Clinical Performance of Cobas 6800 for the Detection of High-Risk Human Papillomavirus in Urine Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Samples and HPV Detection Using Cobas 6800
2.3. LOD Studies
2.4. Stability and Reproducibility Analysis
2.5. Comparative Analysis of ThinPrep and Urine-Based HPV Detection
2.6. Statistical Analysis
3. Results
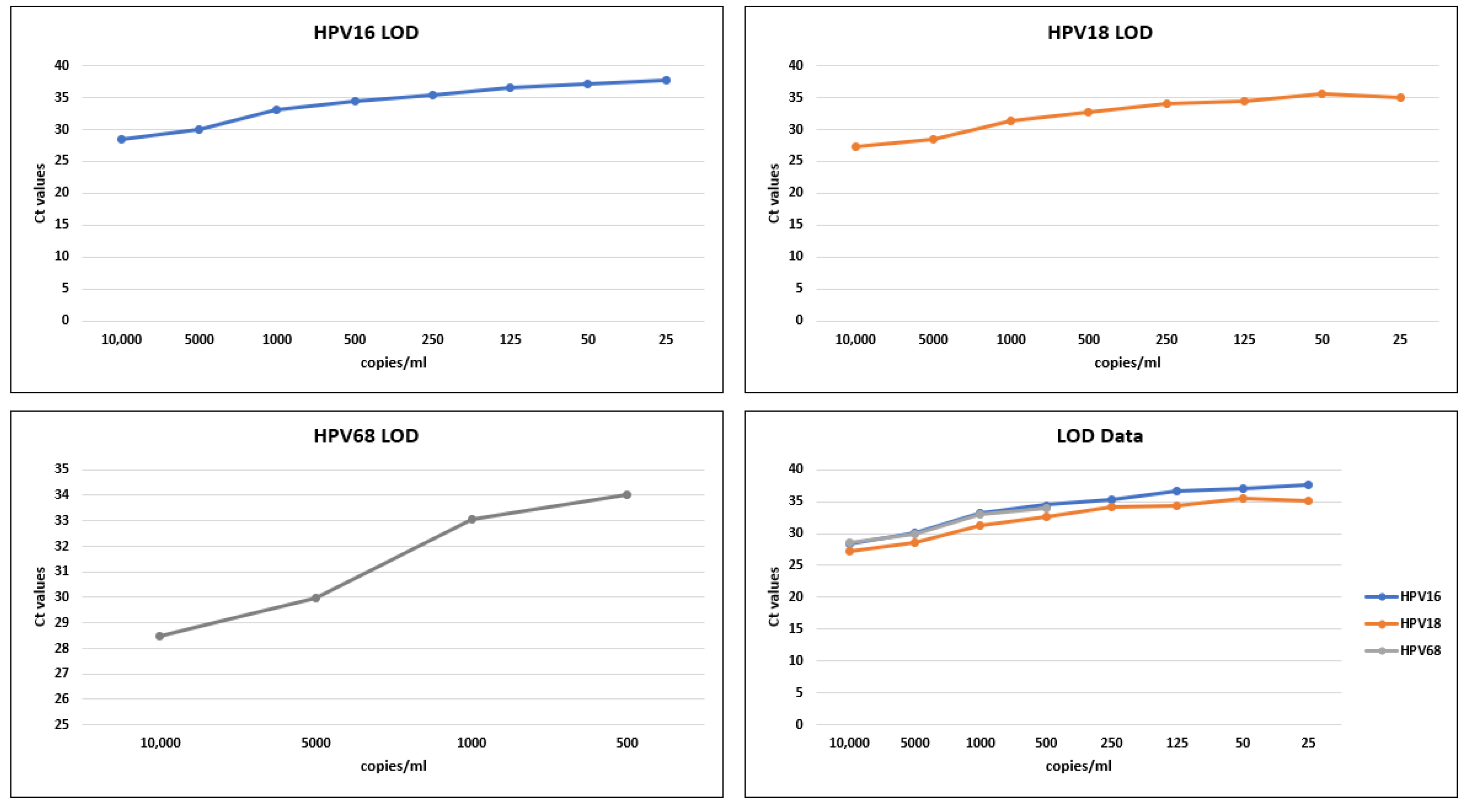
3.1. LOD Analysis
3.2. Analysis of Clinical Performance
3.3. Stability and Reproducibility
3.4. Comparison of ThinPrep and Urine-Based HPV Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, T.R.; Graybill, W.S.; Pierce, J.Y. Morbidity and mortality of vulvar and vaginal cancers: Impact of 2-, 4-, and 9-valent HPV vaccines. Hum. Vaccines Immunother. 2016, 12, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Sah, R.; Muhammad, K.; Waheed, Y. Tracking HPV infection, associated cancer development, and recent treatment efforts—A comprehensive review. Vaccines 2023, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Waheed, Y.; Sah, R.; Muhammad, K. Recent developments in vaccines for viral diseases. Vaccines 2023, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.P.; Crum, C.P. Natural history of cervical neoplasia: Defining progression and its consequence. Clin. Obstet. Gynecol. 2000, 43, 352–362. [Google Scholar] [CrossRef]
- Myers, E.R.; McCrory, D.C.; Nanda, K.; Bastian, L.; Matchar, D. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am. J. Epidemiology 2000, 151, 1158–1171. [Google Scholar] [CrossRef]
- Magalhães, G.M.; Vieira, É.C.; Garcia, L.C.; Carvalho-Leite, D.; de Lourdes Ribeiro, M.; Guedes, A.C.M.; Araújo, M.G. Update on human papilloma virus-part I: Epidemiology, pathogenesis, and clinical spectrum. An. Bras. Dermatol. 2021, 96, 1–16. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dillner, J.; Rebolj, M.; Birembaut, P.; Petry, K.-U.; Szarewski, A.; Munk, C.; De Sanjose, S.; Naucler, P.; Lloveras, B.; Kjaer, S.; et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: Joint European cohort study. BMJ 2008, 337, a1754. [Google Scholar] [CrossRef]
- Ronco, G.; Dillner, J.; Elfström, K.M.; Tunesi, S.; Snijders, P.J.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P.; et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef]
- Frayle, H.; Gori, S.; Rizzi, M.; Graziani, B.N.; Vian, E.; Rossi, P.G.; Del Mistro, A. HPV testing for cervical cancer screening: Technical improvement of laboratory logistics and good clinical performance of the cobas 6800 in comparison to the 4800 system. BMC Women’s Health 2019, 19, 47. [Google Scholar] [CrossRef]
