Experiences, Enablers, and Challenges in Service Delivery and Integration of COVID-19 Vaccines: A Rapid Systematic Review
Abstract
:1. Introduction
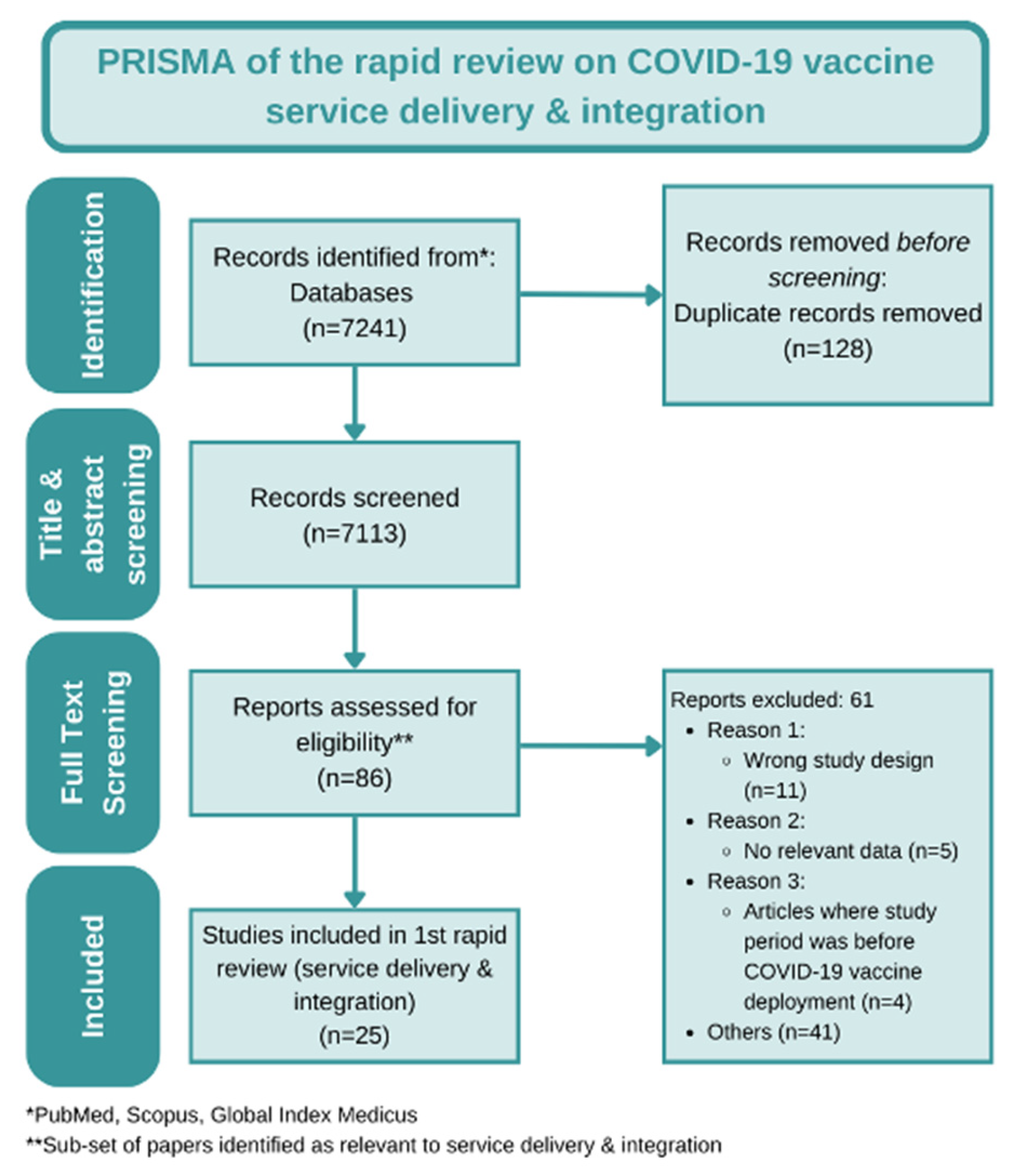
2. Materials and Methods
- The eight domains mentioned above.
