Advances in Alpha Herpes Viruses Vaccines for Human
Abstract
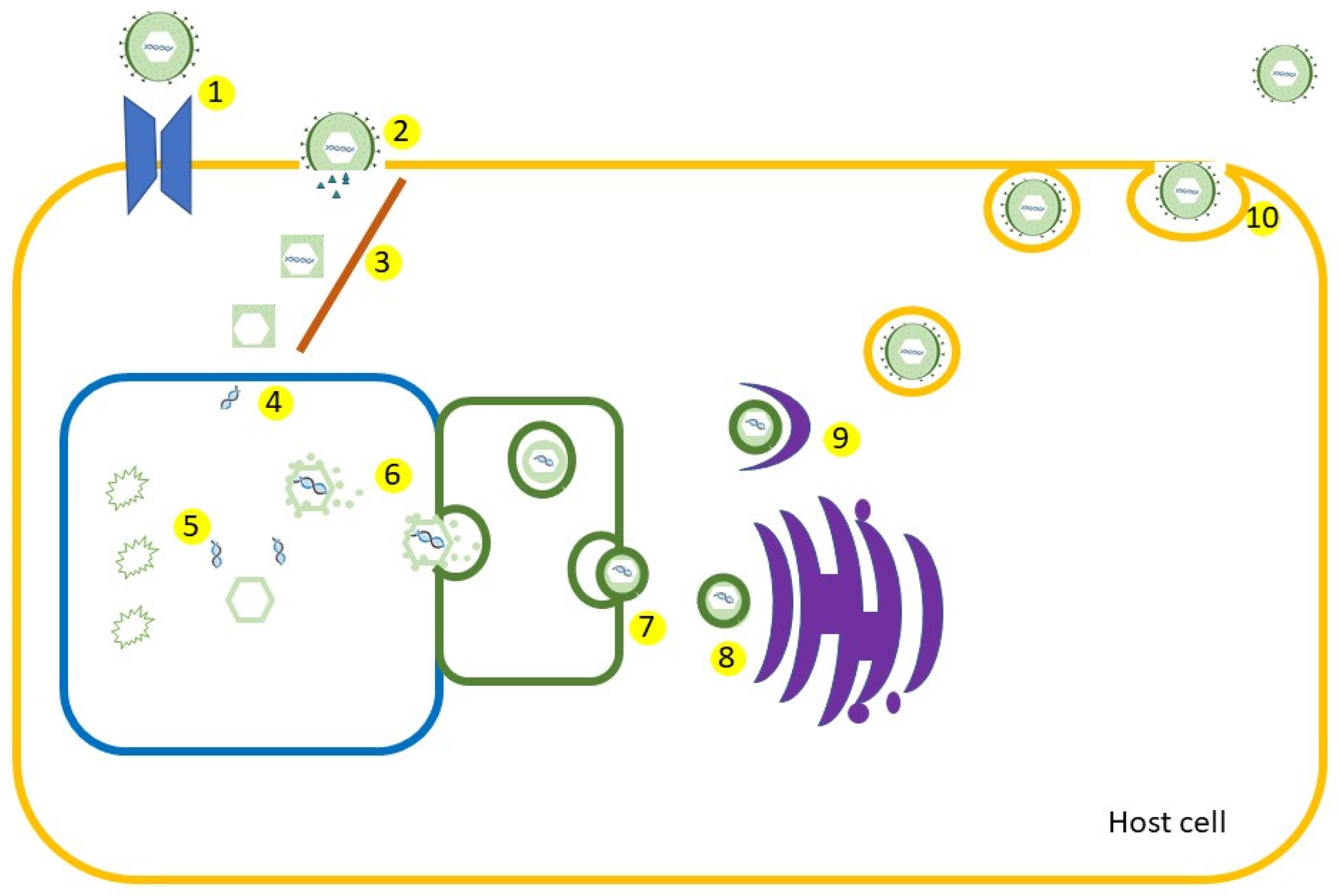
1. Introduction
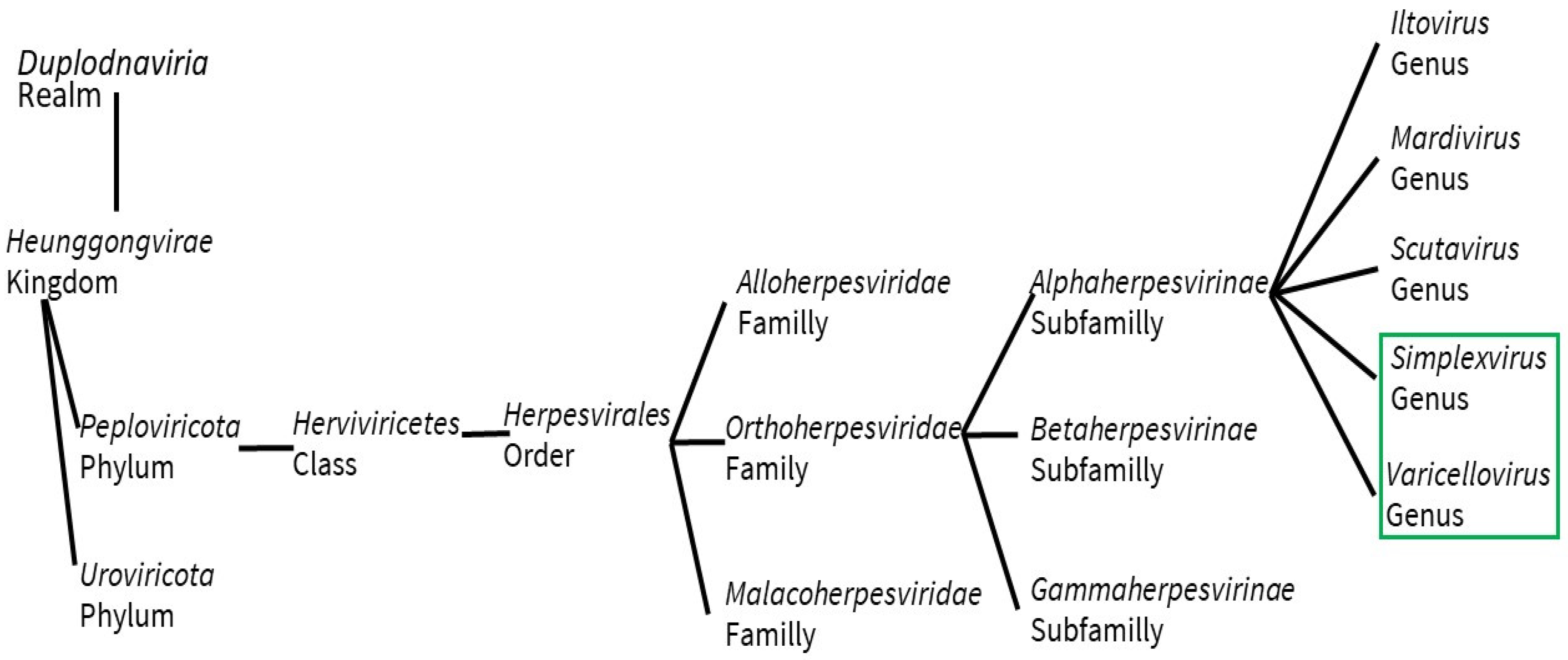
2. Structure, Pathology, and Immune Response
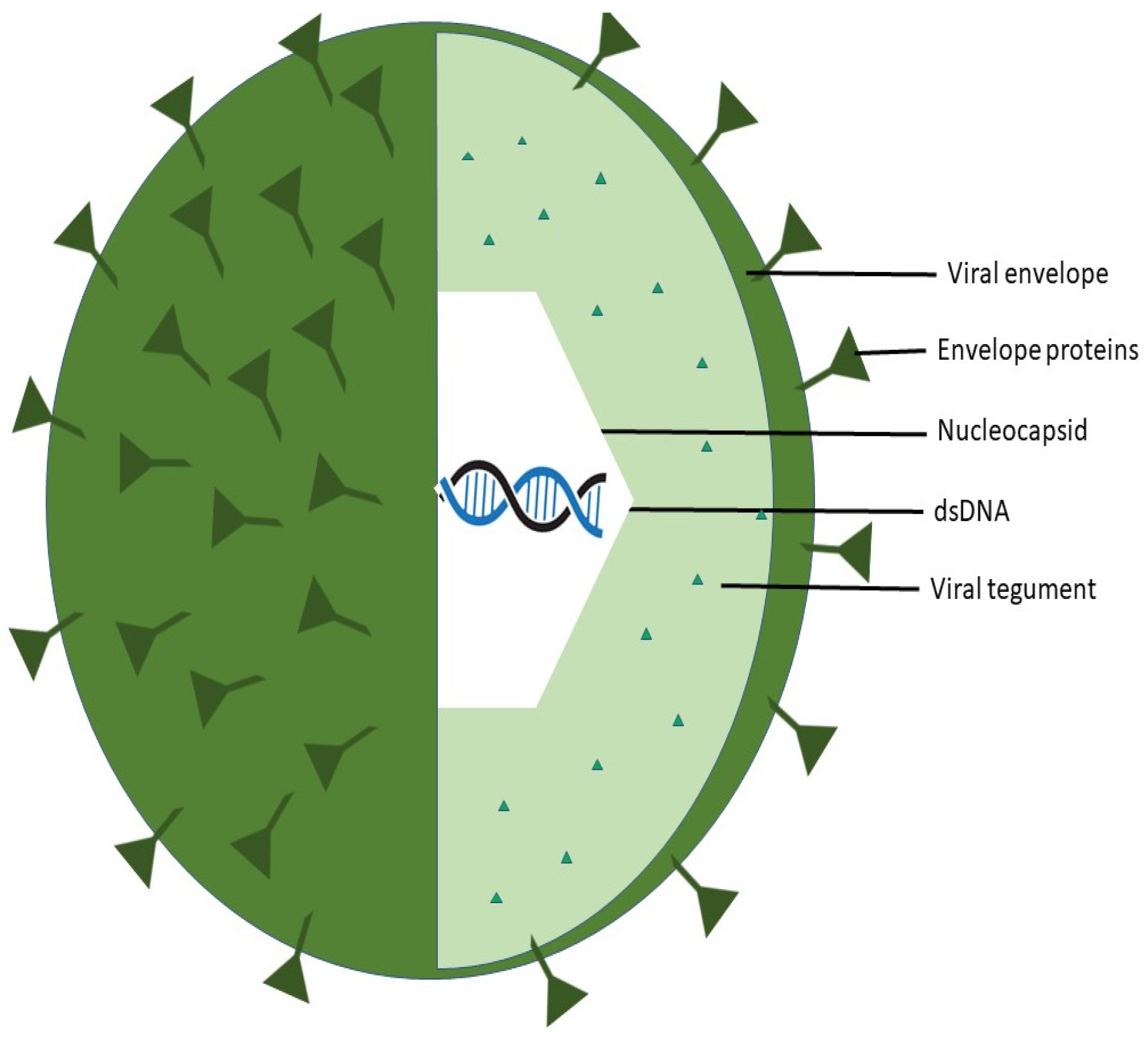
2.1. Structure
2.1.1. Herpes Simplex Viruses (HSV)
2.1.2. Varicella Zoster Virus (VZV)
2.2. Pathology
2.3. Immune Response
3. Vaccines
3.1. Subunit Vaccines
3.2. Live-Attenuated Vaccines
3.3. Naked DNA Vaccines
3.4. Vaccine Vectors
3.5. Replication-Defective Viruses as Vaccines
3.6. Trivalent Subunit Vaccines
3.7. Nucleoside-Modified mRNA Vaccines
3.8. Multivalent DNA Vaccines
3.9. Next-Generation Live-Attenuated Virus Vaccines
3.10. gD2 Deletion Vaccine
3.11. Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visual Taxonomy Browser|ICTV. Available online: https://ictv.global/taxonomy/visual-browser (accessed on 2 June 2023).