- Aretzweiler, G.; Leuchter, S.; Simon, C.O.; Marins, E.; Frontzek, A. Generating timely molecular diagnostic test results: Workflow comparison of the cobas® 6800/8800 to Panther. Expert Rev. Mol. Diagn. 2019, 19, 951–957. [Google Scholar] [CrossRef]
- Dugué, P.-A.; Lynge, E.; Bjerregaard, B.; Rebolj, M. Non-participation in screening: The case of cervical cancer in Denmark. Prev. Med. 2012, 54, 266–269. [Google Scholar] [CrossRef]
- Hernandez, B.Y.; Wilkens, L.R.; Zhu, X.; Thompson, P.; McDuffie, K.; Shvetsov, Y.B.; Kamemoto, L.E.; Killeen, J.; Ning, L.; Goodman, M.T. Transmission of human papillomavirus in heterosexual couples. Emerg. Infect. Dis. 2008, 14, 888. [Google Scholar] [CrossRef]
- Espersen, M.M.; Holten, I.W. Barriers in screening for cervical cancer. Ugeskr. Laeger 2005, 167, 4371–4374. [Google Scholar]
- Reisner, S.L.; Deutsch, M.B.; Peitzmeier, S.M.; Hughto, J.M.W.; Cavanaugh, T.P.; Pardee, D.J.; McLean, S.A.; Panther, L.A.; Gelman, M.; Mimiaga, M.J.; et al. Test performance and acceptability of self-versus provider-collected swabs for high-risk HPV DNA testing in female-to-male trans masculine patients. PLoS ONE 2018, 13, e0190172. [Google Scholar] [CrossRef]
- Harb, C.Y.; Pass, L.E.; De Soriano, I.C.; Zwick, A.; Gilbert, P.A. Motivators and barriers to accessing sexual health care services for transgender/genderqueer individuals assigned female sex at birth. Transgender Heal. 2019, 4, 58–67. [Google Scholar] [CrossRef]
- Peitzmeier, S.M.; Reisner, S.L.; Harigopal, P.; Potter, J. Female-to-male patients have high prevalence of unsatisfactory Paps compared to non-transgender females: Implications for cervical cancer screening. J. Gen. Intern. Med. 2014, 29, 778–784. [Google Scholar] [CrossRef]
- Potter, J.; Peitzmeier, S.M.; Bernstein, I.; Reisner, S.L.; Alizaga, N.M.; Agénor, M.; Pardee, D.J. Cervical cancer screening for patients on the female-to-male spectrum: A narrative review and guide for clinicians. J. Gen. Intern. Med. 2015, 30, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Agénor, M.; Peitzmeier, S.M.; Bernstein, I.M.; McDowell, M.; Alizaga, N.M.; Reisner, S.L.; Pardee, D.J.; Potter, J. Perceptions of cervical cancer risk and screening among transmasculine individuals: Patient and provider perspectives. Cult. Heal. Sex. 2016, 18, 1192–1206. [Google Scholar] [CrossRef] [PubMed]
