A Three-Dose mRNA COVID-19 Vaccine Regime Produces Both Suitable Immunogenicity and Satisfactory Efficacy in Patients with Solid Cancers
Abstract
:1. Introduction
2. Materials and Methods
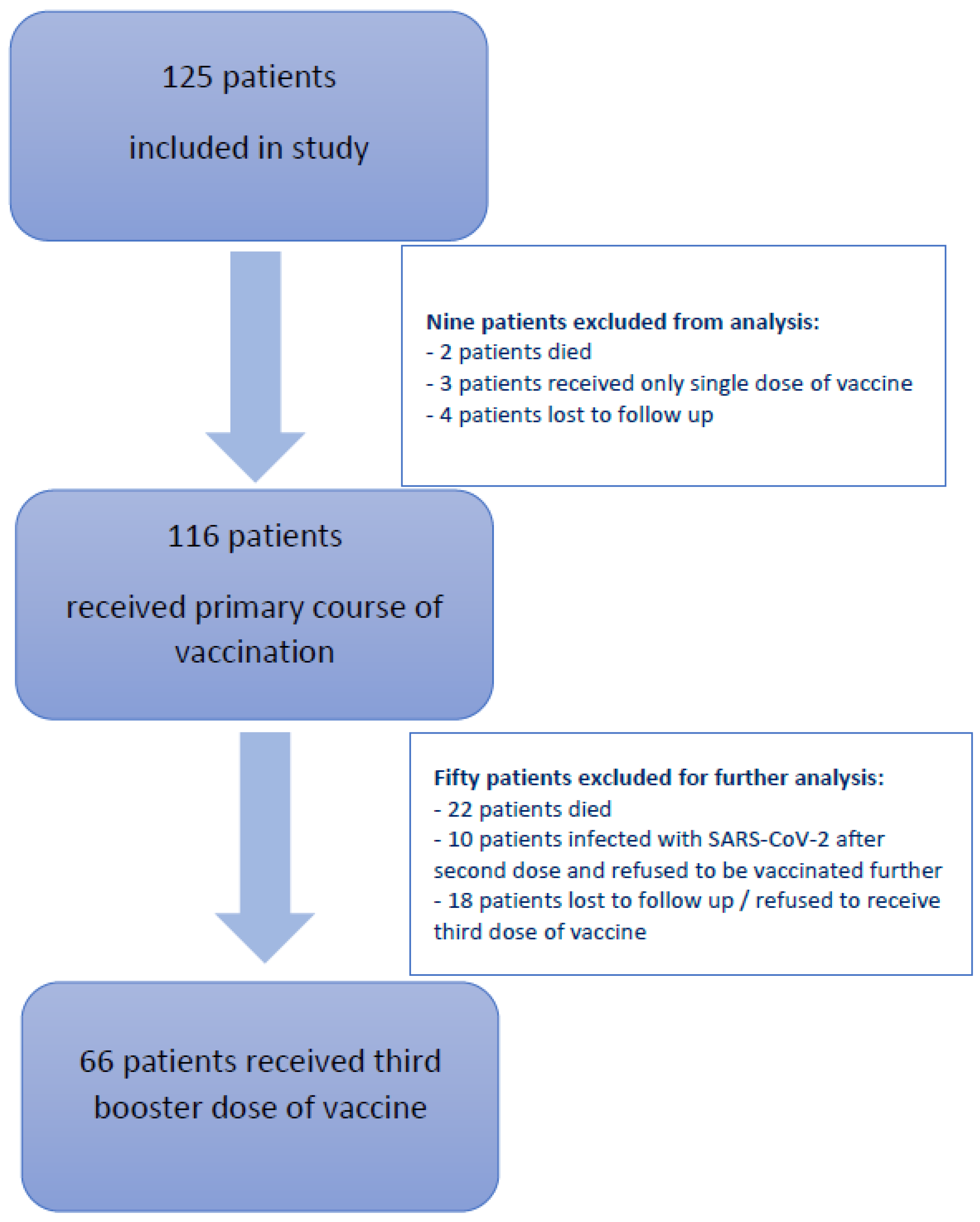
2.1. Study Design and Participants
2.2. Procedures
2.3. Anti-SARS-CoV-2 S1 IgG Antibodies Detection in Serum
2.4. Statistical Analysis and Outcomes
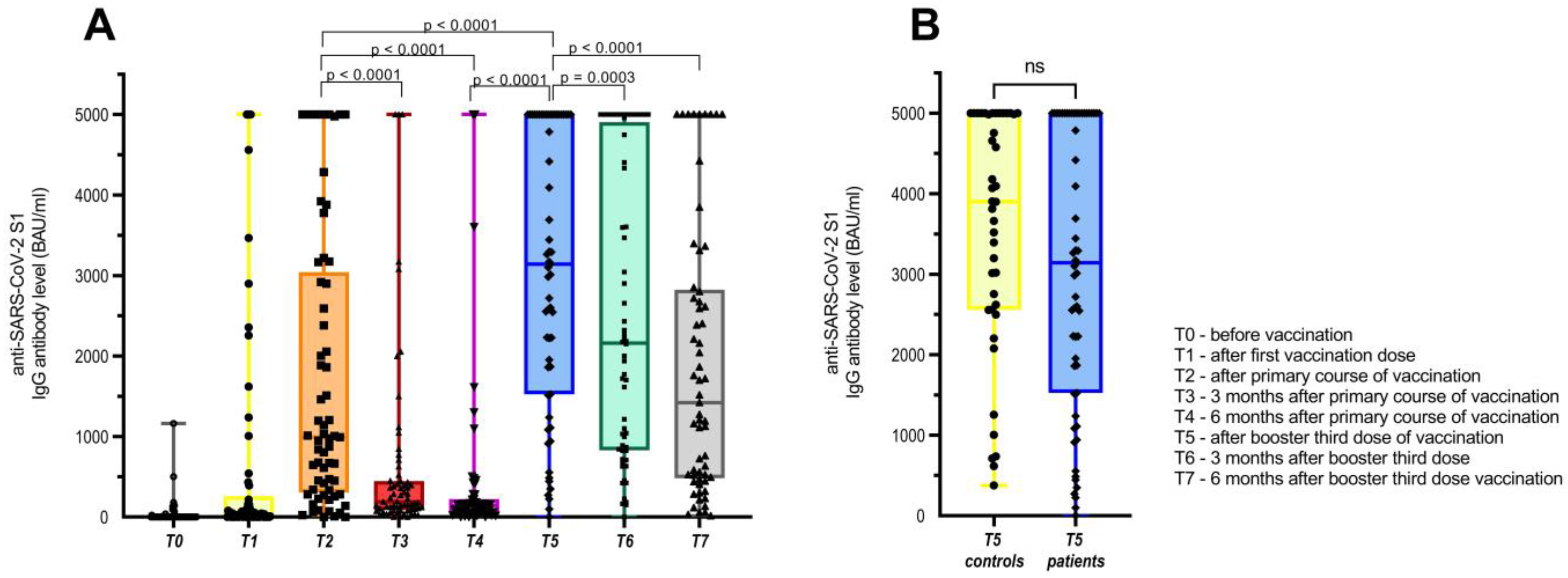
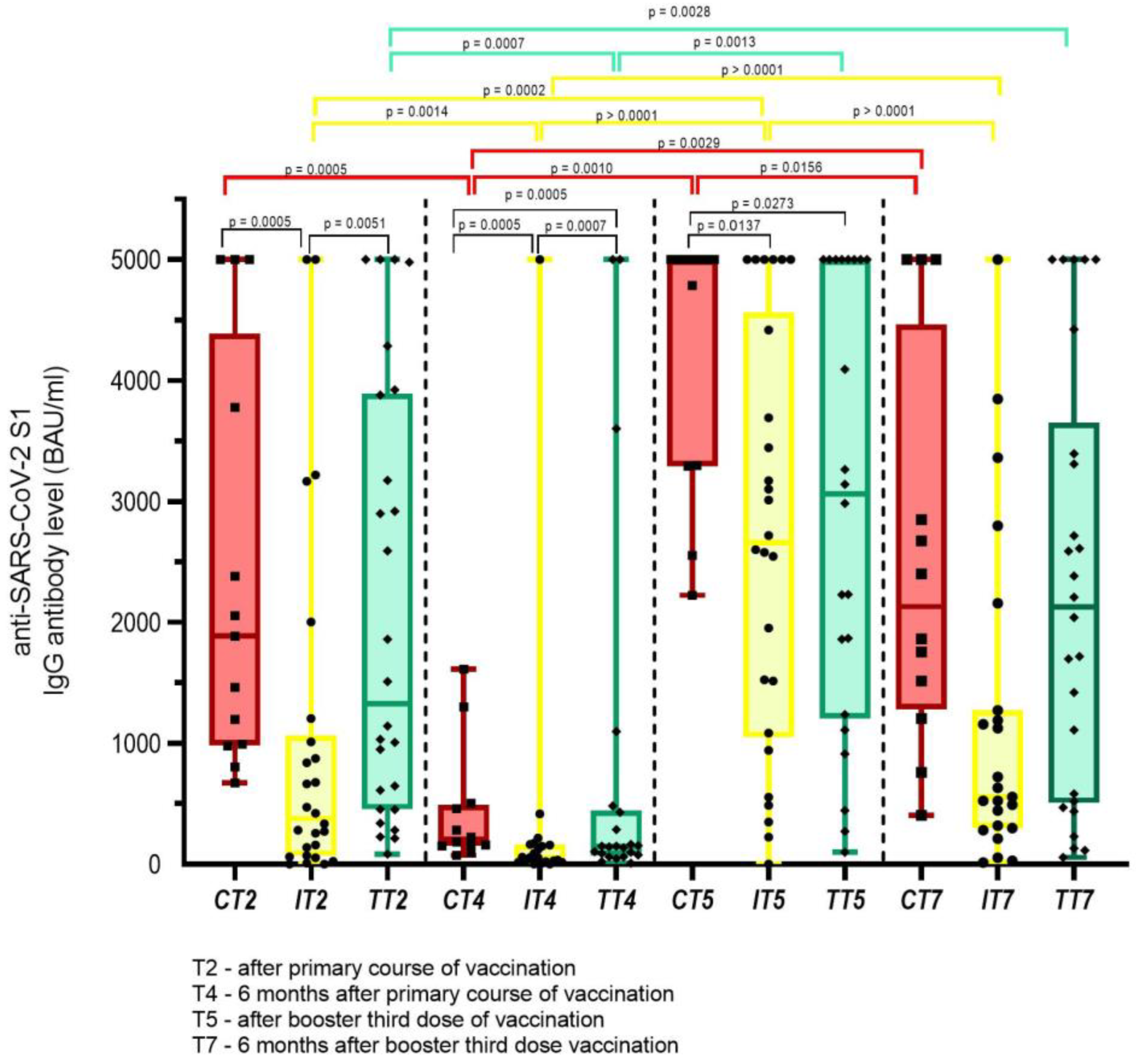
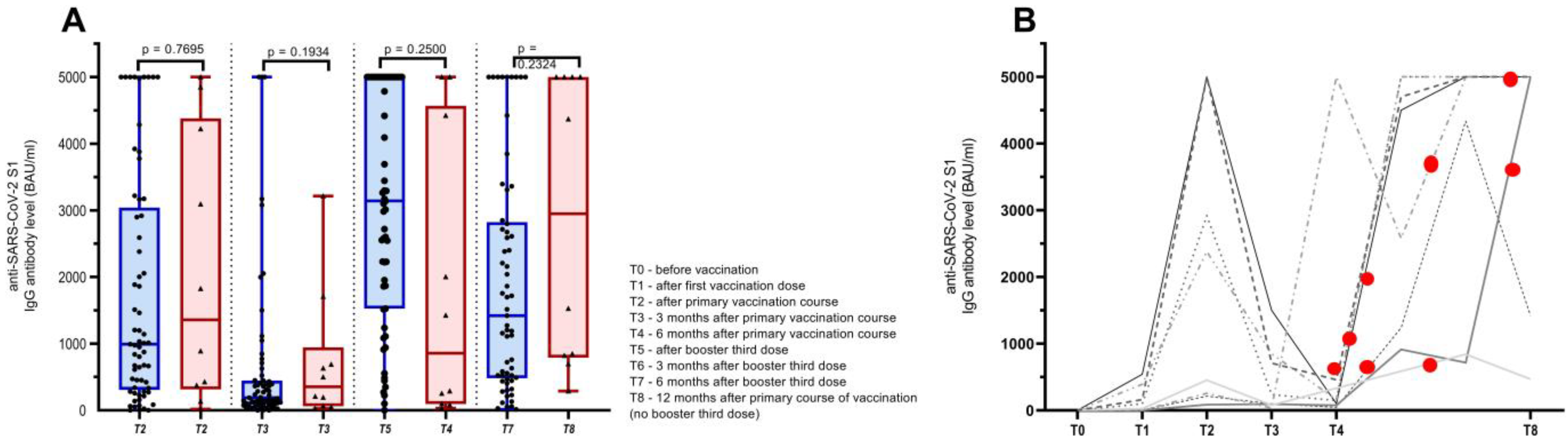
3. Results
3.1. Humoral Immune Response to COVID-19 Booster Third Vaccination
3.2. Safety and Humoral Immune Response of Cancer Patients Vaccinated with Three Doses of COVID-19 Vaccine and Those Infected with SARS-CoV-2 after the Primary Course of Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worldometers.info COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/ (accessed on 10 March 2023).
- Castelo-Branco, L.; Tsourti, Z.; Gennatas, S.; Rogado, J.; Sekacheva, M.; Viñal, D.; Lee, R.; Croitoru, A.; Vitorino, M.; Khallaf, S.; et al. COVID-19 in Patients with Cancer: First Report of the ESMO International, Registry-Based, Cohort Study (ESMO-CoCARE). ESMO Open 2022, 7, 100499. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.S.; Tagliamento, M.; Lambertini, M.; McNally, R.; Romano, M.; Leone, M.; Curigliano, G.; de Azambuja, E. Mortality in Patients with Cancer and Coronavirus Disease 2019: A Systematic Review and Pooled Analysis of 52 Studies. Eur. J. Cancer 2020, 139, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Benderra, M.A.; Aparicio, A.; Leblanc, J.; Wassermann, D.; Kempf, E.; Galula, G.; Bernaux, M.; Canellas, A.; Moreau, T.; Bellamine, A.; et al. Clinical Characteristics, Care Trajectories and Mortality Rate of Sars-Cov-2 Infected Cancer Patients: A Multicenter Cohort Study. Cancers 2021, 13, 4749. [Google Scholar] [CrossRef]