- Papers in the peer-reviewed literature.
- Papers where the reporting period was after the COVID-19 vaccine rollout (starting December 2020).
- Lessons learned from other interventions and not COVID-19 vaccination.
- COVID-19 research protocols.
- COVID-19 vaccine effectiveness studies.
- Articles about the impact of the COVID-19 pandemic (except COVID-19 vaccine rollout) on routine immunization programs and/or other essential services.
- Papers that lack relevant data, i.e., data that can inform COVID vaccine rollout program decision making.
3. Results
3.1. Mass Vaccination Model
3.2. Mobile Vaccination Model
3.3. Fixed-Post Vaccination Model
3.4. Evidence of Integration of COVID-19 Vaccines with Other Health Services
4. Discussion
4.1. Key Learnings on COVID-19 Vaccine Service Delivery Models and Integration
4.2. Adaptations to Strategy
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Section A: Are the Results Valid? | Section B: What Are the Results? | Section C: Will the Results Help Locally? | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Paper | 1. Was There a Clear Statement of the Aims of the Research? | 2. Is a Qualitative Methodology Appropriate? | 3. Was the Research Design Appropriate to Address the Aims of the Research? | 4. Was the Recruitment Strategy Appropriate to the Aims of the Research? | 5. Were the Data Collected in a Way That Addressed the Research Issue? | 6. Has the Relationship between Researcher and Participants Been Adequately Considered? | 7. Have Ethical Issues Been Taken into Consideration? | 8. Was the Data Analysis Sufficiently Rigorous? | 9. Is there a Clear Statement of Findings? | 10. How Valuable Is the Research? |
| Cater | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Further research on providing pregnant people with guidance and support to get the COVID-19 vaccine |
| Cuschieri | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Examines Malta’s vaccination strategies (as a successful case study) to inform other countries how to implement successful vaccination techniques |
| Grech | Yes | Yes | Yes | Yes | Cannot tell | Yes | Yes | Cannot tell | Yes | Examines Malta’s mass vaccination campaign by touring a facility |
| Martinez | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Studies vaccine equity among Hispanic border communities in California to exemplify how equitable access is possible |
| Behrmann | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Provides a background for school-located vaccination clinics for planning and implementation |
| Reddy | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Presents lessons and challenges from mass vaccination campaign from the largest hospital in Africa |
| Behrmann | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Sharing of experiences for insights on school-located vaccination clinics and implementation |
| Alecendor | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Provides insights on the implications of increasing vaccine uptake with the use of a mobile vaccination clinic |
| Andrade | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Explains the implementation process of a pharmacist-led vaccination clinic in a hospital setting |
| Brambilla | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Shares insights into the process of development of a scalable model for mass vaccination clinics |
| Fareed | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Explains the implementation of a COVID-19 vaccination plan in substance use disorder residential settings |
| Fischl | Yes | Yes | Yes | Yes | Yes | Cannot tell | Yes | Cannot tell | Yes | Presents lessons learned and provides insight for mass medical operations |
| Jaffe | Yes | Yes | Cannot tell | Yes | Cannot tell | Cannot tell | Cannot tell | Cannot tell | Yes | Presents the experience of using blood services platforms to facilitate COVID-19 vaccination in Israel |
| Heidari | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Presented lessons learned from integrating COVID-19 vaccination with syringe service and other communicable disease testing programs |
| Mohamed | Yes | Yes | Yes | Yes | Cannot tell | Yes | Yes | Cannot tell | Yes | Explored challenges to COVID-19 vaccination in the Darfur region of Sudan |
| Sanchez | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Presents lessons learned and challenges of developing a COVID-19 vaccination program at a health sciences university |
| Rosen | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Examines factors that enabled Israel to rapidly rollout COVID-19 vaccinations |
| Signorelli | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Provides insights on the planning, implementation, and evaluation of a mass vaccination model utilizing vaccine islands |
| Goga | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Presents lessons learned to strengthen national and global vaccination strategies, based on a vaccine implementation study of healthcare workers in South Africa |
