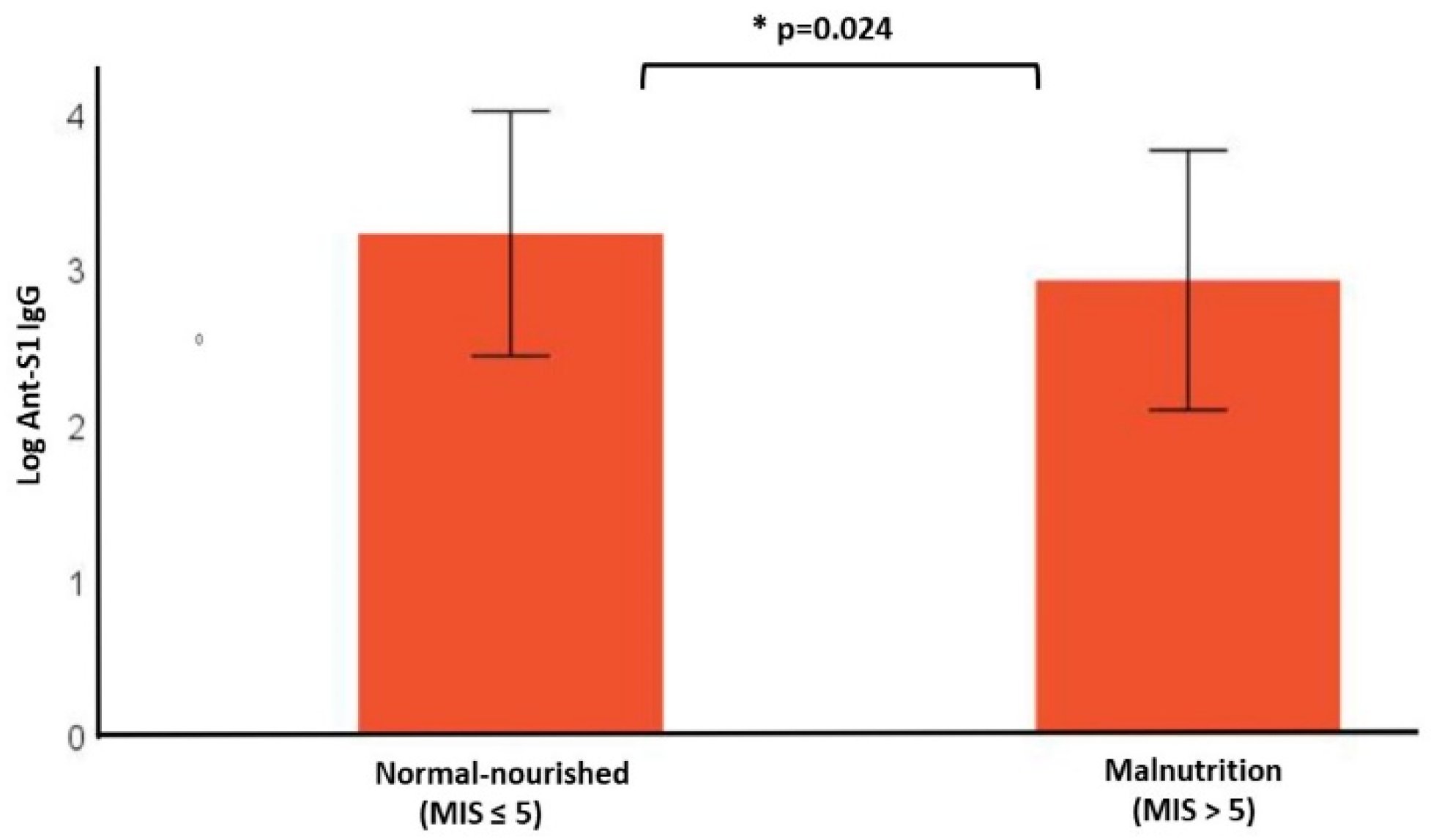
3.2. Anti-S1 Antibody Response
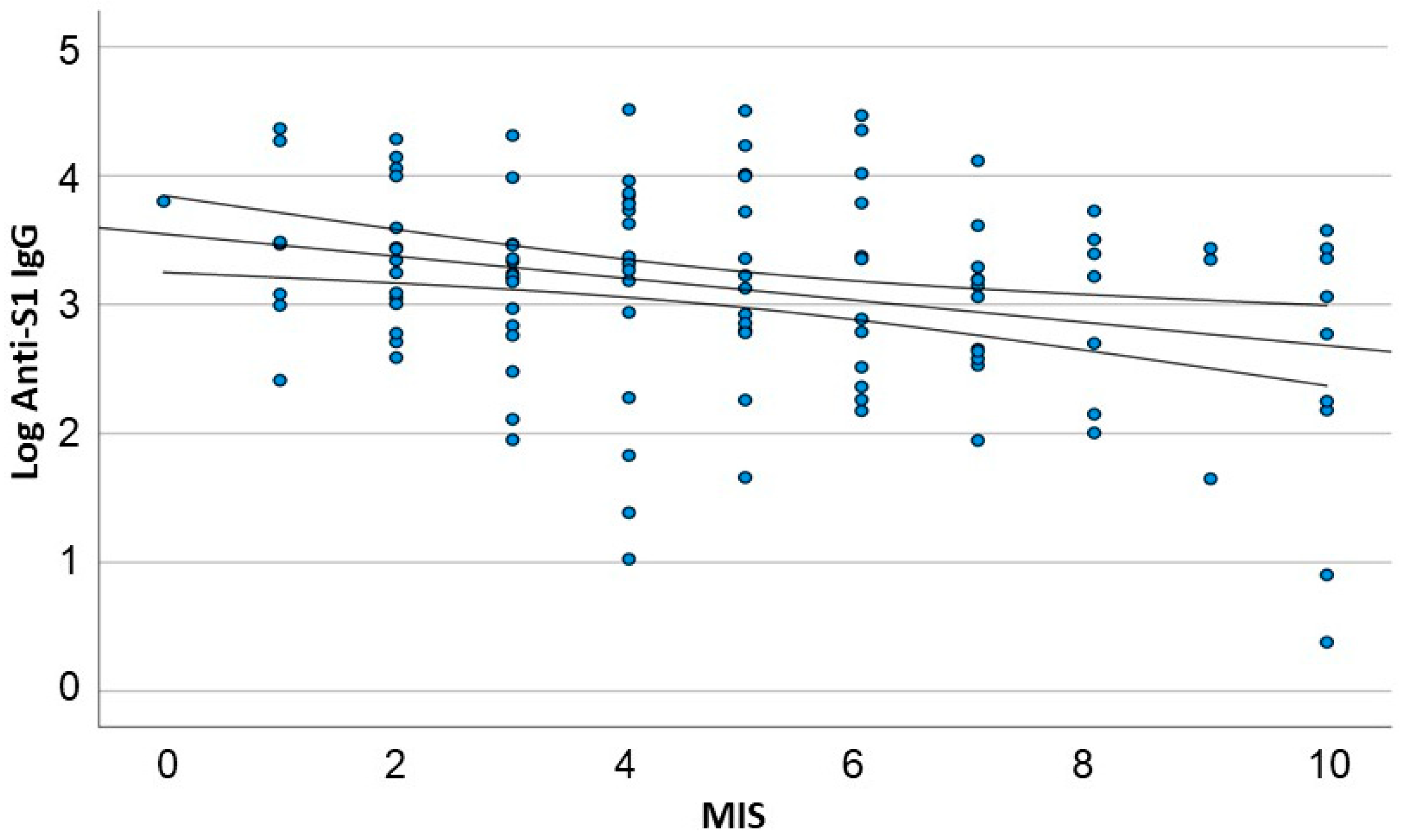
3.3. A Linear Correlation between Antibody Response and Nutritional Status
3.4. A Multivariate Analysis of Predictors of S1-IgG Antibody Levels in Response to BNT162b
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Windpessl, M.; Bruchfeld, A.; Anders, H.-J.; Kramer, H.; Waldman, M.; Renia, L.; Ng, L.F.P.; Xing, Z.; Kronbichler, A. COVID-19 vaccines and kidney disease. Nat. Rev. Nephrol. 2021, 17, 291–293. [Google Scholar] [CrossRef]
- Babel, N.; Hugo, C.; Westhoff, T.H. Vaccination in patients with kidney failure: Lessons from COVID-19. Nat. Rev. Nephrol. 2022, 18, 708–723. [Google Scholar] [CrossRef]
- Fabrizi, F.; Dixit, V.; Martin, P.; Jadoul, M.; Messa, P. Meta-Analysis: The Impact of Nutritional Status on the Immune Response to Hepatitis B Virus Vaccine in Chronic Kidney Disease. Dig. Dis. Sci. 2012, 57, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Krueger, K.M.; Ison, M.G.; Ghossein, C. Practical Guide to Vaccination in All Stages of CKD, Including Patients Treated by Dialysis or Kidney Transplantation. Am. J. Kidney Dis. 2020, 75, 417–425. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Betjes, M.G.H. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 2013, 9, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Rovshan, K.; Mortaza, H.; Aziz, E.; Nasibova, A.; Hasanzadeh, A.; Vahedi, P.; Hosain, H. Overview of the Environmental Distribution, Resistance, Mortality, and Genetic Diversity of New Coronavirus (COVID-19): Review. Adv. Biol. Earth Sci. 2020, 5, 7–12. [Google Scholar]
- Rozen-Zvi, B.; Yahav, D.; Agur, T.; Zingerman, B.; Ben-Zvi, H.; Atamna, A.; Tau, N.; Mashraki, T.; Nesher, E.; Rahamimov, R. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: A prospective cohort study. Clin. Microbiol. Infect. 2021, 27, 1173.e1–1173.e4. [Google Scholar] [CrossRef]