- Thomas, S.J.; Perez, J.L.; Lockhart, S.P.; Hariharan, S.; Kitchin, N.; Xu, X.; Koury, K.; Dychter, S.S.; Lu, C.; Gentile, T.C.; et al. Efficacy and Safety of the BNT162b2 MRNA COVID-19 Vaccine in Participants with a History of Cancer: Subgroup Analysis of a Global Phase 3 Randomized Clinical Trial. Vaccine 2021, 40, 1483–1492. [Google Scholar] [CrossRef]
- Barrière, J.; Chamorey, E.; Adjtoutah, Z.; Castelnau, O.; Mahamat, A.; Marco, S.; Petit, E.; Leysalle, A.; Raimondi, V.; Carles, M. Impaired Immunogenicity of BNT162b2 Anti-SARS-CoV-2 Vaccine in Patients Treated for Solid Tumors. Ann. Oncol. 2021, 32, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Byrne, F.; Cerrone, M.; Schmitt, A.M.; Joharatnam-hogan, N.; Shum, B.; et al. Adaptive Immunity and Neutralizing Antibodies against SARS-CoV-2 Variants of Concern Following Vaccination in Patients with Cancer: The CAPTURE Study. Nat. Cancer 2021, 2, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Oosting, S.F.; van der Veldt, A.A.M.; GeurtsvanKessel, C.H.; Fehrmann, R.S.N.; van Binnendijk, R.S.; Dingemans, A.-M.C.; Smit, E.F.; Hiltermann, T.J.N.; den Hartog, G.; Jalving, M.; et al. MRNA-1273 COVID-19 Vaccination in Patients Receiving Chemotherapy, Immunotherapy, or Chemoimmunotherapy for Solid Tumours: A Prospective, Multicentre, Non-Inferiority Trial. Lancet Oncol. 2021, 2045, 1681–1691. [Google Scholar] [CrossRef]
- Naranbhai, V.; Pernat, C.A.; Gavralidis, A.; St Denis, K.J.; Lam, E.C.; Spring, L.M.; Isakoff, S.J.; Farmer, J.R.; Zubiri, L.; Hobbs, G.S.; et al. Immunogenicity and Reactogenicity of SARS-CoV-2 Vaccines in Patients With Cancer: The CANVAX Cohort Study. J. Clin. Oncol. 2021, 40, 12–23. [Google Scholar] [CrossRef]