- Krishnan, R.; Stuart, P.M. Developments in Vaccination for Herpes Simplex Virus. Front. Microbiol. 2021, 12, 798927. [Google Scholar] [CrossRef] [PubMed]
- Sandgren, K.J.; Truong, N.R.; Smith, J.B.; Bertram, K.; Cunningham, A.L. Vaccines for Herpes Simplex: Recent Progress Driven by Viral and Adjuvant Immunology. In Herpes Simplex Virus: Methods and Protocols; Diefenbach, R.J., Fraefel, C., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2020; pp. 31–56. ISBN 978-1-4939-9814-2. [Google Scholar]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes Simplex Virus: Global Infection Prevalence and Incidence Estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, M.; Kong, M.; Tronstein, E.; Selke, S.; Mikhaylova, A.; Magaret, A.; Huang, M.-L.; Johnston, C.; Corey, L.; Wald, A. Herpes Simplex Virus Type 1 Shedding in Tears, and Nasal and Oral Mucosa of Healthy Adults. Sex. Transm. Dis. 2016, 43, 756–760. [Google Scholar] [CrossRef]
- Eppink, S.T.; Kumar, S.; Miele, K.; Chesson, H.W. Lifetime Medical Costs of Genital Herpes in the United States: Estimates From Insurance Claims. Sex. Transm. Dis. 2021, 48, 266–272. [Google Scholar] [CrossRef]
- CDC Chickenpox for HCPs. Available online: https://www.cdc.gov/chickenpox/hcp/index.html (accessed on 17 January 2023).
- Ayoade, F.; Kumar, S. Varicella Zoster (Chickenpox). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kinchington, P.R.; Leger, A.J.S.; Guedon, J.-M.G.; Hendricks, R.L. Herpes Simplex Virus and Varicella Zoster Virus, the House Guests Who Never Leave. Herpesviridae 2012, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, V.N.; Farouk, I.A.; Zabidi, N.Z.; Puniyamurti, A.; Choo, W.S.; Lal, S.K. Current Vaccine Approaches and Emerging Strategies against Herpes Simplex Virus (HSV). Expert. Rev. Vaccines 2021, 20, 1077–1096. [Google Scholar] [CrossRef]
- Schramlová, J.; Arientová, S.; Hulínská, D. The Role of Electron Microscopy in the Rapid Diagnosis of Viral Infections—Review. Folia Microbiol. 2010, 55, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Kornfeind, E.M.; Visalli, R.J. Human Herpesvirus Portal Proteins: Structure, Function, and Antiviral Prospects. Rev. Med. Virol. 2018, 28, e1972. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I. The Varicella-Zoster Virus Genome. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2010; Volume 342, pp. 1–14. [Google Scholar] [CrossRef]
- Whitley, R.J. Herpesviruses. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 978-0-9631172-1-2. [Google Scholar]
- McElwee, M.; Vijayakrishnan, S.; Rixon, F.; Bhella, D. Structure of the Herpes Simplex Virus Portal-Vertex. PLoS Biol. 2018, 16, e2006191. [Google Scholar] [CrossRef]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and Virulence of Herpes Simplex Virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef]
- Galli, J.D.; Horton, M.; Durr, E.; Heidecker, G.J.; Freed, D.; Fridman, A.; Wang, D.; Zhang, L. Evaluation of HSV-2 GE Binding to IgG-Fc and Application for Vaccine Development. Vaccines 2022, 10, 184. [Google Scholar] [CrossRef]
- Stelitano, D.; Franci, G.; Chianese, A.; Galdiero, S.; Morelli, G.; Galdiero, M. HSV Membrane Glycoproteins, Their Function in Viral Entry and Their Use in Vaccine Studies. In Amino Acids, Peptides and Proteins; Ryadnov, M., Hudecz, F., Eds.; The Royal Society of Chemistry: Cambridge, England, 2019; Volume 43, pp. 14–43. [Google Scholar] [CrossRef]
- Agelidis, A.M.; Shukla, D. Cell Entry Mechanisms of HSV: What We Have Learned in Recent Years. Future Virol. 2015, 10, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Cardone, G.; Heymann, J.B.; Cheng, N.; Trus, B.L.; Steven, A.C. Procapsid Assembly, Maturation, Nuclear Exit: Dynamic Steps in the Production of Infectious Herpesvirions. Adv. Exp. Med. Biol. 2012, 726, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Lussignol, M.; Esclatine, A. Herpesvirus and Autophagy: “All Right, Everybody Be Cool, This Is a Robbery!”. Viruses 2017, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Draganova, E.B.; Valentin, J.; Heldwein, E.E. The Ins and Outs of Herpesviral Capsids: Divergent Structures and Assembly Mechanisms across the Three Subfamilies. Viruses 2021, 13, 1913. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.M.; Avital, N.; Shamay, M.; Kobiler, O. Abortive Herpes Simplex Virus Infection of Nonneuronal Cells Results in Quiescent Viral Genomes That Can Reactivate. Proc. Natl. Acad. Sci. USA 2020, 117, 635–640. [Google Scholar] [CrossRef]
- Sun, J.; Liu, C.; Peng, R.; Zhang, F.-K.; Tong, Z.; Liu, S.; Shi, Y.; Zhao, Z.; Zeng, W.-B.; Gao, G.F.; et al. Cryo-EM Structure of the Varicella-Zoster Virus A-Capsid. Nat. Commun. 2020, 11, 4795. [Google Scholar] [CrossRef]