- Danthu, C.; Hantz, S.; Dahlem, A.; Duval, M.; Ba, B.; Guibbert, M.; El Ouafi, Z.; Ponsard, S.; Berrahal, I.; Achard, J.-M.; et al. Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 32, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Arevalo, H.; Choi, M.; Stefanski, A.-L.; Halleck, F.; Weber, U.; Szelinski, F.; Jahrsdörfer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol. 2021, 6, eabj1031. [Google Scholar] [CrossRef]
- Polvere, J.; Fabbiani, M.; Pastore, G.; Rancan, I.; Rossetti, B.; Durante, M.; Zirpoli, S.; Morelli, E.; Pettini, E.; Lucchesi, S.; et al. B cell response after SARS-CoV-2 mRNA vaccination in people living with HIV. Commun. Med. 2023, 3, 13. [Google Scholar] [CrossRef]
- Campagna, R.; Mazzuti, L.; Guerrizio, G.; Nonne, C.; Migliara, G.; De Vito, C.; Mezzaroma, I.; Chiaretti, S.; Fimiani, C.; Pistolesi, V.; et al. Humoral and T-cell mediated response after administration of mRNA vaccine BNT162b2 in frail populations. Vaccine X 2022, 12, 100246. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, F.; Sicuranza, A.; Ciabattini, A.; Santoni, A.; Pastore, G.; Simoncelli, M.; Polvere, J.; Galimberti, S.; Auddino, S.; Baratè, C.; et al. The Slower Antibody Response in Myelofibrosis Patients after Two Doses of mRNA SARS-CoV-2 Vaccine Calls for a Third Dose. Biomedicines 2021, 9, 1480. [Google Scholar] [CrossRef]
- Chen, J.-J.; Lee, T.H.; Tian, Y.-C.; Lee, C.-C.; Fan, P.-C.; Chang, C.-H. Immunogenicity Rates After SARS-CoV-2 Vaccination in People with End-stage Kidney Disease: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2131749. [Google Scholar] [CrossRef]
- Espi, M.; Charmetant, X.; Barba, T.; Mathieu, C.; Pelletier, C.; Koppe, L.; Chalencon, E.; Kalbacher, E.; Mathias, V.; Ovize, A.; et al. A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney Int. 2021, 101, 390–402. [Google Scholar] [CrossRef]
- Giot, M.; Fourié, T.; Lano, G.; Villarroel, P.M.S.; de Lamballeri, X.; Gully, M.; Samson, L.; Farault, J.; Bouchouareb, D.; Jehel, O.; et al. Spike and neutralizing antibodies response to COVID-19 vaccination in hemodialysis patients. Clin. Kidney J. 2021, 14, 2239–2245. [Google Scholar] [CrossRef]