- Peeters, M.; Verbruggen, L.; Teuwen, L.; Vanhoutte, G.; Vande Kerckhove, S.; Peeters, B.; Raats, S.; Van der Massen, I.; De Keersmaecker, S.; Debie, Y.; et al. Reduced Humoral Immune Response after BNT162b2 Coronavirus Disease 2019 Messenger RNA Vaccination in Cancer Patients under Antineoplastic Treatment. ESMO Open 2021, 6, 100274. [Google Scholar] [CrossRef]
- Addeo, A.; Shah, P.K.; Bordry, N.; Hudson, R.D.; Albracht, B.; Di Marco, M.; Kaklamani, V.; Dietrich, P.Y.; Taylor, B.S.; Simand, P.F.; et al. Immunogenicity of SARS-CoV-2 Messenger RNA Vaccines in Patients with Cancer. Cancer Cell 2021, 39, 1091–1098.e2. [Google Scholar] [CrossRef]
- Palich, R.; Veyri, M.; Vozy, A.; Marot, S.; Gligorov, J.; Benderra, M.A.; Maingon, P.; Morand-Joubert, L.; Adjoutah, Z.; Marcelin, A.G.; et al. High Seroconversion Rate but Low Antibody Titers after Two Injections of BNT162b2 (Pfizer-BioNTech) Vaccine in Patients Treated with Chemotherapy for Solid Cancers. Ann. Oncol. 2021, 32, 1294–1295. [Google Scholar] [CrossRef] [PubMed]
- Trontzas, I.P.; Vathiotis, I.; Economidou, C.; Petridou, I.; Gomatou, G.; Grammoustianou, M.; Tsamis, I.; Syrigos, N.; Anagnostakis, M.; Fyta, E.; et al. Assessment of Seroconversion after SARS-CoV-2 Vaccination in Patients with Lung Cancer. Vaccines 2022, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Janzic, U.; Bidovec-stojkovic, U.; Mohorcic, K.; Mrak, L.; Fokter, N. Solid Cancer Patients Achieve Adequate Immunogenicity and Low Rate of Severe Adverse Events after SARS-CoV-2 Vaccination. Futur. Oncol. 2022, 18, 2537–2550. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.; Antonarelli, G.; Scotté, F.; Spano, J.P.; Barrière, J.; Michot, J.M.; André, F.; Curigliano, G. Seroconversion Rate after Vaccination against COVID-19 in Cancer Patients—A Systematic Review. Ann. Oncol. 2022, 33, 158–168. [Google Scholar] [CrossRef]
