Safety and Immunogenicity of the Inactivated COVID-19 Vaccine Booster in People Living with HIV in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Recruitment of Cases
2.2. Data Collection
2.3. SARS-CoV-2 Antibody Measurement
2.4. Statistical Analysis
2.5. Vaccines
3. Results
3.1. Baseline Characteristics
3.2. Safety of Inactivated SARS-CoV-2 Booster Vaccines in PLWH
3.3. Changes in CD4+ T-Cell Counts
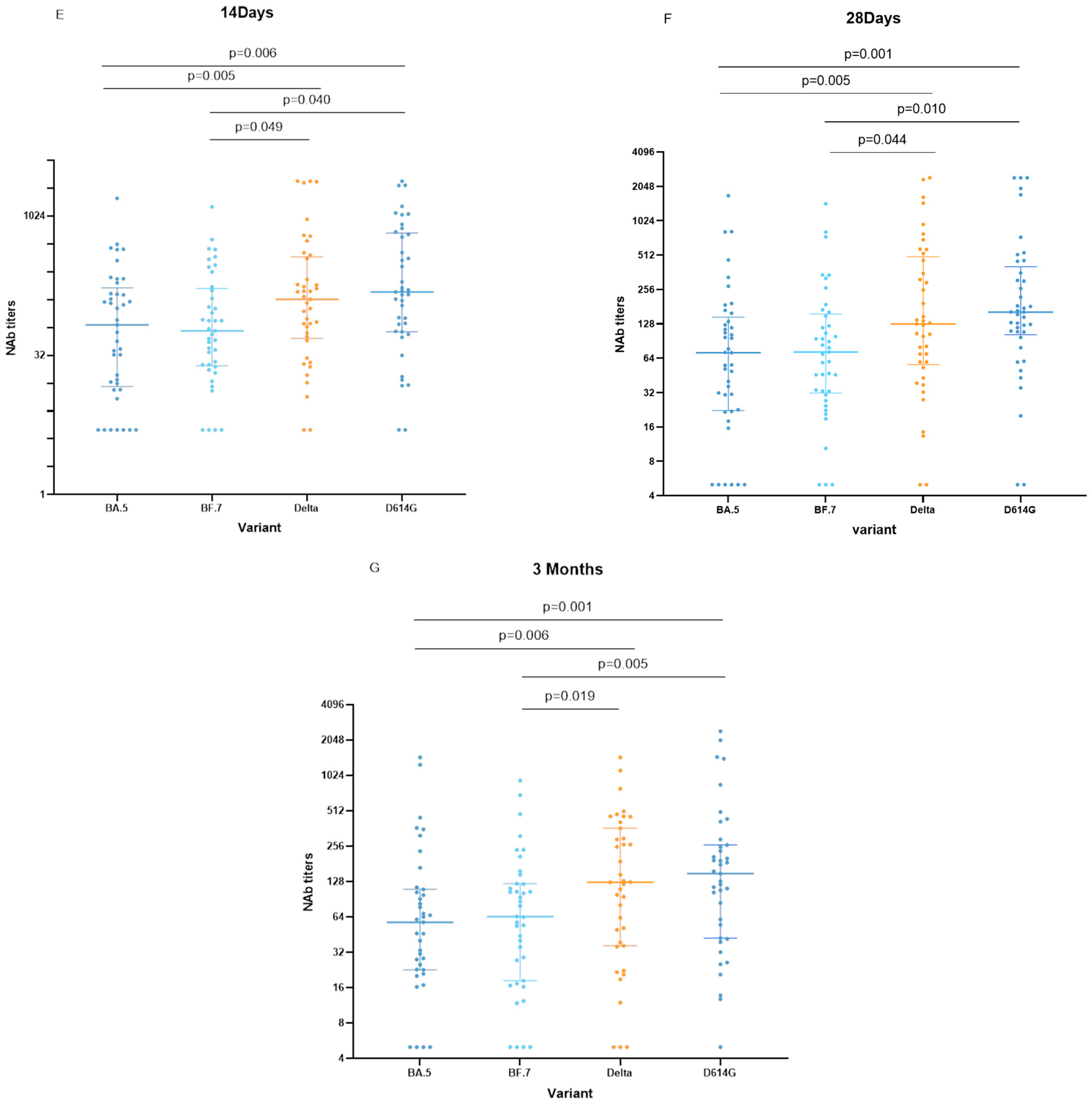
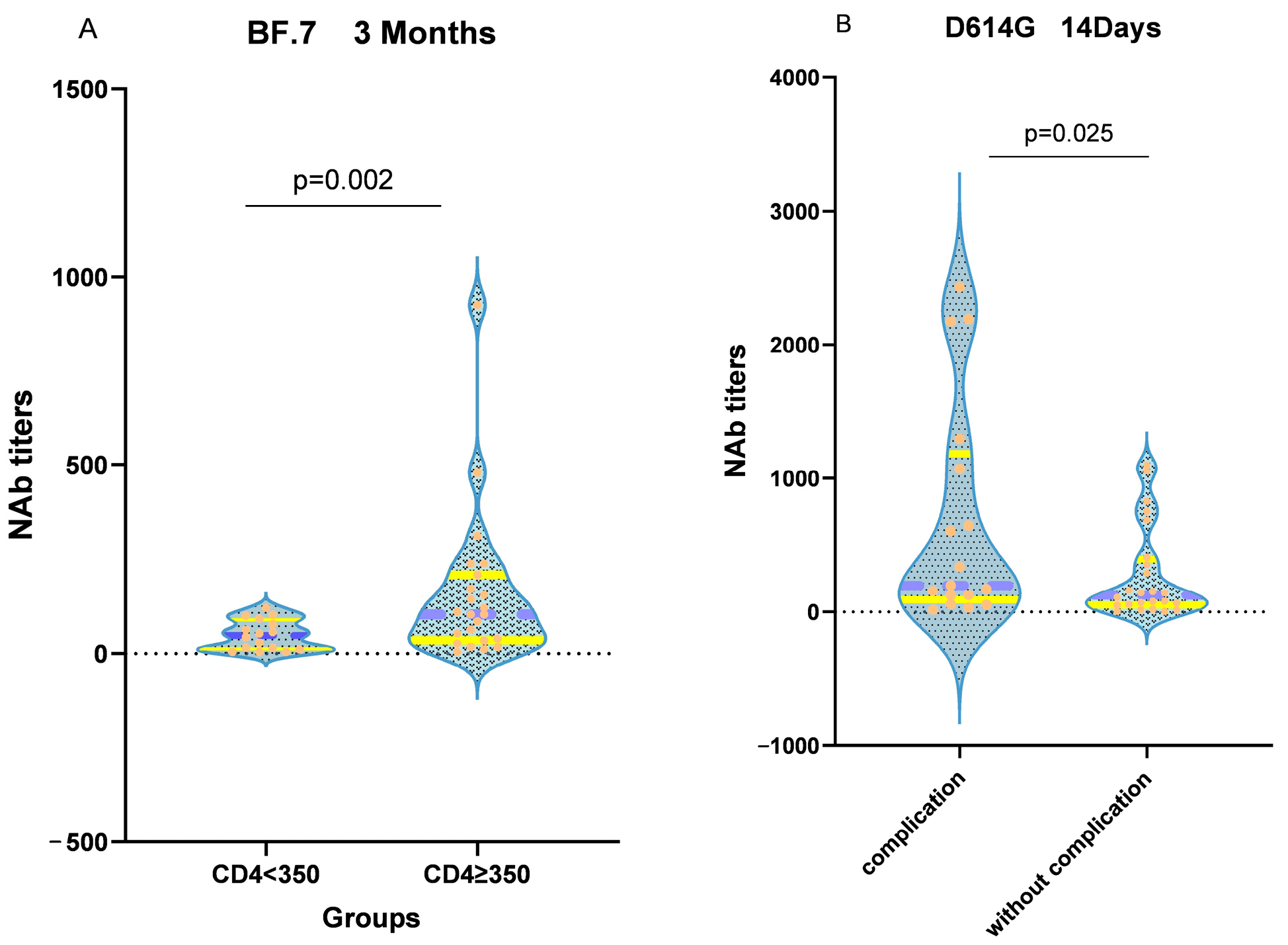
3.4. Neutralizing Activity of the Vaccine
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Geretti, A.M.; Stockdale, A.J.; Kelly, S.H.; Cevik, M.; Collins, S.; Waters, L.; Villa, G.; Docherty, A.; Harrison, E.M.; Turtle, L.; et al. Outcomes of Coronavirus Disease 2019 (COVID-19) Related Hospitalization among People with Human Immunodeficiency Virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): A Prospective Observational Study. Clin. Infect. Dis. 2021, 73, e2095–e2106. [Google Scholar] [CrossRef] [PubMed]
- Kleynhans, J.; Walaza, S.; Martinson, N.A.; Neti, M.; von Gottberg, A.; Bhiman, J.N.; Toi, D.; Amoako, D.G.; Buys, A.; Ndlangisa, K.; et al. Household Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 From Adult Index Cases with and without Human Immunodeficiency Virus in South Africa, 2020–2021: A Case-Ascertained, Prospective, Observational Household Transmission Study. Clin. Infect. Dis. 2022, 76, e71–e81. [Google Scholar] [CrossRef] [PubMed]
- Maponga, T.G.; Jeffries, M.; Tegally, H.; Sutherland, A.; Wilkinson, E.; Lessells, R.J.; Msomi, N.; van Zyl, G.; de Oliveira, T.; Preiser, W. Persistent Severe Acute Respiratory Syndrome Coronavirus 2 Infection with accumulation of mutations in a patient with poorly controlled Human Immunodeficiency Virus infection. Clin. Infect. Dis. 2023, 76, e522–e525. [Google Scholar] [CrossRef]
- Amit, S.; Regev-Yochay, G.; Afek, A.; Kreiss, Y.; Leshem, E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet 2021, 397, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Frater, J.; Ewer, K.J.; Ogbe, A.; Pace, M.; Adele, S.; Adland, E.; Alagaratnam, J.; Aley, P.K.; Ali, M.; Ansari, M.