- Nilyanimit, P.; Chansaenroj, J.; Karalak, A.; Laowahutanont, P.; Junyangdikul, P.; Poovorawan, Y. Comparison of human papillomavirus (HPV) detection in urine and cervical swab samples using the HPV GenoArray Diagnostic assay. PeerJ 2017, 5, e3910. [Google Scholar] [CrossRef] [PubMed]
- D’Hauwers, K.W.; Tjalma, W.A. Screening for human papillomavirus: Is urine useful? Indian J. Cancer 2009, 46, 190. [Google Scholar] [CrossRef]
- Cuzick, J.; Cadman, L.; Ahmad, A.S.; Ho, L.; Terry, G.; Kleeman, M.; Lyons, D.; Austin, J.; Stoler, M.H.; Vibat, C.R.T.; et al. Performance and Diagnostic Accuracy of a Urine-Based Human Papillomavirus Assay in a Referral PopulationPredictors 4 Substudy: HPV Testing from Urine. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1053–1059. [Google Scholar] [CrossRef]
- Tabaac, A.R.; Sutter, M.E.; Wall, C.S.; Baker, K.E. Gender identity disparities in cancer screening behaviors. Am. J. Prev. Med. 2018, 54, 385–393. [Google Scholar] [CrossRef]
- Vorsters, A.; Bergh, J.V.D.; Micalessi, I.; Biesmans, S.; Bogers, J.-P.; Henß, A.; De Coster, I.; Ieven, M.; Van Damme, P. Optimization of HPV DNA detection in urine by improving collection, storage, and extraction. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2005–2014. [Google Scholar] [CrossRef]
- Enerly, E.; Olofsson, C.; Nygård, M. Monitoring human papillomavirus prevalence in urine samples: A review. Clin. Epidemiol. 2013, 5, 67. [Google Scholar] [CrossRef]
- Sundström, K.; Lamin, H.; Dillner, J. Validation of the cobas 6800 human papillomavirus test in primary cervical screening. PLoS ONE 2021, 16, e0247291. [Google Scholar] [CrossRef]
- Lloveras, B.; Gomez, S.; Alameda, F.; Bellosillo, B.; Mojal, S.; Muset, M.; Parra, M.; Palomares, J.C.; Serrano, S. HPV testing by cobas HPV test in a population from Catalonia. PLoS ONE 2013, 8, e58153. [Google Scholar] [CrossRef]
- Pathak, N.; Dodds, J.; Zamora, J.; Khan, K.S. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: Systematic review and meta-analysis. BMJ 2014, 349, g5264. [Google Scholar] [CrossRef]
- Burroni, E.; Bonanni, P.; Sani, C.; Lastrucci, V.; Carozzi, F. Human papillomavirus prevalence in paired urine and cervical samples in women invited for cervical cancer screening. J. Med Virol. 2015, 87, 508–515. [Google Scholar] [CrossRef]
- Hagihara, M.; Yamagishi, Y.; Izumi, K.; Miyazaki, N.; Suzuki, T.; Kato, H.; Nishiyama, N.; Koizumi, Y.; Suematsu, H.; Mikamo, H. Comparison of initial stream urine samples and cervical samples for detection of human papillomavirus. J. Infect. Chemother. 2016, 22, 559–562. [Google Scholar] [CrossRef]