- Thakkar, A.; Pradhan, K.; Jindal, S.; Cui, Z.; Rockwell, B.; Shah, A.P.; Packer, S.; Sica, R.A.; Sparano, J.; Goldstein, D.Y.; et al. Patterns of Seroconversion for SARS-CoV-2 IgG in Patients with Malignant Disease and Association with Anticancer Therapy. Nat. Cancer 2021, 2, 392–399. [Google Scholar] [CrossRef]
- Terpos, E.; Gavriatopoulou, M.; Fotiou, D.; Giatra, C.; Asimakopoulos, I.; Dimou, M.; Sklirou, A.D.; Ntanasis-Stathopoulos, I.; Darmani, I.; Briasoulis, A.; et al. Poor Neutralizing Antibody Responses in 132 Patients with Cll, Nhl and Hl after Vaccination against Sars-Cov-2: A Prospective Study. Cancers 2021, 13, 4480. [Google Scholar] [CrossRef]
- Rzymski, P.; Pazgan-Simon, M.; Kamerys, J.; Moniuszko-Malinowska, A.; Sikorska, K.; Wernik, J.; Zarębska-Michaluk, D.; Supronowicz, Ł.; Sobala-Szczygieł, B.; Skrzat-Klapaczyńska, A.; et al. Severe Breakthrough COVID-19 Cases during Six Months of Delta Variant (B.1.617.2) Domination in Poland. Vaccines 2022, 10, 557. [Google Scholar] [CrossRef]
- Eberhardt, K.A.; Dewald, F.; Heger, E.; Gieselmann, L.; Vanshylla, K.; Wirtz, M.; Kleipass, F.; Johannis, W.; Schommers, P.; Gruell, H.; et al. Evaluation of a New Spike (S)-Protein-Based Commercial Immunoassay for the Detection of Anti-SARS-CoV-2 IgG. Microorganisms 2021, 9, 733. [Google Scholar] [CrossRef]
- Barrière, J.; Carles, M.; Audigier-Valette, C.; Re, D.; Adjtoutah, Z.; Seitz-Polski, B.; Gounant, V.; Descamps, D.; Zalcman, G. Third Dose of Anti-SARS-CoV-2 Vaccine for Patients with Cancer: Should Humoral Responses Be Monitored? A Position Article. Eur. J. Cancer 2022, 162, 182–193. [Google Scholar] [CrossRef]