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: A single-arm substudy of a Phase 2/3 clinical trial. Lancet HIV 2021, 8, e474–e485. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hu, Y.; Xu, M.; Chen, Z.; Yang, W.; Jiang, Z.; Li, M.; Jin, H.; Cui, G.; Chen, P.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021, 21, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; Wu, C.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef]
- Guo, W.; Duan, K.; Zhang, Y.; Yuan, Z.; Zhang, Y.B.; Wang, Z.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18 years or older: A randomized, double-blind, placebo-controlled, phase 1/2 trial. EClinicalMedicine 2021, 38, 101010. [Google Scholar] [CrossRef]
- Schuster, D.J.; Karuna, S.; Brackett, C.; Wesley, M.; Li, S.S.; Eisel, N.; Tenney, D.; Hilliard, S.; Yates, N.L.; Heptinstall, J.R.; et al. HVTN 405/HPTN 1901 Study Team. Lower SARS-CoV-2-specific humoral immunity in PLWH-1 recovered from nonhospitalized COVID-19. JCI Insight 2022, 7, e158402. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, H.R.; Mwimanzi, F.; Cheung, P.K.; Sang, Y.; Yaseen, F.; Umviligihozo, G.; Kalikawe, R.; Speckmaier, S.; Moran-Garcia, N.; Datwani, S.; et al. People with Human Immunodeficiency Virus Receiving Suppressive Antiretroviral Therapy Show Typical Antibody Durability after Dual Coronavirus Disease 2019 Vaccination and Strong Third Dose Responses. J. Infect. Dis. 2023, 227, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yu, X.; Han, Y.; Fang, Q.; Shen, C.; Liu, H.; Wang, P.; Wang, Y. Safety and Immunogenicity of Inactivated COVID-19 Vaccines among PLWH in China. Infect. Drug Resist. 2022, 15, 2091–2100. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, M.; Lin, W.; Dong, W.; Xu, J. Evolutionary analysis of Omicron variant BF.7 and BA.5.2 pandemic in China. J. Biosaf. Biosecurity 2023, 5, 14–20. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, L.; Feng, Z.; Xu, H.; Li, F.; Shen, Y.; Zhang, D.; Liu, W.J.; Gao, G.F.; Wang, Q. Characterisation of SARS-CoV-2 variants in Beijing during 2022: An epidemiological and phylo-genetic analysis. Lancet 2023, 401, 664–672. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Kandi, V.; Sarangi, A.K.; Verma, S.; Tuli, H.S.; Chakraborty, S.; Chakraborty, C.; Dhama, K. The recently emerged BA.4 and BA.5 lineages of Omicron and their global health concerns amid the ongoing wave of COVID-19 pandemic—Correspondence. Int. J. Surg. 2022, 103, 106698. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Lundgreen, K.A.; Apostolidis, S.A.; Baxter, A.E.; Giles, J.R.; Mathew, D.; Pattekar, A.; Reynaldi, A.; Khoury, D.S.; et al. Efficient recall of Omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell 2022, 185, 1875–1887.e8. [Google Scholar] [CrossRef]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185, 2434–2451.e17. [Google Scholar] [CrossRef]
- Nie, J.; Li, Q.; Wu, J.; Zhao, C.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.; Qin, H.; Wang, M.; et al. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Li, Q.; Wu, J.; Zhao, C.; Hao, H.; Liu, H.; Zhang, L.; Nie, L.; Qin, H.; Wang, M.; et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat. Protoc. 2020, 15, 3699–3715. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Zhang, H.; Yao, P.; Xu, N.; Sun, Y.; Lu, H.; Xu, F.; Liao, Y.; Yang, J.; Mao, H.; et al. Immunogenicity and immune-persistence of the CoronaVac or Covilo inactivated COVID-19 Vaccine: A 6-month population-based cohort study. Front. Immunol. 2022, 13, 939311. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, J.; Wang, S.; Ye, Y.; Li, M.; Liu, Y.; Chen, B.; Lai, Y.; Li, L.; Zhuang, L.; et al. Impacts of delta and omicron variants on inactivated SARS-CoV-2 vaccine-induced T cell responses in patients with autoimmune diseases and healthy controls. Clin. Transl. Med. 2023, 13, e1171. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Tauzin, A.; Nicolas, A.; Ding, S.; Benlarbi, M.; Medjahed, H.; Chatterjee, D.; Dionne, K.; Gong, S.Y.; Gendron-Lepage, G.; Bo, Y.; et al. Spike recognition and neutralization of SARS-CoV-2 Omicron subvariants elicited after the third dose of mRNA vaccine. Cell Rep. 2023, 42, 111998. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef]
- Liu, Y.; Han, J.; Li, X.; Chen, D.; Zhao, X.; Qiu, Y.; Zhang, L.; Xiao, J.; Li, B.; Zhao, H. COVID-19 Vaccination in People Living with HIV (PLWH) in China: A Cross Sectional Study of Vaccine Hesitancy, Safety, and Immunogenicity. Vaccines 2021, 9, 1458. [Google Scholar] [CrossRef]