- Sahasrabuddhe, V.V.; Gravitt, P.E.; Dunn, S.T.; Brown, D.; Allen, R.A.; Eby, Y.J.; Smith, K.; Zuna, R.E.; Zhang, R.R.; Gold, M.A.; et al. Comparison of human papillomavirus detections in urine, vulvar, and cervical samples from women attending a colposcopy clinic. J. Clin. Microbiol. 2014, 52, 187–192. [Google Scholar] [CrossRef]
- Rao, A.; Young, S.; Erlich, H.; Boyle, S.; Krevolin, M.; Sun, R.; Apple, R.; Behrens, C. Development and characterization of the cobas human papillomavirus test. J. Clin. Microbiol. 2013, 51, 1478–1484. [Google Scholar] [CrossRef]
- Eklund, C.; Zhou, T.; Dillner, J. Global proficiency study of human papillomavirus genotyping. J. Clin. Microbiol. 2010, 48, 4147–4155. [Google Scholar] [CrossRef]
- Estrade, C.; Sahli, R. Updating the PGMY primers and probes for improved detection of HPV68a: Validation of version 2 of the PGMY-CHUV assay. J. Clin. Microbiol. 2014, 52, 4033–4035. [Google Scholar] [CrossRef]
- Saville, M.; Sultana, F.; Malloy, M.J.; Velentzis, L.S.; Caruana, M.; Ip, E.L.O.; Keung, M.H.T.; Canfell, K.; Brotherton, J.M.L.; Hawkes, D. Clinical Validation of the cobas HPV Test on the cobas 6800 System for the Purpose of Cervical Screening. J. Clin. Microbiol. 2019, 57, e01239-18. [Google Scholar] [CrossRef]
- Meijer, C.J.; Berkhof, J.; Castle, P.E.; Hesselink, A.T.; Franco, E.L.; Ronco, G.; Arbyn, M.; Bosch, F.X.; Cuzick, J.; Dillner, J.; et al. Guidelines for human papillomavirus DNA test requirements for primary cervical cancer screening in women 30 years and older. Int. J. Cancer 2009, 124, 516–520. [Google Scholar] [CrossRef]
- Xavier, S.D.; Castro, T.M.P.P.G.; Filho, I.B.; de Carvalho, J.M.; Framil, V.M.D.S.; Syrjänen, K.J. Prevalence of human papillomavirus (HPV) DNA in oral mucosa of men with anogenital HPV infection. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, 732–737. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Tortolero-Luna, G.; Ferrer, E.; Burchell, A.N.; de Sanjose, S.; Kjaer, S.K.; Muñoz, N.; Schiffman, M.; Bosch, F.X. Epidemiology of human papillomavirus infection in men, cancers other than cervical and benign conditions. Vaccine 2008, 26 (Suppl. 10), K17–K28. [Google Scholar] [CrossRef] [PubMed]
| Total Samples/Calls | HPV 16 | HPV 18 | HPV 68 |
|---|---|---|---|
| 120 | 120 | 90 | |
| True Positive | 84 | 85 | 54 |