- Immundiagnostik, A.G. IDK Anti-SARS-CoV-2 IgG ELISA (Manual). 2021. Available online: https://www.idkna.com/docs/k5004_2021-08-10_idk_sars-cov-2-igg.pdf (accessed on 2 March 2023).
- WHO. WHO Living Guidance for Clinical Management of COVID-19; World Health Organization: Geneva, Switzerland, 2021; pp. 1–116.
- Debie, Y.; Vandamme, T.; Goossens, M.E. Antibody Titres before and after a Third Dose of the SARS-CoV-2 BNT162b2 Vaccine in Patients with Cancer. Eur. J. Cancer 2021, 163, 177–179. [Google Scholar] [CrossRef]
- Naranbhai, V.; St. Denis, K.J.; Lam, E.C.; Ofoman, O.; Garcia-Beltran, W.F.; Mairena, C.B.; Bhan, A.K.; Gainor, J.F.; Balazs, A.B.; Iafrate, A.J. Neutralization Breadth of SARS-CoV-2 Viral Variants Following Primary Series and Booster SARS-CoV-2 Vaccines in Patients with Cancer Graphical. Cancer Cell 2022, 40, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.C.; Thakkar, A.; Campbell, S.T.; Forest, S.K.; Pradhan, K.; Gonzalez-Lugo, J.D.; Quinn, R.; Bhagat, T.D.; Choudhary, G.S.; McCort, M.; et al. Efficacy of Booster Doses in Augmenting Waning Immune Responses to COVID-19 Vaccine in Patients with Cancer. Cancer Cell 2022, 40, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, Y.; Grinshpun, A.; Ben-Dov, I.Z.; Oiknine Djian, E.; Wolf, D.G.; Kadouri, L. Assessment of Response to a Third Dose of the SARS-CoV-2 BNT162b2 MRNA Vaccine in Patients With Solid Tumors Undergoing Active Treatment. JAMA Oncol. 2022, 8, 301–302. [Google Scholar] [CrossRef]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Schmitt, A.M.; Tippu, Z.; Farag, S.; Rogiers, A.; Harvey, R.; et al. Immune Responses Following Third COVID-19 Vaccination Are Reduced in Patients with Hematological Malignancies Compared to Patients with Solid Cancer. Cancer Cell 2022, 40, 114–116. [Google Scholar] [CrossRef]