- Kang, L.; Shang, W.; Gao, P.; Wang, Y.; Liu, J.; Liu, M. Immunogenicity and Safety of COVID-19 Vaccines among People Living with HIV: A System-atic Review and Meta-Analysis. Vaccines 2022, 10, 1569. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef]
- Lyudovyk, O.; Kim, J.Y.; Qualls, D.; Hwee, M.A.; Lin, Y.-H.; Boutemine, S.R.; Elhanati, Y.; Solovyov, A.; Douglas, M.; Chen, E.; et al. Impaired humoral immunity is associated with prolonged COVID-19 despite robust CD8 T cell responses. Cancer Cell 2022, 40, 738–753.e5. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Estes, S.K.; Ali, R.A.; Gandhi, A.A.; Yalavarthi, S.; Shi, H.; Sule, G.; Gockman, K.; Madison, J.A.; Zuo, M.; et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020, 12, eabd3876. [Google Scholar] [CrossRef] [PubMed]
- Luczkowiak, J.; Rivas, G.; Labiod, N.; Lasala, F.; Rolo, M.; Lora-Tamayo, J.; Mancheno-Losa, M.; Rial-Crestelo, D.; Pérez-Rivilla, A.; Folgueira, M.D.; et al. Cross neutralization of SARS-CoV-2 omicron subvariants after repeated doses of COVID-19 mRNA vaccines. J. Med. Virol. 2022, 95, e28268. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zou, S.; Ming, F.; Zhang, Z.; Xing, Z.; Wu, S.; Guo, W.; Tang, W.; Liang, K. Early Efficacy and Safety of the Third Dose Inactivated COVID-19 Vaccine Among People Living With HIV. Am. J. Ther. 2022, 90, e1–e3. [Google Scholar] [CrossRef]
- Bessen, C.; Plaza-Sirvent, C.; Simsek, A.; Bhat, J.; Marheinecke, C.; Urlaub, D.; Bonowitz, P.; Busse, S.; Schumann, S.; Blanco, E.V.; et al. Impact of SARS-CoV-2 vaccination on systemic immune responses in people living with HIV. Front Immunol. 2022, 13, 1049070. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, Y.; Huo, Y.; Wang, L.; Liang, S.; Yu, M.; Lan, X.; Song, M.; Zhang, X.; Yan, Y.; et al. The safety and immunogenicity of a two-dose schedule of CoronaVac, and the immune persistence of vaccination for six months, in people living with HIV: A multicenter prospective cohort study. Front. Immunol. 2023, 14, 1129651. [Google Scholar] [CrossRef]