- Nordén, R.; Nilsson, J.; Samuelsson, E.; Risinger, C.; Sihlbom, C.; Blixt, O.; Larson, G.; Olofsson, S.; Bergström, T. Recombinant Glycoprotein E of Varicella Zoster Virus Contains Glycan-Peptide Motifs That Modulate B Cell Epitopes into Discrete Immunological Signatures. Int. J. Mol. Sci. 2019, 20, 954. [Google Scholar] [CrossRef]
- Abendroth, A.; Kinchington, P.R.; Slobedman, B. Varicella Zoster Virus Immune Evasion Strategies. Curr. Top. Microbiol. Immunol. 2010, 342, 155–171. [Google Scholar] [CrossRef]
- Zerboni, L.; Sen, N.; Oliver, S.L.; Arvin, A.M. Molecular Mechanisms of Varicella Zoster Virus Pathogenesis. Nat. Rev. Microbiol. 2014, 12, 197–210. [Google Scholar] [CrossRef]
- Vleck, S.E.; Oliver, S.L.; Brady, J.J.; Blau, H.M.; Rajamani, J.; Sommer, M.H.; Arvin, A.M. Structure–Function Analysis of Varicella-Zoster Virus Glycoprotein H Identifies Domain-Specific Roles for Fusion and Skin Tropism. Proc. Natl. Acad. Sci. USA 2011, 108, 18412–18417. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, A.S.; Negru, Ș.M.; Preda, M.; Mihăilă, R.I.; Komporaly, I.A.; Dumitrescu, E.A.; Lungulescu, C.V.; Kajanto, L.A.; Georgescu, B.; Radu, E.A.; et al. Biochemical and Metabolical Pathways Associated with Microbiota-Derived Butyrate in Colorectal Cancer and Omega-3 Fatty Acids Implications: A Narrative Review. Nutrients 2022, 14, 1152. [Google Scholar] [CrossRef] [PubMed]
- Erttmann, S.F.; Swacha, P.; Aung, K.M.; Brindefalk, B.; Jiang, H.; Härtlova, A.; Uhlin, B.E.; Wai, S.N.; Gekara, N.O. The Gut Microbiota Prime Systemic Antiviral Immunity via the CGAS-STING-IFN-I Axis. Immunity 2022, 55, 847–861.e10. [Google Scholar] [CrossRef] [PubMed]
- Tognarelli, E.I.; Palomino, T.F.; Corrales, N.; Bueno, S.M.; Kalergis, A.M.; González, P.A. Herpes Simplex Virus Evasion of Early Host Antiviral Responses. Front. Cell. Infect. Microbiol. 2019, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, P.; Manolescu, L.S.C. Association of Hepatitis B Infection in Patients with HIV Encephalopathy. Rom. Biotechnol. Lett. 2012, 17, 7817–7824. [Google Scholar]
- Malik, S.; Sah, R.; Ahsan, O.; Muhammad, K.; Waheed, Y. Insights into the Novel Therapeutics and Vaccines against Herpes Simplex Virus. Vaccines 2023, 11, 325. [Google Scholar] [CrossRef]
- Radu, M.C.; Boeru, C.; Marin, M.; Manolescu, L.S. SARS-CoV-2 Infection in Seven Childbearing Women at the Moment of Delivery, a Romanian Experience. Cureus 2021, 13, e12811. [Google Scholar] [CrossRef]
- Chew, T.; Taylor, K.E.; Mossman, K.L. Innate and Adaptive Immune Responses to Herpes Simplex Virus. Viruses 2009, 1, 979–1002. [Google Scholar] [CrossRef]
- Cocoş, R.; Mahler, B.; Turcu-Stiolica, A.; Stoichiță, A.; Ghinet, A.; Shelby, E.-S.; Bohîlțea, L.C. Risk of Death in Comorbidity Subgroups of Hospitalized COVID-19 Patients Inferred by Routine Laboratory Markers of Systemic Inflammation on Admission: A Retrospective Study. Viruses 2022, 14, 1201. [Google Scholar] [CrossRef]
- Madavaraju, K.; Koganti, R.; Volety, I.; Yadavalli, T.; Shukla, D. Herpes Simplex Virus Cell Entry Mechanisms: An Update. Front. Cell. Infect. Microbiol. 2021, 10, 617578. [Google Scholar] [CrossRef]
- Ma, F.; Lf, D.; Ei, T.; Pa, G. Herpes Simplex Virus Interference with Immunity: Focus on Dendritic Cells. Virulence 2021, 12, 2583–2607. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Jones, E.A.; Orzalli, M.H.; Knipe, D.M. Innate Immune Mechanisms and Herpes Simplex Virus Infection and Disease. Adv. Anat. Embryol. Cell Biol. 2017, 223, 49–75. [Google Scholar] [CrossRef] [PubMed]
- Hare, D.N.; Collins, S.E.; Mukherjee, S.; Loo, Y.-M.; Gale, M.; Janssen, L.J.; Mossman, K.L. Membrane Perturbation-Associated Ca2+ Signaling and Incoming Genome Sensing Are Required for the Host Response to Low-Level Enveloped Virus Particle Entry. J. Virol. 2016, 90, 3018–3027. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Wei, B. Immune Response of T Cells during Herpes Simplex Virus Type 1 (HSV-1) Infection. J. Zhejiang Univ. Sci. B 2017, 18, 277–288. [Google Scholar] [CrossRef]
- Cairns, T.M.; Huang, Z.-Y.; Whitbeck, J.C.; Ponce de Leon, M.; Lou, H.; Wald, A.; Krummenacher, C.; Eisenberg, R.J.; Cohen, G.H. Dissection of the Antibody Response against Herpes Simplex Virus Glycoproteins in Naturally Infected Humans. J. Virol. 2014, 88, 12612–12622. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.M.; Sen, G.C. Innate Immune Responses to Herpesvirus Infection. Cells 2021, 10, 2122. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, K.; Li, J.; Zheng, C. Herpes Simplex Virus 1 Ubiquitin-Specific Protease UL36 Inhibits Beta Interferon Production by Deubiquitinating TRAF3. J. Virol. 2013, 87, 11851–11860. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Su, C.; Zheng, C. Herpes Simplex Virus 1 Tegument Protein UL41 Counteracts IFIT3 Antiviral Innate Immunity. J. Virol. 2016, 90, 11056–11061. [Google Scholar] [CrossRef]
- Truong, N.R.; Smith, J.B.; Sandgren, K.J.; Cunningham, A.L. Mechanisms of Immune Control of Mucosal HSV Infection: A Guide to Rational Vaccine Design. Front. Immunol. 2019, 10, 373. [Google Scholar] [CrossRef]