- Zeng, C.; Evans, J.P.; Chakravarthy, K.; Qu, P.; Reisinger, S.; Song, N.J.; Rubinstein, M.P.; Shields, P.G.; Li, Z.; Liu, S.L. COVID-19 MRNA Booster Vaccines Elicit Strong Protection against SARS-CoV-2 Omicron Variant in Patients with Cancer. Cancer Cell 2022, 40, 117–119. [Google Scholar] [CrossRef]
- Di Noia, V.; Pimpinelli, F.; Renna, D.; Maccallini, M.T.; Gariazzo, L.; Riva, F.; Sperandio, E.; Giannarelli, D.; Cognetti, F. Potentiation of Humoral Response to the BNT162b2 Vaccine after the Third Dose in Patients with Solid Cancer. Ann. Oncol. 2022, 33, 564–565. [Google Scholar] [CrossRef]
- Lasagna, A.; Bergami, F.; Lilleri, D.; Percivalle, E.; Quaccini, M.; Alessio, N.; Comolli, G.; Sarasini, A.; Sammartino, J.C.; Ferrari, A.; et al. Immunogenicity and Safety after the Third Dose of BNT162b2 Anti-SARS-CoV-2 Vaccine in Patients with Solid Tumors on Active Treatment: A Prospective Cohort Study. ESMO Open 2022, 7, 100458. [Google Scholar] [CrossRef]
- Fenioux, C.; Teixeira, L.; Fourati, S.; Melica, G.; Lelievre, J.D.; Gallien, S.; Zalcman, G.; Pawlotsky, J.M.; Tournigand, C. SARS-CoV-2 Antibody Response to 2 or 3 Doses of the BNT162b2 Vaccine in Patients Treated with Anticancer Agents. JAMA Oncol. 2022, 8, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Di Noia, V.; Pimpinelli, F.; Renna, D.; Campo, F.; Cosimati, A.; Torchia, A.; Marcozzi, B.; Massacci, A.; Pallocca, M.; Pellini, R.; et al. Duration of Humoral Response to the Third Dose of BNT162b2 Vaccine in Patients with Solid Cancer: Is Fourth Dose Urgently Needed? Eur. J. Cancer 2022, 176, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Lasagna, A.; Bergami, F.; Lilleri, D.; Percivalle, E.; Quaccini, M.; Serra, F.; Comolli, G.; Sarasini, A.; Sammartino, J.C.; Ferrari, A.; et al. Six-Month Humoral and Cellular Immune Response to the Third Dose of BNT162b2 Anti-SARS-CoV-2 Vaccine in Patients with Solid Tumors: A Longitudinal Cohort Study with a Focus on the Variants of Concern. ESMO Open 2022, 7, 100574. [Google Scholar] [CrossRef]