| Noack | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Discusses development of a multilingual app to provide information regarding COVID-19 vaccination to promote vaccines to groups with limited language proficiency |
| Jin | Yes | Yes | Yes | Yes | Cannot tell | Yes | Yes | Cannot tell | Yes | Examines the creation of a temporary COVID-19 vaccination clinic |
| Abdul-Mutakabbir | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Presents strategies for effectively reaching the Black community with COVID-19 vaccinations |
| Section A: Are the Results of the Trial Valid? | Section B: What Are the Results? | Section C: Will the Results Help Locally? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paper | 1. Did the study address a clearly focused issue? | 2. Did the authors use an appropriate method to answer their question? | 3. Were the cases recruited in an acceptable way? | 4. Were the controls selected in an acceptable way? | 5. Was the exposure accurately measured to minimize bias? | 6. (a) Aside from the experimental exposure, were the groups treated equally? | 6. (b) Have the authors taken account of the potential confounding factors in the design and/or in their analysis? | 7. How large was the treatment effect? | 8. How precise was the estimate of the treatment effect? | 9. Do you believe the results? | 10. Can the results be applied to the local population? | 11. Do the results of this study fit with other available evidence? |
| Berry | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Multiple AORs reported. Some significant, others not | 4 significant results. For those, ranges were (1.0, 1.3), (1.1, 6.6), (1.2, 8.9), (1.5, 19.6) | Yes | Yes | Cannot tell |
| Section A: Are the Results of the Study Valid? | Section B: What Are the Results? | Section C: Will the Results Help Locally? | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paper | 1. Did the study address a clearly focused issue? | 2. Was the cohort recruited in an acceptable way? | 3. Was the exposure accurately measured to minimise bias? | 4. Was the outcome accurately measured to minimise bias? | 5. (a) Have the authors identified all important confounding factors? | 5. (b) Have they taken account of the confounding factors in the design and/or analysis? | 6. (a) Was the follow up of subjects complete enough? | 6. (b) Was the follow up of subjects long enough? | 7. What are the results of this study? | 8. How precise are the results? | 9. Do you believe the results? | 10. Can the results be applied to the local population? | 11. Do the results of this study fit with other available evidence? | 12. What are the implications of this study for practice? |
| Hirshberg | Yes | Yes | Yes | Yes | No | No | Yes | Cannot tell | 3% vs. 11% vaccine uptake before and after the exposure | Not significant (p = 0.22) | No | Cannot tell | Cannot tell | Can inform design of future research projects |
| Section A: Is the Basic Study Design Valid for a Randomized Controlled Trial? | Section B: Was the Study Methodologically Sound? | Section C: What Are the Results? | Section D: Will the Results Help Locally? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paper | 1. Did the study address a clearly focused research question? | 2. Was the assignment of participants to interventions randomized? | 3. Were all participants who entered the study accounted for at its conclusion? | 4. (a) Were the participants ‘blind’ to intervention they were given? | 4. (b) Were the investigators ‘blind’ to the intervention they were giving to participants? | 4. (c) Were the people assessing/analyzing outcome/s ‘blinded’? | 5. Were the study groups similar at the start of the randomized controlled trial? | 6. Apart from the experimental intervention, did each study group receive the same level of care (that is, were they treated equally)? | 7. Were the effects of intervention reported comprehensively? | 8. Was the precision of the estimate of the intervention or treatment effect reported? | 9. Do the benefits of the experimental intervention outweigh the harms and costs? | 10. Can the results be applied to your local population/in your context? | 11. Would the experimental intervention provide greater value to the people in your care than any of the existing interventions? |
| Berry | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | No | Cannot tell | Cannot tell |
References
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; et al. Coronavirus Pandemic (COVID-19). Our World Data 2020, 7, 345. [Google Scholar]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global Impact of the First Year of COVID-19 Vaccination: A Mathematical Modelling Study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- International Monetary Fund. IMF-WHO COVID-19 Vaccine Tracker. Available online: https://www.imf.org/en/Topics/imf-and-covid19/IMF-WHO-COVID-19-Vaccine-Tracker (accessed on 15 February 2023).
