Symptom-Specific Hospital Contacts in 12–18-Year-Olds Vaccinated against COVID-19: A Danish Register-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Study Design and Population
2.3. Study Size, Sampling Strategy, and Follow-Up Periods
2.4. Data Sources
2.5. Variables
2.6. Statistical Approach
3. Results
3.1. Characteristics of the Study Population
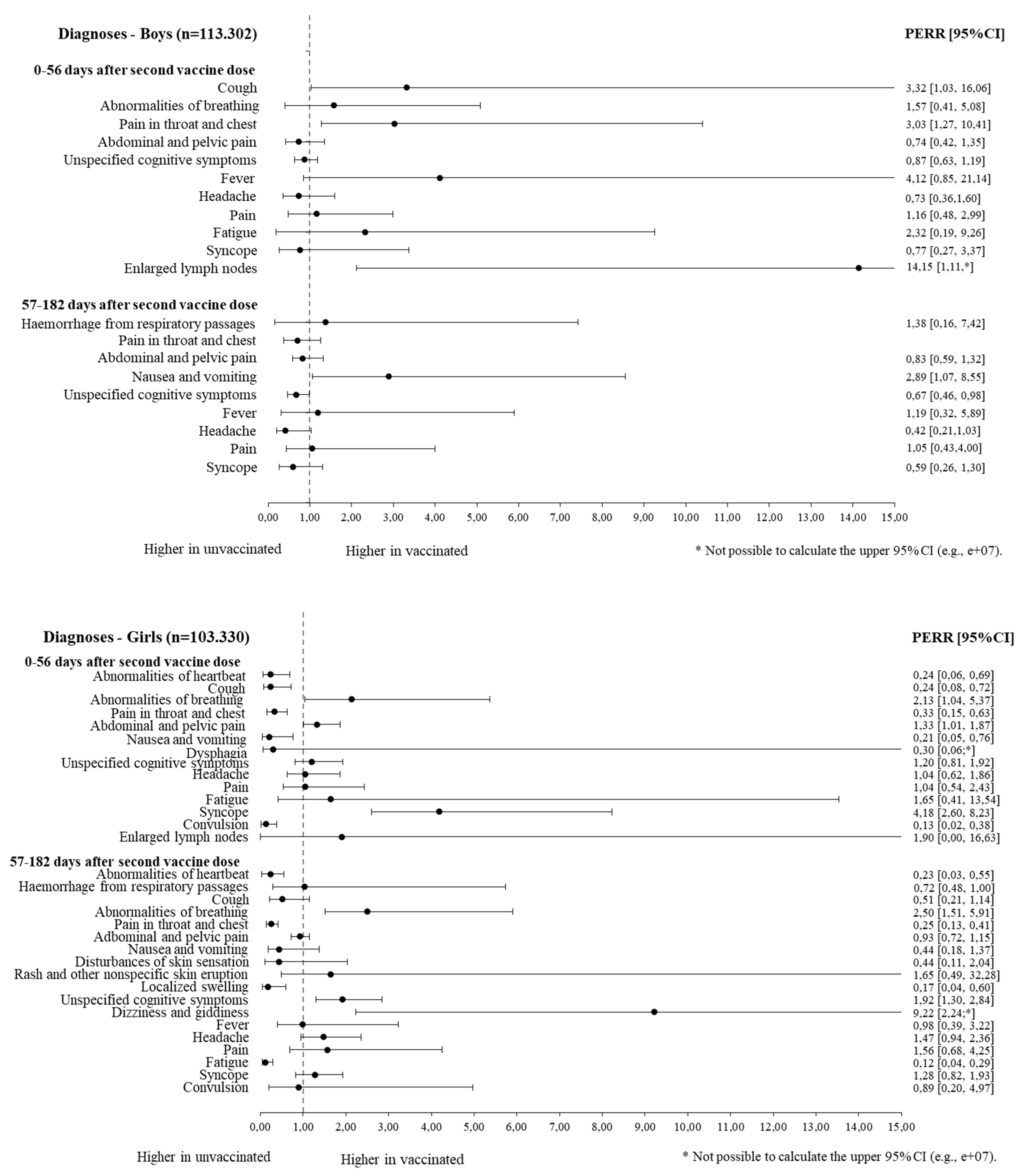
3.2. Symptom-Specific Hospital Contacts 0–56 Days after Second Vaccine Dose (Short Follow-Up)
3.3. Symptom-Specific Hospital Contacts 57–182 Days after Second Vaccine Dose (Long Follow-Up)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frenck, R.W.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef]
- Tano, E.; San Martin, S.; Girgis, S.; Martinez-Fernandez, Y.; Sanchez Vegas, C. Perimyocarditis in Adolescents after Pfizer-BioNTech COVID-19 Vaccine. J. Pediatric. Infect. Dis. Soc. 2021, 10, 962–966. [Google Scholar] [CrossRef]
- Dionne, A.; Sperotto, F.; Chamberlain, S.; Baker, A.L.; Powell, A.J.; Prakash, A.; Castellanos, D.A.; Saleeb, S.F.; de Ferranti, S.D.; Newburger, J.W.; et al. Association of Myocarditis with BNT162b2 Messenger RNA COVID-19 Vaccine in a Case Series of Children. JAMA Cardiol. 2021, 6, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Malamud, E.; Otallah, S.I.; Caress, J.B.; Lapid, D.J. Guillain-Barré Syndrome After COVID-19 Vaccination in an Adolescent. Pediatr. Neurol. 2022, 126, 9–10. [Google Scholar] [CrossRef]
- Yousaf, A.R.; Cortese, M.M.; Taylor, A.W.; Broder, K.R.; Oster, M.E.; Wong, J.M.; Guh, A.Y.; McCormick, D.W.; Kamidani, S.; Schlaudecker, E.P.; et al. Reported cases of multisystem inflammatory syndrome in children aged 12–20 years in the USA who received a COVID-19 vaccine, December, 2020, through August, 2021: A surveillance investigation. Lancet Child. Adolesc. Health 2022, 6, 303. [Google Scholar] [CrossRef]
- García-Vera, C.; Castejón-Ramírez, S.; Miranda, L.E.; Abadía, R.H.; García Ventura, M.; García Ventura, E.; García Ventura, P.; Baeta Ruiz, Á.; Mengual Gil, J.M. COVID-19 in children: Clinical and epidemiological spectrum in the community. Eur. J. Pediatr. 2022, 1, 3. [Google Scholar] [CrossRef]