| False Positive | 0 | 0 | 0 |
| True Negative | 30 | 30 | 30 |
| False Negative | 6 | 5 | 6 |
| Estimated Sensitivity | =100% × TP/(TP + FN) | =100% × TP/(TP + FN) | =100% × TP/(TP + FN) |
| 93% | 94% | 90% | |
| Estimated Specificity | 100% × TN/(FP + TN) | 100% × TN/(FP + TN) | 100% × TN/(FP + TN) |
| 100% | 100% | 100% | |
| Overall Percent Agreement | =100% × (TP + TN)/Total | =100% × (TP + TN)/Total | =100% × (TP + TN)/Total |
| 95% | 95% | 93% | |
| Accuracy | 95% | 95% | 93% |
| Clinical Samples | Positive | Negative | Invalid | |
|---|---|---|---|---|
| Male | 110 | 2 | 108 | 0 |
| Female | 63 | 7 | 56 | 0 |
| Total Samples | 173 | 9 | 164 | 0 |
| HPV16 | HPV18 | |||||||||||||
| Samples | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
| Sample 1 | 30.09 | 31.49 | 32.05 | 32.72 | IM | 34.58 | 34.09 | 28.49 | 28.9 | 29.35 | 29.78 | IM | 30.99 | 31.16 |
| Sample 2 | 30.39 | 31.45 | 31.74 | 32.56 | IM | 33.99 | 33.56 | 28.86 | 29.21 | 29.43 | 29.64 | IM | 29.93 | 30.48 |
| Sample 3 | 30.24 | 31.41 | 31.92 | 32.74 | IM | 34.82 | 33.64 | 28.68 | 28.77 | 29.33 | 29.76 | IM | 30.66 | 30.89 |
| Sample 4 | 30.56 | 31.87 | 31.74 | 32.67 | IM | 34.7 | 33.63 | 28.72 | 29.07 | 29.31 | 29.97 | IM | 30.41 | 30.59 |
| Sample 5 | 30.46 | 31.49 | 32.09 | 32.74 | IM | 34.79 | 33.73 | 28.77 | 29.3 | 29.4 | 29.77 | IM | 30.95 | 30.54 |
| Sample 6 | 30.28 | 31.43 | 31.88 | 32.79 | 33.44 | 34.37 | 33.73 | 28.74 | 28.89 | 29.34 | 29.98 | 30.61 | 30.78 | 30.8 |
| Sample 7 | 30.33 | 31.1 | 31.84 | 32.71 | 33.26 | 35.13 | 33.79 | 28.66 | 28.46 | 29.35 | 29.94 | 30.15 | 30.71 | 30.84 |
| Sample 8 | 30.12 | 31.42 | 31.84 | 32.59 | 33.47 | 34.3 | 33.73 | 28.8 | 29.1 | 29.14 | 30.12 | 30.94 | 30.64 | 30.63 |
| Sample 9 | 30.29 | 31.28 | 31.69 | 32.47 | 33.05 | 34.84 | 34.1 | 28.87 | 28.7 | 29.17 | 29.57 | 30.23 | 30.9 | 31.12 |
| Sample 10 | 30.79 | 31.37 | 31.61 | 32.53 | 32.96 | 34.83 | 33.42 | 29.14 | 29.18 | 29.1 | 29.86 | 30.21 | 31.01 | 30.6 |
| Sample 11 | 30.32 | 31.44 | 31.83 | 32.69 | 33.43 | 34.99 | 33.73 | 28.49 | 28.9 | 29.05 | 29.69 | 29.97 | 30.49 | 30.85 |
| Sample 12 | 30.38 | 31.74 | 31.68 | 32.47 | 33.51 | 35.08 | 33.54 | 28.72 | 29.34 | 29.27 | 29.49 | 30.36 | 30.88 | 31.18 |
| Sample 13 | 30.28 | 31.17 | 31.69 | 32.55 | 33.43 | 34.99 | 33.71 | 28.76 | 28.81 | 29.19 | 29.51 | 30.97 | 30.88 | 30.83 |
| Sample 14 | 30.31 | 31.4 | 31.64 | 32.36 | 33.35 | 34.84 | 33.91 | 28.66 | 28.9 | 29.18 | 29.68 | 30.47 | 30.3 | 30.69 |