- Forman, R.; Shah, S.; Jeurissen, P.; Jit, M.; Mossialos, E. COVID-19 Vaccine Challenges: What Have We Learned so Far and What Remains to Be Done? Health Policy 2021, 125, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in Ensuring Global Access to COVID-19 Vaccines: Production, Affordability, Allocation, and Deployment. Lancet Lond. Engl. 2021, 397, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Peacocke, E.F.; Heupink, L.F.; Frønsdal, K.; Dahl, E.H.; Chola, L. Global Access to COVID-19 Vaccines: A Scoping Review of Factors That May Influence Equitable Access for Low and Middle-Income Countries. BMJ Open 2021, 11, e049505. [Google Scholar] [CrossRef]
- Wonodi, C.; Obi-Jeff, C.; Adewumi, F.; Keluo-Udeke, S.C.; Gur-Arie, R.; Krubiner, C.; Jaffe, E.F.; Bamiduro, T.; Karron, R.; Faden, R. Conspiracy Theories and Misinformation about COVID-19 in Nigeria: Implications for Vaccine Demand Generation Communications. Vaccine 2022, 40, 2114–2121. [Google Scholar] [CrossRef]
- Majid, U.; Ahmad, M.; Zain, S.; Akande, A.; Ikhlaq, F. COVID-19 Vaccine Hesitancy and Acceptance: A Comprehensive Scoping Review of Global Literature. Health Promot. Int. 2022, 37, daac078. [Google Scholar] [CrossRef]
- Caserotti, M.; Girardi, P.; Rubaltelli, E.; Tasso, A.; Lotto, L.; Gavaruzzi, T. Associations of COVID-19 Risk Perception with Vaccine Hesitancy over Time for Italian Residents. Soc. Sci. Med. 2021, 272, 113688. [Google Scholar] [CrossRef]
- Poon, Y.-S.R.; Lin, Y.P.; Griffiths, P.; Yong, K.K.; Seah, B.; Liaw, S.Y. A Global Overview of Healthcare Workers’ Turnover Intention amid COVID-19 Pandemic: A Systematic Review with Future Directions. Hum. Resour. Health 2022, 20, 70. [Google Scholar] [CrossRef]
- World Health Organization. WHO SAGE Roadmap for Prioritizing Uses of COVID-19 Vaccines: An Approach to Optimize the Global Impact of COVID-19 Vaccines, Based on Public Health Goals, Global and National Equity, and Vaccine Access and Coverage Scenarios. Available online: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Vaccines-SAGE-Prioritization-2022.1 (accessed on 15 February 2023).
- World Health Organization Africa. Africa COVID-19 Vaccination Dashboard. Available online: https://app.powerbi.com/view?r=eyJrIjoiOTI0ZDlhZWEtMjUxMC00ZDhhLWFjOTYtYjZlMGYzOWI4NGIwIiwidCI6ImY2MTBjMGI3LWJkMjQtNGIzOS04MTBiLTNkYzI4MGFmYjU5MCIsImMiOjh9 (accessed on 15 February 2023).