- Baek, Y.J.; Lee, Y.-J.; Park, S.R.; Kim, K.H.; Beom, S.-H.; Lee, C.-K.; Shin, S.J.; Rha, S.Y.; Kim, S.; Lee, K.H.; et al. Immunogenicity and Safety of Vaccines Against Coronavirus Disease in Actively Treated Patients with Solid Tumors: A Prospective Cohort Study. Cancer Res. Treat. 2023, 1. [Google Scholar] [CrossRef] [PubMed]
- Al Hajji, Y.; Taylor, H.; Starkey, T.; Lee, L.Y.W.; Tilby, M. Antibody Response to a Third Booster Dose of SARS-CoV-2 Vaccination in Adults with Haematological and Solid Cancer: A Systematic Review. Br. J. Cancer 2022, 127, 1827–1836. [Google Scholar] [CrossRef]
- Shroff, R.T.; Chalasani, P.; Wei, R.; Pennington, D.; Quirk, G.; Schoenle, M.V.; Peyton, K.L.; Uhrlaub, J.L.; Ripperger, T.J.; Jergović, M.; et al. Immune Responses to Two and Three Doses of the BNT162b2 MRNA Vaccine in Adults with Solid Tumors. Nat. Med. 2021, 27, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Fan, F.; Xu, X.; Zhao, G.; Zhang, X.; Zhao, H.; Wang, L.; Wang, B.; Gao, X.-M. A COVID-19 DNA Vaccine Candidate Elicits Broadly Neutralizing Antibodies Against Multiple SARS-CoV-2 Variants Including the Currently Circulating Omicron BF.5, BF.7, BQ.1 and XBB. Vaccines 2023, 11, 778. [Google Scholar] [CrossRef] [PubMed]
| N = 66 | |
|---|---|
| Age in years, median (range) | 64 (43–83) |
| Sex, n (%) | |
| 27 (41%) |
| 39 (59%) |
| Cancer type, n (%) | |
| NSCLC † | 53 (80%) |
| 7 (11%) |
| 3 (5%) |
| 2 (3%) |
| 1 (1%) |
| Stage, n (%) | |
| 15 (23%) |
| 5 (7%) |
| 46 (70%) |
| Anticancer therapy, n (%) | |
| 13 (20%) |
| 26 (40%) |
| 27 (40%) |