- Tsabouri, S.; Makis, A.; Kosmeri, C.; Siomou, E. Risk Factors for Severity in Children with Coronavirus Disease 2019: A Comprehensive Literature Review. Pediatr. Clin. N. Am. 2021, 68, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.K.; Dam Nielsen, S.; Nygaard, U.; Bundgaard, H.; Palm, P.; Rotvig, C.; Christensen, A.V. Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): A national, cross-sectional study. Lancet Child Adolesc. Health 2022, 6, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.; Pinto Pereira, S.M.; Shafran, R.; de Stavola, B.L.; Rojas, N.; McOwat, K.; Simmons, R.; Zavala, M.; O’mahoney, L.; Chalder, T.; et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): A national matched cohort study. Lancet Child Adolesc. Health 2022, 6, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Bussières, E.L.; Malboeuf-Hurtubise, C.; Meilleur, A.; Mastine, T.; Hérault, E.; Chadi, N.; Montreuil, M.; Généreux, M.; Camden, C.; Team, P.-C.; et al. Consequences of the COVID-19 Pandemic on Children’s Mental Health: A Meta-Analysis. Front. Psychiatry 2021, 12, 691659. [Google Scholar] [CrossRef]
- Mckinnon, B.; Quach, C.; Dubé, È.; Tuong, C.; Zinszer, K. Social inequalities in COVID-19 vaccine acceptance and uptake for children and adolescents in Montreal, Canada. Vaccine 2020, 39, 7140–7145. [Google Scholar] [CrossRef]
- Robinson, E.; Jones, A.; Lesser, I.; Daly, M. International estimates of intended uptake and refusal of COVID-19 vaccines: A rapid systematic review and meta-analysis of large nationally representative samples. Vaccine 2021, 39, 2024–2034. [Google Scholar] [CrossRef] [PubMed]
- Heide-Jørgensen, U.; Adelborg, K.; Kahlert, J.; Sørensen, H.T.; Pedersen, L. Sampling strategies for selecting general population comparison cohorts. Clin. Epidemiol. 2018, 10, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Grove Krause, T.; Jakobsen, S.; Haarh, M.; Mølbak, K. The Danish vaccination register. Eurosurveillance 2012, 17, 2. [Google Scholar] [CrossRef]
- Voldstedlund, M.; Haarh, M.; Mølbak, K. The Danish microbiology database (MIBA) 2010 to 2013. Eurosurveillance 2014, 19, 20667. [Google Scholar] [CrossRef]
- Schmidt, M.; Schmidt, S.A.J.; Sandegaard, J.L.; Ehrenstein, V.; Pedersen, L.; Sørensen, H.T. The Danish National patient registry: A review of content, data quality, and research potential. Clin. Epidemiol. 2015, 7, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Pro Medicin. Copenhagen: Dansk Lægemiddel Information A/S (Danish Medicine Information). Available online: https://pro.medicin.dk/ (accessed on 1 December 2022).