- Garritty, C.; Gartlehner, G.; Nussbaumer-Streit, B.; King, V.J.; Hamel, C.; Kamel, C.; Affengruber, L.; Stevens, A. Cochrane Rapid Reviews Methods Group Offers Evidence-Informed Guidance to Conduct Rapid Reviews. J. Clin. Epidemiol. 2021, 130, 13–22. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme. CASP Checklists—Critical Appraisal Skills Programme. Available online: https://casp-uk.net/casp-tools-checklists/ (accessed on 15 February 2023).
- Reddy, D.L.; Dangor, Z.; Lala, N.; Johnstone, J.; Maswabi, L.; Tsitsi, J.M.L. COVID-19 Mass Vaccination Campaign for Healthcare Workers in a Low-Resource Setting: A Clinician-Driven Initiative. S. Afr. Med. J. 2021, 111, 610. [Google Scholar] [CrossRef]
- Brambilla, A.; Mangili, S.; Macchi, M.; Trucco, P.; Perego, A.; Capolongo, S. Covid-19 Massive Vaccination Center Layouts: A Modular and Scalable Model for Lombardy Region, Italy. Acta Biomed. Atenei Parm. 2021, 92, e2021446. [Google Scholar] [CrossRef]
- Fischl, B.; Patterson, A.T.; Baxter, J.; Watson, J.; Hemsworth, J.; Valentine, D.; Wessler, J.; Wong, D. Planning Considerations and Lessons Learned from a COVID-19 Mass Community Vaccination Center. Mil. Med. 2022, 187, 17–22. [Google Scholar] [CrossRef]
- Signorelli, C.; Odone, A.; Gianfredi, V.; Capraro, M.; Kacerik, E.; Chiecca, G.; Scardoni, A.; Minerva, M.; Mantecca, R.; Musarò, P.; et al. Application of the “Immunization Islands” Model to Improve Quality, Efficiency and Safety of a COVID-19 Mass Vaccination Site. Ann. Ig. Med. Prev. Comunita 2021, 33, 499–512. [Google Scholar]
- Grech, V.; Souness, J.; Agius, S. Mass Population Vaccination for COVID-19 in Malta. J. Vis. Commun. Med. 2021, 44, 181–187. [Google Scholar] [CrossRef]
- Jin, H.; Chu, J.; Zhao, W.; Ye, Q.; Zhan, M.; Han, X.; Lu, L.; Liu, J.; Li, Z.; Cui, M. Temporary Vaccination Clinic for COVID-19 in Zhuhai, China. Hum. Vaccines Immunother. 2021, 17, 3478–3480. [Google Scholar] [CrossRef]
- Cuschieri, S.; Agius, S.; Souness, J.; Brincat, A.; Grech, V. The Fastest National COVID Vaccination in Europe—Malta’s Strategies. Health Sci. Rev. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Rosen, B.; Waitzberg, R.; Israeli, A. Israel’s Rapid Rollout of Vaccinations for COVID-19. Isr. J. Health Policy Res. 2021, 10, 6. [Google Scholar] [CrossRef]
- Alcendor, D.J.; Juarez, P.D.; Matthews-Juarez, P.; Simon, S.; Nash, C.; Lewis, K.; Smoot, D. Meharry Medical College Mobile Vaccination Program: Implications for Increasing COVID-19 Vaccine Uptake among Minority Communities in Middle Tennessee. Vaccines 2022, 10, 211. [Google Scholar] [CrossRef]
- Heidari, O.; Meyer, D.; O’Conor, K.J.; Cargill, V.; Patch, M.; Farley, J.E. COVID-19 Vaccination and Communicable Disease Testing Services’ Integration within a Syringe Services Program: A Program Brief. J. Assoc. Nurses AIDS Care 2022, 33, 348–352. [Google Scholar] [CrossRef]