- Agur, T.; Ben-Dor, N.; Herman-Edelstein, M.; Steinmetz, T.; Lichtenberg, S.; Schneider, S.; Yahav, D.; Rozen-Zvi, B.; Zingerman, B. Longevity of Humoral Response Six Months Following BNT162b2 Vaccine in Dialysis Patients. Front. Med. 2022, 9, 781888. [Google Scholar] [CrossRef] [PubMed]
- Herman-Edelstein, M.; Ben-Dor, N.; Agur, T.; Guetta, T.; Raiter, A.; Meisel, E.; Alkeesh, W.; Ori, Y.; Rozen-Zvi, B.; Zingerman, B. BNT162b2 Booster Vaccination Induced Immunity against SARS-CoV-2 Variants among Hemodialysis Patients. Vaccines 2022, 10, 967. [Google Scholar] [CrossRef]
- Fiorino, F.; Ciabattini, A.; Sicuranza, A.; Pastore, G.; Santoni, A.; Simoncelli, M.; Polvere, J.; Galimberti, S.; Baratè, C.; Sammartano, V.; et al. The third dose of mRNA SARS-CoV-2 vaccines enhances the spike-specific antibody and memory B cell response in myelofibrosis patients. Front. Immunol. 2022, 13, 1017863. [Google Scholar] [CrossRef] [PubMed]
- Agur, T.; Ben-Dor, N.; Goldman, S.; Lichtenberg, S.; Herman-Edelstein, M.; Yahav, D.; Rozen-Zvi, B.; Zingerman, B. Antibody response to mRNA SARS-CoV-2 vaccine among dialysis patients—a prospective cohort study. Nephrol. Dial. Transplant. 2021, 36, 1347–1349. [Google Scholar] [CrossRef] [PubMed]
- Agur, T.; Zingerman, B.; Ben-Dor, N.; Alkeesh, W.; Steinmetz, T.; Rachamimov, R.; Korzets, A.; Rozen-Zvi, B.; Herman-Edelstein, M. Humoral Response to the Third Dose of BNT162b2 COVID-19 Vaccine among Hemodialysis Patients. Nephron 2023, 147, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Broseta, J.J.; Rodríguez-Espinosa, D.; Rodríguez, N.; Mosquera, M.d.M.; Marcos, M.; Egri, N.; Pascal, M.; Soruco, E.; Bedini, J.L.; Bayés, B.; et al. Humoral and Cellular Responses to mRNA-1273 and BNT162b2 SARS-CoV-2 Vaccines Administered to Hemodialysis Patients. Am. J. Kidney Dis. 2021, 78, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Van Praet, J.; Reynders, M.; De Bacquer, D.; Viaene, L.; Schoutteten, M.K.; Caluwé, R.; Doubel, P.; Heylen, L.; De Bel, A.V.; Van Vlem, B.; et al. Predictors and Dynamics of the Humoral and Cellular Immune Response to SARS-CoV-2 mRNA Vaccines in Hemodialysis Patients: A Multicenter Observational Study. J. Am. Soc. Nephrol. 