- Reeg, D.B.; Hofmann, M.; Neumann-Haefelin, C.; Thimme, R.; Luxenburger, H. SARS-CoV-2-Specific T Cell Responses in Immunocompromised Indi-viduals with Cancer, HIV or Solid Organ Transplants. Pathogens 2023, 12, 244. [Google Scholar] [CrossRef]
- Lv, Z.; Li, Q.; Feng, Z.; Zheng, X.; Yang, H.; Gu, Q.; Gu, Q.; Ying, S.; Qi, Y.; Li, X.; et al. Inactivated SARS-CoV-2 vaccines elicit immunogenicity and T-cell responses in people living with HIV. Int. Immunopharmacol. 2022, 102, 108383. [Google Scholar] [CrossRef]
- Wu, S.; Zou, S.; Ming, F.; Wu, M.; Guo, W.; Xing, Z.; Zhang, Z.; Liu, J.; Tang, W.; Liang, K. Humoral immune response to inactivated COVID-19 vaccination at the 3rd month among people living with HIV. BMC Infect. Dis. 2023, 23, 34. [Google Scholar] [CrossRef]
- Netto, L.C.; Ibrahim, K.Y.; Picone, C.M.; Alves, A.P.P.S.; Aniceto, E.V.; Santiago, M.R.; Parmejani, P.S.S.; Aikawa, N.E.; Medeiros-Ribeiro, A.C.; Pasoto, S.G.; et al. Safety and immunogenicity of CoronaVac in people living with HIV: A prospective cohort study. Lancet HIV 2022, 9, e323–e331. [Google Scholar] [CrossRef]
- Cao, Y.; Yisimayi, A.; Bai, Y.; Huang, W.; Li, X.; Zhang, Z.; Yuan, T.; An, R.; Wang, J.; Xiao, T.; et al. Humoral immune response to circulating SARS-CoV-2 variants elicited by inactivated and RBD-subunit vaccines. Cell Res. 2021, 31, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, Y.; Yero, A.; Mboumba Bouassa, R.S.; Comeau, E.; Samarani, S.; Brumme, Z.L.; Hull, M.; Crawley, A.M.; Langlois, M.-A.; Angel, J.B.; et al. SARS-CoV-2 Vaccine-Induced T-Cell Response after Three Doses in Peo-ple Living with HIV on Antiretroviral Therapy Compared to Seronegative Controls (CTN 328 COVAXHIV Study). Viruses 2023, 15, 575. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | PLWH (n = 41) |
|---|---|
| Age (years), median (range) | 30 (19–60) |
| Gender | |
| Male, n (%) | 40 (97.56) |
| Female, n (%) | 1 (2.44) |
| Weight (kg), median (IQR) | 66 (57–75) |
| Height (cm), mean (range) | 173.27 (160–185) |
| BMI, median (IQR) | 21.97 (17.30–50.70) |
| Days before 3rd dose Vaccination, median (range) | |
| 4 (3–14), n (%) | 41 (100) |
| Days after 3rd dose Vaccination, median (range) | |
| 14 days (11–19 days), n (%) | 41 (100) |
| 28 days (24–32 days), n (%) | 41 (100) |