- Manolescu, L.S.C.; Zugravu, C.; Zaharia, C.N.; Dumitrescu, A.I.; Prasacu, I.; Radu, M.C.; Letiția, G.D.; Nita, I.; Cristache, C.M.; Gales, L.N. Barriers and Facilitators of Romanian HPV (Human Papillomavirus) Vaccination. Vaccines 2022, 10, 1722. [Google Scholar] [CrossRef]
- Manolescu, L.S.C.; Zaharia, C.N.; Dumitrescu, A.I.; Prasacu, I.; Radu, M.C.; Boeru, A.C.; Boidache, L.; Nita, I.; Necsulescu, A.; Medar, C.; et al. COVID-19 Parental Vaccine Hesitancy in Romania: Nationwide Cross-Sectional Study. Vaccines 2022, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Manolescu, L.; Temereanca, A.; Diaconu, C.C.; Ruta, S. Correlation between Resistance Profile and Immunosuppression in Heavily Treated HIV-1 Infected Romanian Patients. Rom. Biotechnol. Lett. 2011, 16, 6439–6449. [Google Scholar] [PubMed]
- Preda, M.; Manolescu, L.C.S. Romania, a Harbour of HIV-1 Subtype F1: Where Are We after 33 Years of HIV-1 Infection? Viruses 2022, 14, 2081. [Google Scholar] [CrossRef] [PubMed]
- Celum, C.; Wald, A.; Lingappa, J.R.; Magaret, A.S.; Wang, R.S.; Mugo, N.; Mujugira, A.; Baeten, J.M.; Mullins, J.I.; Hughes, J.P.; et al. Acyclovir and Transmission of HIV-1 from Persons Infected with HIV-1 and HSV-2. N. Engl. J. Med. 2010, 362, 427–439. [Google Scholar] [CrossRef]
- Schiffer, J.T.; Swan, D.A.; Corey, L.; Wald, A. Rapid Viral Expansion and Short Drug Half-Life Explain the Incomplete Effectiveness of Current Herpes Simplex Virus 2-Directed Antiviral Agents. Antimicrob. Agents Chemother. 2013, 57, 5820–5829. [Google Scholar] [CrossRef]
- Sarkar, B.; Ullah, M.A. Designing Novel Subunit Vaccines against Herpes Simplex Virus-1 Using Reverse Vaccinology Approach. bioRxiv 2020. bioRxiv:2020.01.10.901678. Available online: https://www.biorxiv.org/content/10.1101/2020.01.10.901678v1.full (accessed on 2 June 2023). [CrossRef]
- Wang, X.; Xie, G.; Liao, J.; Yin, D.; Guan, W.; Pan, M.; Li, J.; Li, Y. Design and Evaluation of a Multi-Epitope Assembly Peptide (MEAP) against Herpes Simplex Virus Type 2 Infection in BALB/c Mice. Virol. J. 2011, 8, 232. [Google Scholar] [CrossRef]
- Skoberne, M.; Cardin, R.; Lee, A.; Kazimirova, A.; Zielinski, V.; Garvie, D.; Lundberg, A.; Larson, S.; Bravo, F.J.; Bernstein, D.I.; et al. An Adjuvanted Herpes Simplex Virus 2 Subunit Vaccine Elicits a T Cell Response in Mice and Is an Effective Therapeutic Vaccine in Guinea Pigs. J. Virol. 2013, 87, 3930–3942. [Google Scholar] [CrossRef]
- Burke, R.L. Development of a Herpes Simplex Virus Subunit Glycoprotein Vaccine for Prophylactic and Therapeutic Use [with Discussion]. Rev. Infect. Dis. 1991, 13, S906–S911. [Google Scholar] [CrossRef]
- Bourne, N.; Bravo, F.J.; Francotte, M.; Bernstein, D.I.; Myers, M.G.; Slaoui, M.; Stanberry, L.R. Herpes Simplex Virus (HSV) Type 2 Glycoprotein D Subunit Vaccines and Protection against Genital HSV-1 or HSV-2 Disease in Guinea Pigs. J. Infect. Dis. 2003, 187, 542–549. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Cardin, R.D.; Bravo, F.J.; Hamouda, T.; Pullum, D.A.; Cohen, G.; Bitko, V.; Fattom, A. Intranasal Nanoemulsion-Adjuvanted HSV-2 Subunit Vaccine Is Effective as a Prophylactic and Therapeutic Vaccine Using the Guinea Pig Model of Genital Herpes. Vaccine 2019, 37, 6470–6477. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Srivastava, R.; Vahed, H.; Roy, S.; Walia, S.S.; Kim, G.J.; Fouladi, M.A.; Yamada, T.; Ly, V.T.; Lam, C.; et al. Human Asymptomatic Epitope Peptide/CXCL10-Based Prime/Pull Vaccine Induces Herpes Simplex Virus-Specific Gamma Interferon-Positive CD107+ CD8+ T Cells That Infiltrate the Corneas and Trigeminal Ganglia of Humanized HLA Transgenic Rabbits and Protect against Ocular Herpes Challenge. J. Virol. 2018, 92, e00535-18. [Google Scholar] [CrossRef] [PubMed]
- Odegard, J.M.; Flynn, P.A.; Campbell, D.J.; Robbins, S.H.; Dong, L.; Wang, K.; Ter Meulen, J.; Cohen, J.I.; Koelle, D.M. A Novel HSV-2 Subunit Vaccine Induces GLA-Dependent CD4 and CD8 T Cell Responses and Protective Immunity in Mice and Guinea Pigs. Vaccine 2016, 34, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Wald, A.; Koelle, D.M.; Fife, K.; Warren, T.; Leclair, K.; Chicz, R.M.; Monks, S.; Levey, D.L.; Musselli, C.; Srivastava, P.K. Safety and Immunogenicity of Long HSV-2 Peptides Complexed with RhHsc70 in HSV-2 Seropositive Persons. Vaccine 2011, 29, 8520–8529. [Google Scholar] [CrossRef]
- Hook, L.M.; Awasthi, S.; Dubin, J.; Flechtner, J.; Long, D.; Friedman, H.M. A Trivalent GC2/GD2/GE2 Vaccine for Herpes Simplex Virus Generates Antibody Responses That Block Immune Evasion Domains on GC2 Better than Natural Infection. Vaccine 2019, 37, 664–669. [Google Scholar] [CrossRef]
- Meignier, B.; Longnecker, R.; Roizman, B. In Vivo Behavior of Genetically Engineered Herpes Simplex Viruses R7017 and R7020: Construction and Evaluation in Rodents. J. Infect. Dis. 1988, 158, 602–614. [Google Scholar] [CrossRef]