2021, 32, 3208–3220. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults—Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.-J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl. 1), S1–S107. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the Protein-Energy Wasting Syndrome in Chronic Kidney Disease: A Consensus Statement From the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Eyerich, S.; Sarin, T.; Klatt, K.C. Nutrition and the Immune System: A Complicated Tango. Nutrients 2020, 12, 818. [Google Scholar] [CrossRef]
- Garagarza, C.A.; Valente, A.T.; Oliveira, T.S.; Caetano, C.G. Effect of personalized nutritional counseling in maintenance hemodialysis patients. Hemodial. Int. 2015, 19, 412–418. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Cano, N.J.; Budde, K.; Chazot, C.; Kovesdy, C.P.; Mak, R.H.; Mehrotra, R.; Raj, D.S.; Sehgal, A.R.; Stenvinkel, P.; et al. Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat. Rev. Nephrol. 2011, 7, 369–384. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Ikizler, T.A.; Block, G.; Avram, M.M.; Kopple, J.D. Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am. J. Kidney Dis. 2003, 42, 864–881. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A Malnutrition-Inflammation Score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Barril, G.; Nogueira, A.; Cigarrán, S.; La Torre, J.; Sanchez, R.; de Santos, A.; Hadad, F.; Amair, R.; Romaniouk, I.; Truissar, I. Differences in Malnutrition Inflammation Score of Hemodialysis Patients Associated With Hemodialysis Factors. A Spanish Multicenter Epidemiologic Study. J. Ren. Nutr. 2023, 33, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.C.B.; Pimentel, G.D. Is body weight or muscle strength correlated with the Malnutrition Inflammation Score (MIS)? A cross-sectional study in hemodialysis patients. Clin. Nutr. ESPEN 2019, 33, 276–278. [Google Scholar] [CrossRef]
- Qureshi, A.R.; Alvestrand, A.; Danielsson, A.; Divino-Filho, J.C.; Gutierrez, A.; Lindholm, B.; Bergström, J. Factors predicting malnutrition in hemodialysis patients: A cross-sectional study. Kidney Int. 1998, 53, 773–782. [Google Scholar] [CrossRef]