| 3 months (85–94 days), n (%) | 39 (95.12) |
| 6 months (176–190 days), n (%) | 39 (95.12) |
| ART use, n (%) | |
| 2NRTIs + NNRTIs | 27 (48.20) |
| 2NRTIs + INSTIs | 11 (19.70) |
| 2NRTIs + PIs | 3 (5.40) |
| COVID-19 Vaccine, n (%) | |
| COVILO | 24 (58.5) |
| CoronaVac | 17 (41.5) |
| Time of HIV diagnosis, median (range), years | 2 (0.5–9) |
| HIV viral load before vaccination, median (IQR), copies/mL | 20 (20–29,509) |
| CD4+ T-cell counts before vaccination, median (IQR), cells/uL | 403 (13–827) |
| CD8+ T-cell counts before vaccination, median (IQR), cells/uL | 732 (432–2189) |
| CD4/CD8 ratio before vaccination, median (IQR) | 0.50 (0.30–0.79) |
| Comorbidities, n (%) | |
| Herpes zoster | 1 (2.4) |
| Herpes simplex | 2 (4.8) |
| Sphagnum | 1 (2.4) |
| Syphilis | 6 (14.6) |
| Condyloma acuminatum | 6 (14.6) |
| Tuberculosis | 1 (2.4) |
| Pneumocystis carinii pneumonia | 1 (2.4) |
| Thrush | 1 (2.4) |
| Eczema | 1 (2.4) |
| Diabetes | 1 (2.4) |
| Hyperlipidemia | 5 (12.2) |
| Chronic hepatitis | 1 (2.4) |
| Hypertension | 1 (2.4) |
| Laboratory examination | |
| White blood cell count (109/L) median (IQR) | 5.70 (4.60–6.86) |
| Red blood cell count (109/L) median (IQR) | 4.87 (4.50–5.20) |
| Platelet count (109/L) median (IQR) | 254 (213–274) |
| Aspartate aminotransferase (U/L) median (IQR) | 25.40 (18.60–30.15) |
| Alanine aminotransferase (U/L) median (IQR) | 28.60 (13.00–159.00) |
| Blood urea nitrogen (mmol/L) mean (range) | 4.48 (2.92–6.93) |
| Serum creatinine (μmol/L) mean (range) | 73.90 (44.40–105.70) |
| Triglyceride (mmol/L) median (IQR) | 1.46 (0.87–2.87) |
| Uric acid (μmol/L) mean (range) | 367.98 (205.00–603.00) |
| Blood glucose (mmol/L) median (IQR) | 5.55 (5.27–5.78) |
| Variable | PLWH (n = 41) |
|---|---|
| Overall adverse events within 7 days | 35 (85.4%) |
| Overall adverse events within 28 days | 35 (85.4%) |
| Local adverse events | |
| Pain | 22 (53.7%) |
| Redness | 16 (39.0%) |
| Itch | 6 (14.6%) |
| Induration | / |
| Swelling | 16 (39%) |
| Systemic adverse events | |
| Lethargy | 6 (14.6%) |
| Fever | / |
| Headache | / |
| Muscle and joint pain | 3 (7.3%) |
| Nausea | 2 (4.9%) |
| Thrombotic events or bleeding | / |