| Sample 15 | 30.16 | 31.53 | 31.69 | 32.45 | 33.25 | 34.9 | 33.63 | 28.51 | 29.1 | 29.19 | 29.62 | 30.09 | 30.87 | 30.58 |
| Sample 16 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Sample 17 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Sample 18 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Sample 19 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Sample 20 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| Average | 30.33 | 31.43 | 31.8 | 32.6 | 33.32 | 34.74 | 33.73 | 28.72 | 28.98 | 29.25 | 29.76 | 30.4 | 30.69 | 30.78 |
| HPV68 | β globulin | |||||||||||||
| Samples | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 |
| Sample 1 | 29.96 | 30.44 | 30.99 | 31.47 | IM | 32.63 | 32.72 | 30 | 31.21 | 31.13 | 31.58 | N/A | N/A | 32 |
| Sample 2 | 30.08 | 30.76 | 30.98 | 31.42 | IM | 31.56 | 32.22 | 30.09 | 31.29 | 30.77 | 31.56 | N/A | 32.7 | 31.41 |
| Sample 3 | 29.9 | 30.24 | 31.02 | 31.58 | IM | 32.65 | 32.45 | 29.73 | 30.98 | 30.72 | 31.33 | N/A | 33.8 | 32.07 |
| Sample 4 | 30.29 | 30.67 | 30.81 | 31.68 | IM | 32.51 | 32.22 | 30.05 | 31.54 | 30.78 | 31.46 | N/A | 33.26 | 31.85 |
| Sample 5 | 30.29 | 30.7 | 31.15 | 31.46 | IM | 32.76 | 32.32 | 30.69 | 31.15 | 30.78 | 31.1 | N/A | 33.94 | 31.66 |
| Sample 6 | 30.21 | 30.46 | 31.01 | 31.64 | 32.28 | 32.31 | 32.3 | 30.08 | 31.27 | 31.05 | 31.63 | 31.97 | 33.61 | 31.87 |
| Sample 7 | 30.05 | 30.14 | 31.13 | 31.65 | 32.05 | 32.74 | 32.19 | 29.68 | 30.61 | 31.32 | 31.25 | 31.59 | 33.82 | 32.27 |
| Sample 8 | 29.88 | 30.68 | 30.81 | 31.63 | 32.36 | 32.55 | 32.4 | 29.55 | 30.79 | 30.51 | 31.18 | 32.03 | 33.42 | 32.35 |
| Sample 9 | 30.07 | 30.28 | 30.99 | 31.32 | 32.07 | 32.72 | 32.7 | 29.88 | 30.32 | 30.62 | 31.24 | 31.77 | N/A | 32.73 |
| Sample 10 | 30.44 | 30.35 | 30.63 | 31.39 | 31.89 | 32.72 | 32.46 | 30.46 | 29.18 | 30.58 | 31.41 | 31.49 | N/A | 31.66 |
| Sample 11 | 30.03 | 30.52 | 31.1 | 31.44 | 31.85 | 32.39 | 32.34 | 30.12 | 31 | 30.73 | 31.81 | 31.86 | 33.7 | 32.54 |
| Sample 12 | 30.22 | 30.8 | 30.88 | 31.41 | 32.27 | 32.65 | 32.22 | 30.29 | 31.84 | 30.39 | 31.75 | 32.47 | 33.77 | 31.75 |
| Sample 13 | 30.14 | 30.64 | 30.87 | 31.47 | 32.04 | 32.64 | 32.3 | 29.92 | 31.39 | 30.56 | 31.35 | 32.34 | 33.7 | 31.86 |
| Sample 14 | 29.98 | 30.31 | 30.57 | 31.26 | 32.3 | 32.57 | 32.52 | 29.64 | 31.13 | 30.55 | 31.6 | 32.47 | 33.59 | 32.09 |
| Sample 15 | 29.88 | 30.58 | 30.97 | 31.39 | 31.99 | 32.7 | 31.66 | 29.36 | 30.83 | 31.11 | 31.56 | 31.48 | N/A | 32.16 |