- Abdul-Mutakabbir, J.C.; Casey, S.; Jews, V.; King, A.; Simmons, K.; Hogue, M.D.; Belliard, J.C.; Peverini, R.; Veltman, J. A Three-Tiered Approach to Address Barriers to COVID-19 Vaccine Delivery in the Black Community. Lancet Glob. Health 2021, 9, e749–e750. [Google Scholar] [CrossRef] [PubMed]
- Noack, E.M.; Schäning, J.; Müller, F. A Multilingual App for Providing Information to SARS-CoV-2 Vaccination Candidates with Limited Language Proficiency: Development and Pilot. Vaccines 2022, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.D.; Baier, R.R.; Syme, M.; Gouskova, N.; Bishnoi, C.; Patel, U.; Leitson, M.; Gharpure, R.; Stone, N.D.; Link-Gelles, R.; et al. Strategies Associated with COVID-19 Vaccine Coverage among Nursing Home Staff. J. Am. Geriatr. Soc. 2022, 70, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.D.; Goldfeld, K.S.; McConeghy, K.; Gifford, D.; Davidson, H.E.; Han, L.; Syme, M.; Gandhi, A.; Mitchell, S.L.; Harrison, J.; et al. Evaluating the Findings of the IMPACT-C Randomized Clinical Trial to Improve COVID-19 Vaccine Coverage in Skilled Nursing Facilities. JAMA Intern. Med. 2022, 182, 324. [Google Scholar] [CrossRef]
- Cater, K.; Yazbek, J.; Morris, P.; Watts, K.; Whitehouse, C. Developing a Fast-Track COVID-19 Vaccination Clinic for Pregnant People. Br. J. Midwifery 2022, 30, 41–46. [Google Scholar] [CrossRef]
- Martinez, M.E.; Nodora, J.N.; McDaniels-Davidson, C.; Crespo, N.C.; Edward, A.A. Equitable COVID-19 Vaccination for Hispanics in the United States: A Success Story from California Border Communities. Int. J. Environ. Res. Public. Health 2022, 19, 535. [Google Scholar] [CrossRef]
- Hirshberg, J.S.; Huysman, B.C.; Oakes, M.C.; Cater, E.B.; Odibo, A.O.; Raghuraman, N.; Kelly, J.C. Offering Onsite COVID-19 Vaccination to High-Risk Obstetrical Patients: Initial Findings. Am. J. Obstet. Gynecol. MFM 2021, 3, 100478. [Google Scholar] [CrossRef]
- Behrmann, E.; Turner, O.; Magee, M. The Landscape of State and Local School-Located Vaccination Clinics: Practices, Policies, and Lessons Learned for Providing COVID-19 and Routine Vaccinations. NASN Sch. Nurse 2022, 37, 3S–14S. [Google Scholar] [CrossRef]
- Behrmann, E.; Kenney, O.; Magee, M. Key Challenges and Opportunities for Implementing School Located Vaccination Clinics for COVID-19 and Influenza: Roundtables with School Nurses and Immunization Programs. NASN Sch. Nurse Print 2022, 37, 15S–23S. [Google Scholar] [CrossRef]
- Andrade, J.; Slaby, M.; DeAngelis, J.; Connors, J.; Truong, J.; Ciaramella, C.; DiGregorio, R. Implementation of a Pharmacist-Led COVID-19 Vaccination Clinic at a Community Teaching Hospital. Am. J. Health. Syst. Pharm. 2021, 78, 1038–1042. [Google Scholar] [CrossRef]
- Fareed, A.; Fareed, M. Implementation of COVID-19 Vaccination in Substance Use Disorder Residential Settings. Addict. Disord. Treat. 2021, 20, 601–604. [Google Scholar] [CrossRef]