- Lund, L.C.; Hallas, J.; Nielsen, H.; Koch, A.; Mogensen, S.H.; Brun, N.C.; Christiansen, C.F.; Thomsen, R.W.; Pottegård, A. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: A Danish population-based cohort study. Lancet Infect. Dis. 2021, 21, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Kildegaard, H.; Lund, L.C.; Højlund, M.; Stensballe, L.G.; Pottegård, A. Risk of adverse events after COVID-19 in Danish children and adolescents and effectiveness of BNT162b2 in adolescents: Cohort study. BMJ 2022, 377, e068898. [Google Scholar] [CrossRef]
- Weiner, M.G.; Xie, D.; Tannen, R.L. Replication of the Scandinavian Simvastatin Survival Study using a primary care medical record database prompted exploration of a new method to address unmeasured confounding. Pharmacoepidemiol. Drug Saf. 2008, 17, 661–670. [Google Scholar] [CrossRef]
- Uddin, M.J.; Groenwold, R.H.H.; van Staa, T.P.; de Boer, A.; Belitser, S.V.; Hoes, A.W.; Roes, K.C.; Klungel, O.H. Performance of prior event rate ratio adjustment method in pharmacoepidemiology: A simulation study. Pharmacoepidemiol. Drug Saf. 2015, 24, 468–477. [Google Scholar] [CrossRef]
- Haukoos, J.S.; Lewis, R.J. Advanced statistics: Bootstrapping confidence intervals for statistics with “difficult” distributions. Acad. Emerg. Med. 2005, 12, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.K.; Wallach-Kildemoes, H.; Rasmussen, L.R.; Nygaard, U.; Bundgaard, H.; Petersen, M.N.S.; Hammer, C.B.; Ersbøll, A.K.; Thygesen, L.C.; Nielsen, S.D. Short- and Long-Term Self-Reported Symptoms in Adolescents Aged 12–19 Years after Vaccination against SARS-CoV-2 Compared to Adolescents Not Vaccinated-A Danish Retrospective Cohort Study. Vaccines 2022, 10, 1863. [Google Scholar] [CrossRef] [PubMed]
- Flora, J.; Khan, W.; Jin, J.; Jin, D.; Hussain, A.; Dajani, K.; Khan, B. Usefulness of Vaccine Adverse Event Reporting System for Machine-Learning Based Vaccine Research: A Case Study for COVID-19 Vaccines. Int. J. Mol. Sci. 2022, 23, 8235. [Google Scholar] [CrossRef] [PubMed]
- Ljung, R.; Xu, Y.Y.; Sundström, A.; Leach, S.; Hallberg, E.; Bygdell, M.; Larsson, M.; Arthurson, V.; Gisslén, M.; Gedeborg, R.; et al. Association between SARS-CoV-2 vaccination and healthcare contacts for menstrual disturbance and bleeding in women before and after menopause: Nationwide, register based cohort study. BMJ 2023, 381, e074778. [Google Scholar] [CrossRef]