| Variable | Days before 3rd Dose of Vaccination | 28 Days after 3rd Dose of Vaccination | p-Value |
|---|---|---|---|
| White blood cell count (109/L) median (IQR) | 5.70 (4.60–6.86) | 5.45 (4.8–6.15 | 0.323 |
| Red blood cell count (109/L) median (IQR) | 4.87 (4.50–5.20) | 4.83 (4.56–5.17) | 0.835 |
| Platelet count (109/L) median (IQR) | 254 (213–274) | 243 (220.5–280) | 0.766 |
| Aspartate aminotransferase (U/L) median (IQR) | 25.40 (18.60–30.15) | 21.10 (16.95–28.8) | 0.389 |
| Alanine aminotransferase (U/L) median (IQR) | 28.60 (19.50–43.75) | 27.60 (16.7–38.20) | 0.424 |
| Triglyceride (mmol/L) median (IQR) | 1.46 (0.87–2.87) | 1.30 (0.98–2.67) | 0.973 |
| Blood urea nitrogen (mmol/L) mean (range) | 4.48 (2.92–6.93) | 4.74 (2.70–8.25) | 0.163 |
| Serum creatinine (μmol/L) mean (range) | 73.90 (44.40–105.70) | 76.86 (53.30–101.70) | 0.073 |
| Uric acid (μmol/L) mean (range) | 367.98 (205.00–603.00) | 387.56 (222.00–620.00) | 0.265 |
| Blood glucose (mmol/L) median (IQR) | 5.55 (5.265–5.78) | 5.38 (5.06–5.69) | 0.492 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Y.; Han, X.; Cui, X.; Wang, P.; Wang, X.; Liu, H.; Wang, Y.; Zhu, N.; Li, Y.; Lin, Y.; et al. Safety and Immunogenicity of the Inactivated COVID-19 Vaccine Booster in People Living with HIV in China. Vaccines 2023, 11, 1019. https://doi.org/10.3390/vaccines11061019
Yi Y, Han X, Cui X, Wang P, Wang X, Liu H, Wang Y, Zhu N, Li Y, Lin Y, et al. Safety and Immunogenicity of the Inactivated COVID-19 Vaccine Booster in People Living with HIV in China. Vaccines. 2023; 11(6):1019. https://doi.org/10.3390/vaccines11061019
Chicago/Turabian StyleYi, Yunyun, Xiaoxu Han, Xinyu Cui, Peng Wang, Xin Wang, Hui Liu, Yuqi Wang, Na Zhu, Yanyan Li, Yingying Lin, and et al. 2023. "Safety and Immunogenicity of the Inactivated COVID-19 Vaccine Booster in People Living with HIV in China" Vaccines 11, no. 6: 1019. https://doi.org/10.3390/vaccines11061019
APA StyleYi, Y., Han, X., Cui, X., Wang, P., Wang, X., Liu, H., Wang, Y., Zhu, N., Li, Y., Lin, Y., & Li, X. (2023). Safety and Immunogenicity of the Inactivated COVID-19 Vaccine Booster in People Living with HIV in China. Vaccines, 11(6), 1019. https://doi.org/10.3390/vaccines11061019