| Sample 16 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 30.79 | 31.84 | 32.02 | 31.51 | 32.1 | 31.27 | 31.5 |
| Sample 17 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 31.65 | 31.52 | 31.65 | 31.09 | 32.7 | 31 | 32.18 |
| Sample 18 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 31.77 | 31.2 | 31.23 | 31.51 | 32.3 | 31.82 | 32 |
| Sample 19 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 31.28 | 31.09 | 32.16 | 29.7 | 32.12 | 31.72 | 31.72 |
| Sample 20 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 30.93 | 31.82 | 31.23 | 32.46 | 32.03 | 31.72 | 31.3 |
| Average | 30.09 | 30.5 | 30.92 | 31.48 | 32.11 | 32.54 | 32.33 | 29.96 | 30.97 | 30.77 | 31.4 | 31.9 | 33.57 | 32 |
| HPV16 | HPV18 | HPV68 | ||||
|---|---|---|---|---|---|---|
| Sample Type | ThinPrep | Urine | ThinPrep | Urine | ThinPrep | Urine |
| Total Samples/Calls | 30 | 120 | 30 | 120 | 30 | 90 |
| True Positive | 30 | 84 | 30 | 85 | 30 | 54 |
| False Positive | 0 | 0 | 0 | 0 | 0 | 0 |
| True Negative | 30 | 30 | 30 | 30 | 30 | 30 |
| False Negative | 0 | 6 | 0 | 5 | 0 | 6 |
| Sensitivity = 100% × TP/(TP + FN) | 100% | 93% | 100% | 94% | 100% | 90% |
| Specificity = 100% × TN/(FP + TN) | 100% | 100% | 100% | 100% | 100% | 100% |
| Accuracy = 100% × (TP + TN)/Total | 100% | 95% | 100% | 95% | 100% | 93% |
| Overall Percent Agreement | 100% | 95% | 100% | 95% | 100% | 93% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajjar, B.J.; Raheel, U.; Manina, R.; Simpson, J.; Irfan, M.; Waheed, Y. Clinical Performance of Cobas 6800 for the Detection of High-Risk Human Papillomavirus in Urine Samples. Vaccines 2023, 11, 1071. https://doi.org/10.3390/vaccines11061071
Hajjar BJ, Raheel U, Manina R, Simpson J, Irfan M, Waheed Y. Clinical Performance of Cobas 6800 for the Detection of High-Risk Human Papillomavirus in Urine Samples. Vaccines. 2023; 11(6):1071. https://doi.org/10.3390/vaccines11061071
Chicago/Turabian StyleHajjar, Brian Joseph, Ummar Raheel, Rachel Manina, Jovanie Simpson, Muhammad Irfan, and Yasir Waheed. 2023. "Clinical Performance of Cobas 6800 for the Detection of High-Risk Human Papillomavirus in Urine Samples" Vaccines 11, no. 6: 1071. https://doi.org/10.3390/vaccines11061071
APA StyleHajjar, B. J., Raheel, U., Manina, R., Simpson, J., Irfan, M., & Waheed, Y. (2023). Clinical Performance of Cobas 6800 for the Detection of High-Risk Human Papillomavirus in Urine Samples. Vaccines, 11(6), 1071. https://doi.org/10.3390/vaccines11061071