- Hause, A.M.; Gee, J.; Baggs, J.; Abara, W.E.; Marquez, P.; Thompson, D.; Su, J.R.; Licata, C.; Rosenblum, H.G.; Myers, T.R.; et al. COVID-19 Vaccine Safety in Adolescents Aged 12–17 Years—United States, December 14, 2020–July 16, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1053–1058. [Google Scholar] [CrossRef]
- Petra, K.; Bettina, S. Gender and Health in Adolescence; WHO: Geneva, Switzerland, 1999; Available online: https://apps.who.int/iris/handle/10665/108178 (accessed on 23 May 2023).
- Mori, M.; Yokoyama, A.; Shichida, A.; Sasuga, K.; Maekawa, T.; Moriyama, T. Impact of Sex and Age on mRNA COVID-19 Vaccine-Related Side Effects in Japan. Microbiol. Spectr. 2022, 10, e01309-22. [Google Scholar] [CrossRef] [PubMed]
- Mansanguan, S.; Charunwatthana, P.; Piyaphanee, W.; Dechkhajorn, W.; Poolcharoen, A.; Mansanguan, C. Cardiovascular Effects of the BNT162b2 mRNA COVID-19 Vaccine in Adolescents. Trop. Med. Infect. Dis. 2022, 7, 196. [Google Scholar] [CrossRef]
- Nygaard, U.; Holm, M.; Bohnstedt, C.; Chai, Q.; Schmidt, L.S.; Hartling, U.B.; Petersen, J.J.H.; Thaarup, J.; Bjerre, J.; Vejlstrup, N.G.; et al. Population-based Incidence of Myopericarditis after COVID-19 Vaccination in Danish Adolescents. Pediatr. Infect. Dis. J. 2022, 41, E25–E28. [Google Scholar] [CrossRef]
- Olusanya, O.A.; Bednarczyk, R.A.; Davis, R.L.; Shaban-Nejad, A. Addressing Parental Vaccine Hesitancy and Other Barriers to Childhood/Adolescent Vaccination Uptake During the Coronavirus (COVID-19) Pandemic. Front. Immunol. 2021, 12, 663074. [Google Scholar] [CrossRef]

| Second Dose of the Vaccine a | |||
|---|---|---|---|
| Vaccinated | Unvaccinated According to Follow-Up | ||
| 0–56 Days | 57–182 Days | ||
| All n (%) b | (n = 105,316) | (n = 105,316) | (n = 105,316) |
| Unique individuals, n (%) c | 105,316 (100.0%) | 21,833 (20.7%) | 14,007 (13.3%) |
| Sex (girl, %) | 51,665 (49.1%) | 51,665 (49.1%) | 51,665 (49.1%) |
| Age (mean, SD) | 15.5 (1.9) | 15.4 (2.1) | 15.4 (2.1) |
| 12–15 years (%) | 46,733 (44.4%) | 46,733 (44.4%) | 46,733 (44.4%) |
| 16–18 years (%) | 58,583 (55.6%) | 58,583 (55.6%) | 58,583 (55.6%) |
| Prevalent health condition, n/yes (%) d | |||
| Any of the listed somatic diseases | 13,971 (13.3%) | 12,324 (11.7%) | 12,197 (11.6%) |
| Any registered psychiatric diagnosis | 8377 (8.0%) | 10,284 (9.8%) | 10,468 (9.9%) |