- Ho, L.-C.; Wang, H.-H.; Peng, Y.-S.; Chiang, C.-K.; Huang, J.-W.; Hung, K.-Y.; Hu, F.-C.; Wu, K.-D. Clinical Utility of Malnutrition-Inflammation Score in Maintenance Hemodialysis Patients: Focus on Identifying the Best Cut-Off Point. Am. J. Nephrol. 2008, 28, 840–846. [Google Scholar] [CrossRef]
- Laboratories, A. SARS-CoV-2 IgG II Quant Assay User Manual, Abbott Laboratories, Diagnostics Division. 2020. Available online: https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2 (accessed on 15 May 2023).
- Knezevic, I.; Mattiuzzo, G.; Page, M.; Minor, P.; Griffiths, E.; Nuebling, M.; Moorthy, V. WHO International Standard for evaluation of the antibody response to COVID-19 vaccines: Call for urgent action by the scientific community. Lancet Microbe 2021, 3, e235–e240. [Google Scholar] [CrossRef] [PubMed]
- Boaz, M.; Azoulay, O.; Schwartz, I.F.; Schwartz, D.; Assady, S.; Kristal, B.; Benshitrit, S.; Yanai, N.; Weinstein, T. Malnutrition Risk in Hemodialysis Patients in Israel: Results of the Status of Nutrition in Hemodialysis Patients Survey Study. Nephron 2019, 141, 166–176. [Google Scholar] [CrossRef]
- Carrero, J.J.; Thomas, F.; Nagy, K.; Arogundade, F.; Avesani, C.M.; Chan, M.; Chmielewski, M.; Cordeiro, A.C.; Espinosa-Cuevas, A.; Fiaccadori, E.; et al. Global Prevalence of Protein-Energy Wasting in Kidney Disease: A Meta-analysis of Contemporary Observational Studies from the International Society of Renal Nutrition and Metabolism. J. Ren. Nutr. 2018, 28, 380–392. [Google Scholar] [CrossRef]
- Dramé, M.; Godaert, L. The Obesity Paradox and Mortality in Older Adults: A Systematic Review. Nutrients 2023, 15, 1780. [Google Scholar] [CrossRef]
- Ahmadian, E.; Khatibi, S.M.H.; Soofiyani, S.R.; Abediazar, S.; Shoja, M.M.; Ardalan, M.; Vahed, S.Z. COVID-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev. Med. Virol. 2021, 31, e2176. [Google Scholar] [CrossRef]