| Positive SARS-CoV-2 IgG antibodies prior to vaccination, n (%) ‡ | |
| 57 (86%) |
| 9 (14%) |
| Type of primary vaccination received, n (%) | |
| 60 (91%) |
| 2 (3%) |
| 4 (6%) |
| Type of booster third-dose vaccination received, n (%) | |
| 64 (97%) |
| 2 (3%) |
| Median time between primary course of vaccination and third booster dose received, n (range) | 216 days (153–300) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janzic, U.; Bidovec-Stojkovic, U.; Korosec, P.; Mohorcic, K.; Mrak, L.; Caks, M.; Ravnik, M.; Skof, E.; Rijavec, M. A Three-Dose mRNA COVID-19 Vaccine Regime Produces Both Suitable Immunogenicity and Satisfactory Efficacy in Patients with Solid Cancers. Vaccines 2023, 11, 1017. https://doi.org/10.3390/vaccines11061017
Janzic U, Bidovec-Stojkovic U, Korosec P, Mohorcic K, Mrak L, Caks M, Ravnik M, Skof E, Rijavec M. A Three-Dose mRNA COVID-19 Vaccine Regime Produces Both Suitable Immunogenicity and Satisfactory Efficacy in Patients with Solid Cancers. Vaccines. 2023; 11(6):1017. https://doi.org/10.3390/vaccines11061017
Chicago/Turabian StyleJanzic, Urska, Urska Bidovec-Stojkovic, Peter Korosec, Katja Mohorcic, Loredana Mrak, Marina Caks, Maja Ravnik, Erik Skof, and Matija Rijavec. 2023. "A Three-Dose mRNA COVID-19 Vaccine Regime Produces Both Suitable Immunogenicity and Satisfactory Efficacy in Patients with Solid Cancers" Vaccines 11, no. 6: 1017. https://doi.org/10.3390/vaccines11061017
APA StyleJanzic, U., Bidovec-Stojkovic, U., Korosec, P., Mohorcic, K., Mrak, L., Caks, M., Ravnik, M., Skof, E., & Rijavec, M. (2023). A Three-Dose mRNA COVID-19 Vaccine Regime Produces Both Suitable Immunogenicity and Satisfactory Efficacy in Patients with Solid Cancers. Vaccines, 11(6), 1017. https://doi.org/10.3390/vaccines11061017