| Parents’ socioeconomic position | |||
| Parents’ highest formal education, n (%) | |||
| Basic education | 4737 (4.5%) | 15,230 (14.5%) | 17,631 (16.7%) |
| High school and vocational training | 43,245 (41.1%) | 50,777 (48.2%) | 52,260 (49.6%) |
| Higher education | 57,334 (54.4%) | 39,309 (37.3%) | 35,425 (33.6%) |
| Annual family income, n (%) | |||
| Low (1. tertile) | 27,615 (26.2%) | 54,884 (52.1%) | 59,961 (56.9%) |
| Middle (2. tertile) | 33,168 (31.5%) | 29,734 (28.2%) | 28,450 (27.0%) |
| High (3. tertile) | 43,984 (41.8%) | 19,715 (18.7%) | 15,901 (15.1%) |
| Maternal citizenship, n (%) | |||
| Danish | 97,754 (92.8%) | 82,037 (77.9%) | 78,905 (74.9%) |
| Other Western countries | 3089 (2.9%) | 5922 (5.6%) | 6778 (6.4%) |
| Non-Western countries | 4277 (4.1%) | 16,684 (15.8%) | 18,928 (18.0%) |
| Unknown | 196 (0.2%) | 673 (0.6%) | 705 (0.7%) |
| Girls | Boys | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unvaccinated (Rate) | Vaccinated (Rate) | Unvaccinated (Rate) | Vaccinated (Rate) | ||||||||||
| Before | After | Before | After | PERR Crude | PERR (95% CI) c | Before | After | Before | After | PERR Crude | PERR (95% CI) c | ||
| Short (0–56 days) | |||||||||||||
| Circulatory and respiratory symptoms | R00 Abnormal heartbeat | 1.4 | 5.9 | 2.3 | 1.9 | 0.20 | 0.24 (0.06–0.69) | - | - | - | - | - | - |
| R05 Cough | 0.9 | 4.5 | 2.1 | 2.7 | 0.26 | 0.24 (0.08–0.72) | 1.8 | 1.2 | 0.7 | 1.5 | 2.98 | 3.32 (1.03–16.06) | |
| R06 Abnormalities of breathing | 8.0 | 2.9 | 5.4 | 4.7 | 2.37 | 2.13 (1.04–5.37) | 2.5 | 3.8 | 2.0 | 3.2 | 1.03 | 1.57 (0.41–5.08) | |
| R07 Pain in the throat and chest | 2.3 | 10.9 | 3.4 | 5.9 | 0.37 | 0.33 (0.15–0.63) | 4.6 | 3.5 | 2.6 | 5.3 | 2.70 | 3.03 (1.27–10.41) | |
| Digestive system and abdomen | R10 Abdominal and pelvic pain | 54.88 | 35.52 | 44.17 | 37.73 | 1.32 | 1.33 (1.01–1.87) | 14.27 | 18.09 | 15.38 | 15.12 | 0.78 | 0.74 (0.42–1.35) |
| R11 Nausea and vomiting | 1.87 | 5.80 | 4.37 | 4.29 | 0.32 | 0.21 (0.05–0.76) | - | - | - | - | - | - | |
| R13 Dysphagia | 0.59 | 0.76 | 1.66 | 1.14 | 0.54 | 0.30 (0.06-#) | - | - | - | - | - | - | |
| Cognition, perception, emotional state, and behavior | R41.8 Other and unspecified symptoms and signs involving cognitive functions and awareness | 42.1 | 40.8 | 23.8 | 26.5 | 1.15 | 1.20 (0.81–1.92) | 37.4 | 38.4 | 22.1 | 20.8 | 0.92 | 0.87 (0.63–1.19) |