- Ikeya, T.; Shibutani, M.; Maeda, K.; Sugano, K.; Nagahara, H.; Ohtani, H.; Hirakawa, K. Maintenance of the nutritional prognostic index predicts survival in patients with unresectable metastatic colorectal cancer. J. Cancer Res. Clin. Oncol. 2015, 141, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Qin, W.; Zheng, Y.; Pang, J.; Zhong, N.; Fei, J.; Li, Y.; Jian, X.; Hou, X.; Hu, Z.; et al. Malnutrition Contributes to Low Lymphocyte Count in Early-Stage Coronavirus Disease-2019. Front. Nutr. 2021, 8, 739216. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-Y.; Hung, N.-K.; Hung, S.-C. Association of malnutrition with SARS-CoV-2 vaccine response in patients undergoing hemodialysis. Clin. Nutr. 2022, 41, 2683–2690. [Google Scholar] [CrossRef]
- Rytter, M.J.H.; Kolte, L.; Briend, A.; Friis, H.; Christensen, V.B. The Immune System in Children with Malnutrition—A Systematic Review. PLoS ONE 2014, 9, e105017. [Google Scholar] [CrossRef] [PubMed]
- Bourke, C.D.; Berkley, J.A.; Prendergast, A.J. Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol. 2016, 37, 386–398. [Google Scholar] [CrossRef]
- Sinha, P.; Davis, J.; Saag, L.; Wanke, C.; Salgame, P.; Mesick, J.; Horsburgh, C.; Hochberg, N.S. Undernutrition and Tuberculosis: Public Health Implications. J. Infect. Dis. 2019, 219, 1356–1363. [Google Scholar] [CrossRef]
- Briguglio, M.; Pregliasco, F.E.; Lombardi, G.; Perazzo, P.; Banfi, G. The Malnutritional Status of the Host as a Virulence Factor for New Coronavirus SARS-CoV-2. Front. Med. 2020, 7, 146. [Google Scholar] [CrossRef]
- Detopoulou, P.; Tsouma, C.M.; Papamikos, V.M. COVID-19 and Nutrition: Summary of Official Recommendations. Top. Clin. Nutr. 2022, 37, 187–202. [Google Scholar] [CrossRef]
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92. [Google Scholar] [CrossRef]
- Giefing-Kröll, C.; Berger, P.; Lepperdinger, G.; Grubeck-Loebenstein, B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 2015, 14, 309–321. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Boroumand, A.B.; Forouhi, M.; Karimi, F.; Moghadam, A.S.; Naeini, L.G.; Kokabian, P.; Naderi, D. Immunogenicity of COVID-19 vaccines in patients with diabetes mellitus: A systematic review. Front. Immunol. 2022, 13, 940357. [Google Scholar] [CrossRef]