- Jaffe, E.; Abramovich, I.; Sonkin, R.; Shinar, E. Using Blood Services Platforms to Facilitate COVID-19 Vaccination Programs. Vox Sang. 2022, 117, 288. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Elhadi, Y.A.M.; Mohammed, N.A.; Ekpenyong, A.; Lucero-Prisno, D.E. Exploring Challenges to COVID-19 Vaccination in the Darfur Region of Sudan. Am. J. Trop. Med. Hyg. 2022, 106, 17–20. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Busse, K.; MacKinnon, K.; Henk, L.; MacKinnon, G.E.; Brown, J.; Mauermann, S.; Dobrowski, T.; Jungmann, J.; Bultman, J.; et al. Collective Action: The Medical College of Wisconsin COVID-19 Vaccination Program. WMJ Off. Publ. State Med. Soc. Wis. 2021, 120, 5. [Google Scholar]
- Goga, A.E.; ChB, M.; Bekker, L.-G.; ChB, M.; Garrett, N.; Takuva, S.; Sanne, I.; ChB, M.; Odhiambo, J.; Mayat, F.; et al. Sisonke Phase 3B Open-Label Study: Lessons Learnt for National and Global Vaccination Scale-up during Epidemics. S. Afr. Med. J. 2022, 112, 9. [Google Scholar] [CrossRef]
- Rackimuthu, S.; Hasan, M.M.; Bardhan, M.; Essar, M.Y. COVID-19 Vaccination Strategies and Policies in India: The Need for Further Re-evaluation Is a Pressing Priority. Int. J. Health Plann. Manag. 2022, 37, 1847–1850. [Google Scholar] [CrossRef]
- Dooling, K. Phase 1 Allocation COVID-19 Vaccine: Work Group Considerations. In Proceedings of the Advisory Committee on Immunization Practices, Meeting, Atlanta, GA, USA, 22 September 2020. [Google Scholar]
- Thomas, H.; Angrist, N.; Goldszmidt, R.; Kira, B.; Petherick, A.; Phillips, T.; Webster, S.; Cameron-Blake, E.; Hallas, L.; Majumdar, S.; et al. A Global Panel Database of Pandemic Policies (Oxford COVID-19 Government Response Tracker). Nat. Hum. Behav. 2021, 5, 529–538. [Google Scholar] [CrossRef]
- Carini, E.; Cadeddu, C.; Castagna, C.; Nurchis, M.C.; Lanza, T.E.; Grossi, A.; Barbara, A.; Axelrod, S.; Goletti, M.; Parente, P. Organisational Model and Coverage of At-Home COVID-19 Vaccination in an Italian Urban Context. Vaccines 2021, 9, 1256. [Google Scholar] [CrossRef]
- Bell, D.; Brown, G.W.; Oyibo, W.A.; Ouédraogo, S.; Tacheva, B.; Barbaud, E.; Kalk, A.; Ridde, V.; Paul, E. COVAX—Time to Reconsider the Strategy and Its Target. Health Policy Open 2023, 4, 100096. [Google Scholar] [CrossRef]
- Sexton, J.B.; Adair, K.C.; Proulx, J.; Profit, J.; Cui, X.; Bae, J.; Frankel, A. Emotional Exhaustion among US Health Care Workers before and during the COVID-19 Pandemic, 2019–2021. JAMA Netw. Open 2022, 5, e2232748. [Google Scholar] [CrossRef]
- Social Science in Humanitarian Action Platform. Key Considerations for Integrating COVID-19 Vaccination Services: Insights from Iraq and Syria for the MENA Region. Available online: https://www.socialscienceinaction.org/resources/key-considerations-for-integrating-covid-19-vaccination-services-insights-from-iraq-and-syria-for-the-mena-region/ (accessed on 2 May 2023).