| General symptoms and signs | R50 Fever | - | - | - | - | - | - | 0.6 | 0.6 | 0.5 | 1.8 | 3.68 | 4.12 (0.85–21.14) |
| R51 Headache | 18.0 | 15.1 | 15.7 | 13.4 | 1.01 | 1.04 (0.62–1.86) | 6.1 | 9.7 | 5.9 | 6.0 | 0.63 | 0.73 (0.36–1.60) | |
| R52 Pain | 5.2 | 5.4 | 6.2 | 5.4 | 0.84 | 1.04 (0.54–2.43) | 3.7 | 2.8 | 2.9 | 2.3 | 1.03 | 1.16 (0.48–2.99) | |
| R53 Fatigue | 2.8 | 1.9 | 3.0 | 2.7 | 1.30 | 1.65 (0.41–13.54) | 0.7 | 1.0 | 2.1 | 3.5 | 1.27 | 2.32 (0.19–9.26) | |
| R55 Syncope | 12.6 | 4.7 | 7.0 | 10.7 | 4.12 | 4.18 (2.60–8.23) | 1.8 | 2.7 | 3.2 | 4.4 | 0.93 | 0.77 (0.27–3.37) | |
| R56 Convulsion | 0.5 | 2.7 | 1.7 | 1.0 | 0.12 | 0.13 (0.02–0.38) | - | - | - | - | - | - | |
| R59 Enlarged lymph nodes | 0.7 | 1.0 | 0.6 | 1.3 | 1.37 | 1.90 (0.00–16.63) | 3.0 | 0.7 | 0.3 | 1.3 | 18.18 | 14.15 (2.11-#) | |
| Long (57–182 days) | |||||||||||||
| Circulatory and respiratory symptoms | R00 Abnormal heartbeat | 0.5 | 3.5 | 2.3 | 3.1 | 0.22 | 0.23 (0.03–0.55) | - | - | - | - | - | - |
| R04 Hemorrhage from respiratory passages | 1.6 | 1.3 | 0.5 | 1.1 | 2.60 | 1.03 (0.29–5.74) | 1.0 | 1.2 | 0.9 | 1.1 | 0.96 | 1.38 (0.16–7.42) | |
| R05 Cough | 1.1 | 3.5 | 2.1 | 3.1 | 0.46 | 0.51 (0.21–1.14) | - | - | - | - | - | - | |
| R06 Abnormalities of breathing | 10.7 | 3.8 | 5.1 | 4.4 | 2.46 | 2.50 (1.51–5.91) | - | - | - | - | - | - | |
| R07 Pain in the throat and chest | 2.9 | 14.0 | 3.3 | 4.9 | 0.30 | 0.25 (0.13–0.41) | 2.7 | 8.5 | 2.4 | 3.9 | 0.51 | 0.71 (0.38–1.26) | |
| Digestive system and abdomen | R10 Abdominal and pelvic pain | 61.14 | 69.71 | 42.25 | 42.43 | 0.88 | 0.93 (0.72–1.15) | 16.52 | 23.37 | 15.53 | 15.40 | 0.70 | 0.83 (0.59–1.32) |
| R11 Nausea and vomiting | 5.04 | 10.12 | 4.26 | 4.86 | 0.57 | 0.44 (0.18–1.37) | 4.73 | 2.64 | 2.06 | 3.18 | 2.77 | 2.89 (1.07–8.55) | |
| Skin and subcutaneous tissue | R20 Disturbances of skin sensation | 0.53 | 0.89 | 0.70 | 0.73 | 0.61 | 0.44 (0.11–2.04) | - | - | - | - | - | - |
| R21 Rash and other nonspecific skin eruptions | 2.71 | 1.45 | 1.03 | 1.17 | 2.12 | 1.65 (0.49–32.28) | - | - | - | - | - | - | |
| R22 Localized swelling, mass, and lump of skin and subcutaneous tissue | 0.53 | 2.74 | 0.70 | 0.61 | 0.17 | 0.17 (0.04–0.60) | - | - | - | - | - | - | |
| Cognition, perception, emotional state, and behavior | R41.8 Other and unspecified symptoms and signs involving cognitive functions and awareness | 30.7 | 31.4 | 19.7 | 31.9 | 1.59 | 1.92 (1.30–2.84) | 38.7 | 53.6 | 21.4 | 22.5 | 0.76 | 0.67 (0.46–0.98) |