- Strengert, M.; Becker, M.; Ramos, G.M.; Dulovic, A.; Gruber, J.; Juengling, J.; Lürken, K.; Beigel, A.; Wrenger, E.; Lonnemann, G.; et al. Cellular and humoral immunogenicity of a SARS-CoV-2 mRNA vaccine in patients on haemodialysis. Ebiomedicine 2021, 70, 103524. [Google Scholar] [CrossRef] [PubMed]
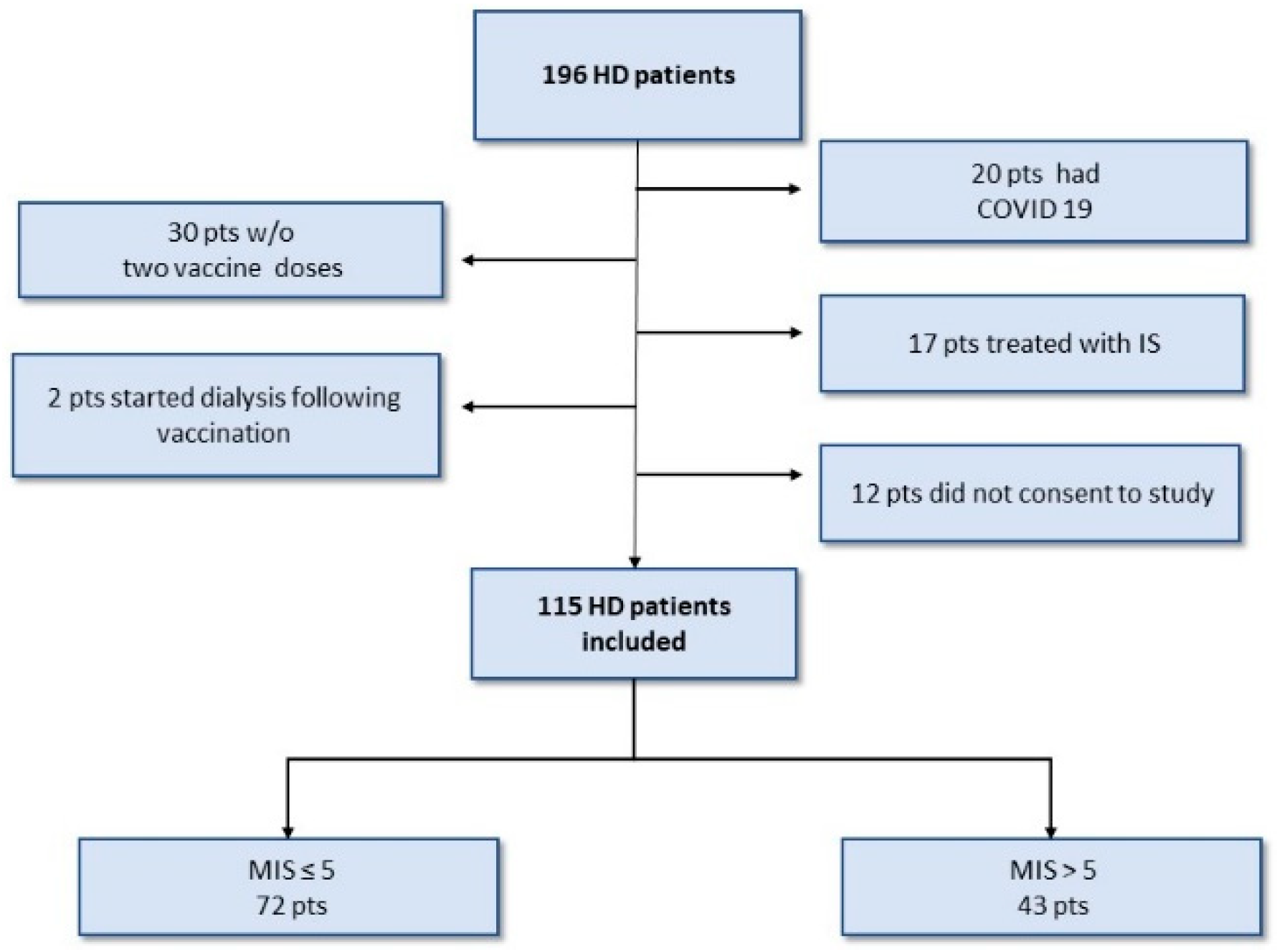
| Variable | All | Normal-Nourished a MIS ≤ 5 | Malnutrition b MIS > 5 | p-Value |
|---|---|---|---|---|
| Number | 115 | 72 (62.6%) | 43 (37.4) | |
| MIS median (IQR) | 5 (0–22) | 3 (0–5) | 7 (6–22) | <0.001 |
| Recipient sex | 0.564 | |||
| Female sex (%) | 39 (33.9) | 23 (31.9) | 16 (37.2) | |
| Male sex (%) | 76 (66.1) | 49 (68.1) | 27 (62.8) | |
| Age (years) mean ± SD | 72.2 ± 12.65 | 69.9 ± 13.25 | 76.05 ± 10.65 | 0.011 |
| Diabetes Mellitus (%) | 67 (58.2) | 41 (56.9) | 26 (60.4) | 0.711 |
| Albumin (g/dL) mean ± SD | 3.94 ± 0.30 | 4.04 ± 0.22 | 3.78 ± 0.34 | <0.001 |
| Hemoglobin (g/dL) mean ± SD | 10.64 ± 1.16 | 10.68 ± 1.22 | 10.58 ± 1.07 | 0.657 |
| Lymphocyte count (K/micl) mean ± SD | 1.28 ± 0.58 | 1.38 ± 0.57 | 1.13 ± 0.57 | 0.029 |
| KT/V mean ± SD | 1.43 ± 0.31 | 1.39 ± 0.30 | 1.48 ± 0.32 | 0.185 |
| nPCR (gr/kg/d) | 1.1 ± 0.26 | 1.11 ± 0.25 | 1.08 ± 0.27 | 0.53 |
| Creatinine (mg/dL) mean ± SD | 6.91 ± 1.95 | 7.15 ± 1.86 | 6.52 ± 2.07 | 0.098 |
| Transferrin (mg/dL) mean ± SD | 39.21 ± 174.27 | 39.71 ± 186.81 | 25.21 ± 150.75 | <0.001 |
| Ferritin (mg/dL) mean ± SD | 699.15 ± 459.96 | 442.66 ± 655.76 | 521.42 ± 781.44 | 0.236 |
| Dry weight (kg) mean ± SD | 72.3 ± 18.4 | 75.12 ± 17.70 | 67.39 ± 19.02 | 0.038 |
| BMI (kg/m2) mean ± SD | 26.63 ± 5.44 | 27.52 ± 5.46 | 25.09 ± 5.10 | 0.021 |
| Dialysis vintage (months) mean ± SD | 33.1 ± 40.89 | 37.85 ± 33.52 | 45.52 ± 32.29 | 0.245 |
| All | Normal-Nourished MIS ≤ 5 | Malnutrition MIS > 5 | p-Value | |
|---|---|---|---|---|
| Patients number (%) | 115 | 72 (62.6%) | 43 (37.3%) | |
| Anti-S1 IgG (AU/mL) (median [IQR]) | 1559.80 (2.4–31,849.3) | 1848 (3.2–31,849.39) | 1151.5 (2.4–29,311.9) | |