- Whitley, R.J.; Kern, E.R.; Chatterjee, S.; Chou, J.; Roizman, B. Replication, Establishment of Latency, and Induced Reactivation of Herpes Simplex Virus Gamma 1 34.5 Deletion Mutants in Rodent Models. J. Clin. Invest. 1993, 91, 2837–2843. [Google Scholar] [CrossRef]
- Halford, W.P.; Püschel, R.; Rakowski, B. Herpes Simplex Virus 2 ICP0− Mutant Viruses Are Avirulent and Immunogenic: Implications for a Genital Herpes Vaccine. PLoS ONE 2010, 5, e12251. [Google Scholar] [CrossRef]
- Mohr, I.; Sternberg, D.; Ward, S.; Leib, D.; Mulvey, M.; Gluzman, Y. A Herpes Simplex Virus Type 1 Γ34.5 Second-Site Suppressor Mutant That Exhibits Enhanced Growth in Cultured Glioblastoma Cells Is Severely Attenuated in Animals. J. Virol. 2001, 75, 5189–5196. [Google Scholar] [CrossRef]
- Prichard, M.N.; Kaiwar, R.; Jackman, W.T.; Quenelle, D.C.; Collins, D.J.; Kern, E.R.; Kemble, G.M.; Spaete, R.R. Evaluation of AD472, a Live Attenuated Recombinant Herpes Simplex Virus Type 2 Vaccine in Guinea Pigs. Vaccine 2005, 23, 5424–5431. [Google Scholar] [CrossRef]
- Stanfield, B.A.; Rider, P.J.F.; Caskey, J.; Del Piero, F.; Kousoulas, K.G. Intramuscular Vaccination of Guinea Pigs with the Live-Attenuated Human Herpes Simplex Vaccine VC2 Stimulates a Transcriptional Profile of Vaginal Th17 and Regulatory Tr1 Responses. Vaccine 2018, 36, 2842–2849. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.C.; Tran, R.K.; Feller, J.; Voellmy, R. Immunization by Replication-Competent Controlled Herpesvirus Vectors. J. Virol. 2018, 92, e00616-18. [Google Scholar] [CrossRef] [PubMed]
- Petro, C.D.; Weinrick, B.; Khajoueinejad, N.; Burn, C.; Sellers, R.; Jacobs, W.R.; Herold, B.C. HSV-2 ΔGD Elicits FcγR-Effector Antibodies That Protect against Clinical Isolates. JCI Insight 2016, 1, e88529. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.D.; Backes, l.M.; Taylor, S.A.; Jiang, Y.; Marchant, A.; Pesola, J.M.; Coen, D.M.; Knipe, D.M.; Ackerman, M.E.; Leib, D.A. Maternal Immunization Confers Protection against Neonatal Herpes Simplex Mortality and Behavioral Morbidity. Sci. Transl. Med. 2019, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Cardin, R.D.; Smith, G.A.; Pickard, G.E.; Sollars, P.J.; Dixon, D.A.; Pasula, R.; Bravo, F.J. The R2 Non-Neuroinvasive HSV-1 Vaccine Affords Protection from Genital HSV-2 Infections in a Guinea Pig Model. NPJ Vaccines 2020, 5, 104. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; He, X.; Yu, F.; Yuan, X.; Hu, W.; Liu, C.; Zhao, F.; Dou, J. Immunization with DNA Vaccine Expressing Herpes Simplex Virus Type 1 GD and IL-21 Protects against Mouse Herpes Keratitis. Immunol. Invest. 2011, 40, 265–278. [Google Scholar] [CrossRef]
- Awasthi, S.; Hook, L.M.; Pardi, N.; Wang, F.; Myles, A.; Cancro, M.P.; Cohen, G.H.; Weissman, D.; Friedman, H.M. Nucleoside-Modified MRNA Encoding HSV-2 Glycoproteins C, D, E Prevents Clinical and Subclinical Genital Herpes. Sci. Immunol. 2019, 4, eaaw7083. [Google Scholar] [CrossRef]
- Shlapobersky, M.; Marshak, J.O.; Dong, L.; Huang, M.; Wei, Q.; Chu, A.; Rolland, A.; Sullivan, S.; Koelle, D.M. Vaxfectin-Adjuvanted Plasmid DNA Vaccine Improves Protection and Immunogenicity in a Murine Model of Genital Herpes Infection. J. Gen. Virol. 2012, 93, 1305–1315. [Google Scholar] [CrossRef]
- Awasthi, S.; Mahairas, G.G.; Shaw, C.E.; Huang, M.-L.; Koelle, D.M.; Posavad, C.; Corey, L.; Friedman, H.M. A Dual-Modality Herpes Simplex Virus 2 Vaccine for Preventing Genital Herpes by Using Glycoprotein C and D Subunit Antigens To Induce Potent Antibody Responses and Adenovirus Vectors Containing Capsid and Tegument Proteins as T Cell Immunogens. J. Virol. 2015, 89, 8497–8509. [Google Scholar] [CrossRef]
- Chiuppesi, F.; Vannucci, L.; De Luca, A.; Lai, M.; Matteoli, B.; Freer, G.; Manservigi, R.; Ceccherini-Nelli, L.; Maggi, F.; Bendinelli, M.; et al. A Lentiviral Vector-Based, Herpes Simplex Virus 1 (HSV-1) Glycoprotein B Vaccine Affords Cross-Protection against HSV-1 and HSV-2 Genital Infections. J. Virol. 2012, 86, 6563–6574. [Google Scholar] [CrossRef]
- Marin, M.; Marti, M.; Kambhampati, A.; Jeram, S.M.; Seward, J.F. Global Varicella Vaccine Effectiveness: A Meta-Analysis. Pediatrics 2016, 137, e20153741. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Broder, K.R.; Marin, M. Severe Varicella in Persons Vaccinated with Varicella Vaccine (Breakthrough Varicella): A Systematic Literature Review. Expert. Rev. Vaccines 2017, 16, 391–400. [Google Scholar] [CrossRef]
- Belshe, R.B.; Leone, P.A.; Bernstein, D.I.; Wald, A.; Levin, M.J.; Stapleton, J.T.; Gorfinkel, I.; Morrow, R.L.A.; Ewell, M.G.; Stokes-Riner, A.; et al. Efficacy Results of a Trial of a Herpes Simplex Vaccine. N. Engl. J. Med. 2012, 366, 34–43. [Google Scholar] [CrossRef]