| R42 Dizziness and giddiness | 5.0 | 0.6 | 3.4 | 3.3 | 7.79 | 9.22 (2.24-#) | - | - | - | - | - | - | |
| General symptoms and signs | R50 Fever | 4.6 | 3.5 | 3.0 | 2.0 | 0.87 | 0.98 (0.39–3.22) | 1.0 | 0.8 | 2.2 | 2.3 | 1.47 | 1.19 (0.32–5.89) |
| R51 Headache | 26.8 | 15.8 | 15.2 | 12.9 | 1.43 | 1.47 (0.94–2.36) | 6.0 | 14.8 | 6.3 | 6.5 | 0.42 | 0.42 (0.21–1.03) | |
| R52 Pain | 7.4 | 5.3 | 5.5 | 4.8 | 1.23 | 1.56 (0.68–4.25) | 2.8 | 2.4 | 3.1 | 2.8 | 1.04 | 1.05 (0.43–4.00) | |
| R53 Fatigue | 0.9 | 6.0 | 1.7 | 1.3 | 0.11 | 0.12 (0.04–0.29) | - | - | - | - | - | - | |
| R55 Syncope | 11.6 | 12.6 | 7.1 | 8.3 | 1.07 | 1.28 (0.82–1.93) | 2.1 | 6.2 | 3.1 | 4.7 | 0.52 | 0.59 (0.26–1.30) | |
| R56 Convulsion | 0.5 | 0.6 | 1.4 | 1.7 | 0.90 | 0.89 (0.20–4.97) | - | - | - | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berg, S.K.; Wallach-Kildemoes, H.; Rasmussen, L.R.; Nygaard, U.; Birk, N.M.; Bundgaard, H.; Ersbøll, A.K.; Thygesen, L.C.; Nielsen, S.D.; Christensen, A.V. Symptom-Specific Hospital Contacts in 12–18-Year-Olds Vaccinated against COVID-19: A Danish Register-Based Cohort Study. Vaccines 2023, 11, 1049. https://doi.org/10.3390/vaccines11061049
Berg SK, Wallach-Kildemoes H, Rasmussen LR, Nygaard U, Birk NM, Bundgaard H, Ersbøll AK, Thygesen LC, Nielsen SD, Christensen AV. Symptom-Specific Hospital Contacts in 12–18-Year-Olds Vaccinated against COVID-19: A Danish Register-Based Cohort Study. Vaccines. 2023; 11(6):1049. https://doi.org/10.3390/vaccines11061049
Chicago/Turabian StyleBerg, Selina Kikkenborg, Helle Wallach-Kildemoes, Line Ryberg Rasmussen, Ulrikka Nygaard, Nina Marie Birk, Henning Bundgaard, Annette Kjær Ersbøll, Lau Caspar Thygesen, Susanne Dam Nielsen, and Anne Vinggaard Christensen. 2023. "Symptom-Specific Hospital Contacts in 12–18-Year-Olds Vaccinated against COVID-19: A Danish Register-Based Cohort Study" Vaccines 11, no. 6: 1049. https://doi.org/10.3390/vaccines11061049
APA StyleBerg, S. K., Wallach-Kildemoes, H., Rasmussen, L. R., Nygaard, U., Birk, N. M., Bundgaard, H., Ersbøll, A. K., Thygesen, L. C., Nielsen, S. D., & Christensen, A. V. (2023). Symptom-Specific Hospital Contacts in 12–18-Year-Olds Vaccinated against COVID-19: A Danish Register-Based Cohort Study. Vaccines, 11(6), 1049. https://doi.org/10.3390/vaccines11061049