| LOG Anti-S1 IgG (mean ± SD) | 3.12 ± 0.78 | 3.25 ± 0.72 | 2.91 ± 0.83 | 0.024 |
| Variable | HD Patients N = 115 | Univariate B (95% CI) | p Value | Multivariate B (95% CI) | p Value |
|---|---|---|---|---|---|
| Age (per year) mean ± SD | 72.2 ± 12.6 | −0.023 | <0.01 | −0.022 (−0.032 to −0.012) | <0.001 |
| Female sex (%) | 39 (33.9) | −0.365 (−0.66 to −0.067) | 0.017 | –0.446 (−0.722 to −0.171) | 0.002 |
| Hemoglobin (g/dL) mean ± SD | 10.64 ± 1.15 | −0.019 (−0.144 to −0.107) | 0.766 | - | |
| Diabetes mellitus | 67 (58.2) | −0.079 (−0.372 to −0.214) | 0.595 | - | |
| KT/V mean ± SD | 1.43 ± 0.30 | 0.208 (−0.271 to −0.688) | 0.391 | 0.578 (0.140 to −1.01) | 0.01 |
| nPCR mean ± SD | 1.1 ± 0.25 | 0.342 (−0.233 to −0.908) | 0.233 | – | – |
| Lymphocyte count (K/micl) mean ± SD | 1.28 ± 0.58 | 0.265 (0.020 to −0.511) | 0.34 | – | – |
| MIS mean ± SD | 4.8 ± 2.56 | −0.087 (−0.141 to −0.032) | 0.02 | –0.066 (−0.117 to −0.015) | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobson-Naftali, M.; Azoulay, O.; Frishman, S.; Godny, L.; Zingerman, B.; Rozen-Zvi, B.; Agur, T. The Humoral Response to SARS-CoV-2 Vaccine in Hemodialysis Patients Is Correlated with Nutritional Status. Vaccines 2023, 11, 1141. https://doi.org/10.3390/vaccines11071141
Jacobson-Naftali M, Azoulay O, Frishman S, Godny L, Zingerman B, Rozen-Zvi B, Agur T. The Humoral Response to SARS-CoV-2 Vaccine in Hemodialysis Patients Is Correlated with Nutritional Status. Vaccines. 2023; 11(7):1141. https://doi.org/10.3390/vaccines11071141
Chicago/Turabian StyleJacobson-Naftali, Merav, Odile Azoulay, Sigal Frishman, Lihi Godny, Boris Zingerman, Benaya Rozen-Zvi, and Timna Agur. 2023. "The Humoral Response to SARS-CoV-2 Vaccine in Hemodialysis Patients Is Correlated with Nutritional Status" Vaccines 11, no. 7: 1141. https://doi.org/10.3390/vaccines11071141
APA StyleJacobson-Naftali, M., Azoulay, O., Frishman, S., Godny, L., Zingerman, B., Rozen-Zvi, B., & Agur, T. (2023). The Humoral Response to SARS-CoV-2 Vaccine in Hemodialysis Patients Is Correlated with Nutritional Status. Vaccines, 11(7), 1141. https://doi.org/10.3390/vaccines11071141