- Belshe, R.B.; Heineman, T.C.; Bernstein, D.I.; Bellamy, A.R.; Ewell, M.; van der Most, R.; Deal, C.D. Correlate of Immune Protection Against HSV-1 Genital Disease in Vaccinated Women. J. Infect. Dis. 2014, 209, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Niță, I.; Nițipir, C.; Toma, Ș.A.; Limbău, A.M.; Pîrvu, E.; Bădărău, I.A.; Suciu, I.; Suciu, G.; Manolescu, L.S.C. Histological Aspects and Quantitative Assessment of Ki67 as Prognostic Factors in Breast Cancer Patients: Result from a Single-Center, Cross Sectional Study. Medicina 2020, 56, 600. [Google Scholar] [CrossRef] [PubMed]
- Dudek, T.; Knipe, D.M. Replication-Defective Viruses as Vaccines and Vaccine Vectors. Virology 2006, 344, 230–239. [Google Scholar] [CrossRef]
- Spaete, R.R.; Frenkel, N. The Herpes Simplex Virus Amplicon: A New Eucaryotic Defective-Virus Cloning-Amplifying Vector. Cell 1982, 30, 295–304. [Google Scholar] [CrossRef]
- Morrison, L.A.; Knipe, D.M. Immunization with Replication-Defective Mutants of Herpes Simplex Virus Type 1: Sites of Immune Intervention in Pathogenesis of Challenge Virus Infection. J. Virol. 1994, 68, 689–696. [Google Scholar] [CrossRef]
- Da Costa, X.; Kramer, M.F.; Zhu, J.; Brockman, M.A.; Knipe, D.M. Construction, Phenotypic Analysis, and Immunogenicity of a UL5/UL29 Double Deletion Mutant of Herpes Simplex Virus 2. J. Virol. 2000, 74, 7963–7971. [Google Scholar] [CrossRef]
- Loudon, P.T.; Blakeley, D.M.; Boursnell, M.E.; Day, D.A.; Duncan, I.A.; Lowden, R.C.; McLean, C.S.; Martin, G.; Miller, J.C.; Shaw, M.L. Preclinical Safety Testing of DISC-HGMCSF to Support Phase I Clinical Trials in Cancer Patients. J. Gene Med. 2001, 3, 458–467. [Google Scholar] [CrossRef]
- Stanfield, B.A.; Bravo, F.J.; Dixon, D.A.; Chouljenko, V.N.; Kousoulas, K.G.; Bernstein, D.I. Cross Protective Efficacy of the Non-Neurotropic Live Attenuated Herpes Simplex Virus Type 1 Vaccine VC-2 Is Enhanced by Intradermal Vaccination and Deletion of Glycoprotein G. Vaccine 2022, 40, 6093–6099. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Cardin, R.D.; Pullum, D.A.; Bravo, F.J.; Kousoulas, K.G.; Dixon, D.A. Duration of Protection from Live Attenuated vs. Sub Unit HSV-2 Vaccines in the Guinea Pig Model of Genital Herpes: Reassessing Efficacy Using Endpoints from Clinical Trials. PLoS ONE 2019, 14, e0213401. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Earwood, J.D.; Bravo, F.J.; Cohen, G.H.; Eisenberg, R.J.; Clark, J.R.; Fairman, J.; Cardin, R.D. Effects of Herpes Simplex Virus Type 2 Glycoprotein Vaccines and CLDC Adjuvant on Genital Herpes Infection in the Guinea Pig. Vaccine 2011, 29, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Saied, A.A.; Chouljenko, V.N.; Subramanian, R.; Kousoulas, K.G. A Replication Competent HSV-1(McKrae) with a Mutation in the Amino-Terminus of Glycoprotein K (GK) Is Unable to Infect Mouse Trigeminal Ganglia after Cornea Infection. Curr. Eye Res. 2014, 39, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.; Hook, L.M.; Naughton, A.; Friedman, H.M.; Awasthi, S. Herpes Simplex Virus Type 2 Trivalent Protein Vaccine Containing Glycoproteins C, D and E Protects Guinea Pigs against HSV-1 Genital Infection. Hum. Vaccines Immunother. 2020, 16, 2109–2113. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Friedman, H.M. An MRNA Vaccine to Prevent Genital Herpes. Transl. Res. 2022, 242, 56–65. [Google Scholar] [CrossRef]
- Awasthi, S.; Friedman, H.M. Status of Prophylactic and Therapeutic Genital Herpes Vaccines. Curr. Opin. Virol. 2014, 6, 6–12. [Google Scholar] [CrossRef]
- Kim, H.C.; Oh, D.S.; Park, J.H.; Kim, H.-J.; Seo, Y.B.; Yoo, H.J.; Jang, H.S.; Shin, J.; Kim, C.W.; Kwon, M.S.; et al. Multivalent DNA Vaccine Protects against Genital Herpes by T-Cell Immune Induction in Vaginal Mucosa. Antivir. Res. 2020, 177, 104755. [Google Scholar] [CrossRef]
- Ramsey, N.L.M.; Visciano, M.; Hunte, R.; Loh, L.N.; Aschner, C.B.; Jacobs, W.R.; Herold, B.C. A Single-Cycle Glycoprotein d Deletion Viral Vaccine Candidate, ∆gd-2, Elicits Polyfunctional Antibodies That Protect against Ocular Herpes Simplex Virus. J. Virol. 2020, 94, e00335-20. [Google Scholar] [CrossRef]
- Corey, L.; Langenberg, A.G.; Ashley, R.; Sekulovich, R.E.; Izu, A.E.; Douglas, J.M.; Handsfield, H.H.; Warren, T.; Marr, L.; Tyring, S.; et al. Recombinant Glycoprotein Vaccine for the Prevention of Genital HSV-2 Infection: Two Randomized Controlled Trials. Chiron HSV Vaccine Study Group. JAMA 1999, 282, 331–340. [Google Scholar] [CrossRef]
- Nandy, A.; Basak, S.C. Bioinformatics in Design of Antiviral Vaccines. Encycl. Biomed. Eng. 2019, 15, 280–290. [Google Scholar] [CrossRef]
- Murata, T. Human Herpesvirus and the Immune Checkpoint PD-1/PD-L1 Pathway: Disorders and Strategies for Survival. Microorganisms 2021, 9, 778. [Google Scholar] [CrossRef] [PubMed]
- Pîrlog, C.-F.; Paroșanu, A.I.; Slavu, C.O.; Olaru, M.; Popa, A.M.; Iaciu, C.; Niță, I.; Moțatu, P.; Horia, C.; Manolescu, L.S.C.; et al. Nivolumab Hypersensitivity Reactions a Myth or Reality in Solid Tumors—A Systematic Review of the Literature. Curr. Oncol. 2022, 29, 9428–9436. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.M.; Lepisto, A.J.; Freeman, M.L.; Sheridan, B.S.; Cherpes, T.L.; Hendricks, R.L. Early CD4+ T Cell Help Prevents Partial CD8+ T Cell Exhaustion and Promotes Maintenance of Herpes Simplex Virus 1 Latency. J. Immunol. 2010, 184, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Twardy, B.S.; Suvas, S. Blocking of PDL-1 Interaction Enhances Primary and Secondary CD8 T Cell Response to Herpes Simplex Virus-1 Infection. PLoS ONE 2012, 7, e39757. [Google Scholar] [CrossRef]
- Spicknall, I.H.; Looker, K.J.; Gottlieb, S.L.; Chesson, H.W.; Schiffer, J.T.; Elmes, J.; Boily, M.-C. Review of Mathematical Models of HSV-2 Vaccination: Implications for Vaccine Development. Vaccine 2019, 37, 7396–7407. [Google Scholar] [CrossRef]
- Freeman, E.E.; White, R.G.; Bakker, R.; Orroth, K.K.; Weiss, H.A.; Buvé, A.; Hayes, R.J.; Glynn, J.R. Population-Level Effect of Potential HSV2 Prophylactic Vaccines on HIV Incidence in Sub-Saharan Africa. Vaccine 2009, 27, 940–946. [Google Scholar] [CrossRef]
- WHO. Preferred Product Characteristics for Herpes Simplex Virus Vaccines. Available online: https://www.who.int/publications-detail-redirect/9789241515580 (accessed on 10 May 2023).
| Types of Vaccine | Study | Mechanism of Action | Advantages and Disadvantages | References |
|---|---|---|---|---|
| Subunit vaccine | CTL, HTL and BCL epitopes | Production of neutralizing antibodies and some can block the immune evasion [18] | Advantage:
| [53] |
| MEAP | [54] | |||
| GEN-003/MM-2 − gD2 truncated + ICP4 fragment (29.2 kD) + Matrix M2 Adjuvant | [55] | |||
| Chiron glycoproteins gB and gD | [56] | |||
| GlaxoSmithKline—purified carboxy-terminal truncated gD2 expressed in CHO cells | [57] | |||
| Bivalent HSV-2 Subunit (gD2 and gB2) + Nanoemulsion adjuvant NE01 | [58] | |||
| CD8+ T cell peptide epitopes (UL44 aa400–408, UL9 aa196–204, and UL25 aa572–580) | [59] | |||
| G103—three recombinantly expressed HSV-2 proteins (gD and the UL19 and UL25 gene products) adjuvanted with synthetic TLR4 agonist glucopyranosyl lipid A (GLA) formulated in stable emulsion | [60] | |||
| 32 synthetic 35mer HSV-2 peptides non-covalently complexed with recombinant human Hsc70 protein (named HerpV, formerly AG-707) | [61] | |||
| intranasal trivalent HSV-2 subunit vaccine (gC2, gD2, and gE2)/alum adjuvant | [62] | |||
| Live-attenuated Vaccines | R7017 and R7020 | They stimulate both humoral and cell-mediated adaptive immune responses as well as innate immunity; can lead to a impairment of virus replication | Advantages:
| [63] |
| herpes simplex virus gamma 1 34.5 deletion mutants | [64] | |||
| ICP0(−) mutant virus (HSV-2 0DeltaNLS) | [65] | |||
| RAV 9395 | [66] | |||
| a vaccine lacking both copies of γ134.5 as well as UL55, UL56 and the US10-12 region of HSV-2 | [67] | |||
| live-attenuated HSV-1 VC2 vaccine strain | [68] | |||
| replication-competent controlled HSV-1 vectors (HSV-GS3 and HSV-GS7) | [69] | |||
| HSV-2 with US6 (gD) deletion | [70] | |||
| replication-defective HSV-2 dl5-29 | [71] | |||
| R2 non-neuroinvasive HSV-1 vaccine (HSV1-GS6264, 5 missense mutations in UL37) | [72] | |||
| DNA vaccines | naked plasmid DNA (pDNA) vaccine expressing herpes simplex virus type 1 (HSV-1) glycoprotein B (gB) | Production of neutralizing antibodies, sIgA and increased activity of natural killer cells and splenocytes [18] | Advantage:
| [73] |
| nucleoside-modified mRNA encoding gC2, gD2, and gE2 + lipid nanoparticles | [74] | |||
| Vaxfectin-adjuvanted plasmid DNA vaccine | [75] | |||
| Viral vector agents | herpes simplex virus 2 (HSV-2) glycoproteins C (gC2) and D (gD2) and UL19 (capsid protein VP5) and UL47 (tegument protein VP13/14) expressed in baculovirus | The delivery of one or more antigens encoded in the context of an unrelated, modified virus induces immune responses against the respective target pathogen | Advantage:
| [76] |
| lentiviral vector-based HSV-1 glycoprotein B vaccine | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preda, M.; Manolescu, L.S.C.; Chivu, R.D. Advances in Alpha Herpes Viruses Vaccines for Human. Vaccines 2023, 11, 1094. https://doi.org/10.3390/vaccines11061094
Preda M, Manolescu LSC, Chivu RD. Advances in Alpha Herpes Viruses Vaccines for Human. Vaccines. 2023; 11(6):1094. https://doi.org/10.3390/vaccines11061094
Chicago/Turabian StylePreda, Madalina, Loredana Sabina Cornelia Manolescu, and Razvan Daniel Chivu. 2023. "Advances in Alpha Herpes Viruses Vaccines for Human" Vaccines 11, no. 6: 1094. https://doi.org/10.3390/vaccines11061094
APA StylePreda, M., Manolescu, L. S. C., & Chivu, R. D. (2023). Advances in Alpha Herpes Viruses Vaccines for Human. Vaccines, 11(6), 1094. https://doi.org/10.3390/vaccines11061094