- Declich, S.; De Ponte, G.; Marchetti, G.; Dente, M.G.; Tosti, M.E.; Tavoschi, L.; Lopalco, P.L.; Russo, M.L.; Marceca, M. Life-Course Vaccinations for Migrants and Refugees: Drawing Lessons from the COVID-19 Vaccination Campaigns. J. Glob. Health 2022, 12, 03064. [Google Scholar] [CrossRef] [PubMed]
- Adepoju, P. As COVID-19 Vaccines Arrive in Africa, Omicron Is Reducing Supply and Increasing Demand. Nat. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
| Author (First) | Country | Reporting Period | Target Population | Strategies Used | Recommendations/Learnings |
|---|---|---|---|---|---|
| Mass Vaccination Model | |||||
| Reddy et al. [15] | South Africa | 17 February to 26 February 2021 | Healthcare workers |
|
|
| Brambilla et al. [16] | Italy | 21 January to 21 February 2021 | General population |
| None reported by authors. |
| Fischl et al. [17] | USA | 24 February to 6 April 2021 | General population |
|
|
| Signorelli et al. [18] | Italy | 20 April to 4 May 2021 | General population |
|
|
| Grech et al. [19] | Malta | 27 December 2020 to 15 April 2021 | General population |
|
|
| Jin et al. [20] | China | March to April 2021 | General population |
|
|
| Cuschieri et al. [21] | Malta | December 2020 to 9 August 2021 | General population |
|
|
| Rosen et al. [22] | Israel | 14 December to 30 December 2020 | General population |
|
|
| Mobile Vaccination Model | |||||
| Alcendor et al. [23] | USA | March to September 2021 | General population—underserved minorities |
|
|
| Heidari et al. [24] | USA | Starting Spring 2021 | Injecting drug users |
|
|
| Abdul—Mutakabbir et al. [25] | USA | 21 January to 20 February 2021 | General population |
|
|
| Noack et al. [26] | Germany | Not specified | General population |
|
|
| Fixed-Post Vaccination Model | |||||
| Berry et al. [27] | USA | 4 February to 2 March 2021 | Healthcare workers and elderly people |
|
|
| Berry et al. [28] | USA | December 2020 to March 2021 | Healthcare workers |
|
|
| Cater et al. [29] | England | 28 June to 30 September 2021 | Pregnant women |
|
|
| Martinez et al. [30] | USA | Inception of COVID-19 vaccination to 20 September 2021 | General population |
|
|
| Hirshberg et al. [31] | USA | 27 April to 20 May 2021 | Pregnant women |
|
|
| Behrmann et al. [32] * | USA | August 2019 to late summer 2021 | School students and general population |
|
|
| Behrmann et al. [33] | USA | Not specified | School students and general population |
|
|
| Andrade et al. [34] | USA | 15 December to 29 December 2020 | Healthcare workers |
|
|
| Fareed et al. [35] | USA | 23 December 2020 to 31 January 2021 | General population |
| None reported by authors. |
| Jaffe et al. [36] | Israel | Not specified | General population |
|
|
| Mohamed et al. [37] | Sudan | Not specified | General population |
|
|
| Sanchez et al. [38] | USA | December 2020 to April 2021 | Healthcare workers |
|
|
| Goga et al. [39] | South Africa | 17 February to 26 May 2021 | Healthcare workers |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabia, S.; Wonodi, C.B.; Vilajeliu, A.; Sussman, S.; Olson, K.; Cooke, R.; Udayakumar, K.; Twose, C.; Ezeanya, N.; Adefarrell, A.A.; et al. Experiences, Enablers, and Challenges in Service Delivery and Integration of COVID-19 Vaccines: A Rapid Systematic Review. Vaccines 2023, 11, 974. https://doi.org/10.3390/vaccines11050974
Nabia S, Wonodi CB, Vilajeliu A, Sussman S, Olson K, Cooke R, Udayakumar K, Twose C, Ezeanya N, Adefarrell AA, et al. Experiences, Enablers, and Challenges in Service Delivery and Integration of COVID-19 Vaccines: A Rapid Systematic Review. Vaccines. 2023; 11(5):974. https://doi.org/10.3390/vaccines11050974
Chicago/Turabian StyleNabia, Sarah, Chizoba Barbara Wonodi, Alba Vilajeliu, Sabine Sussman, Katharine Olson, Rianna Cooke, Krishna Udayakumar, Claire Twose, Nwamaka Ezeanya, Adewumi Adetola Adefarrell, and et al. 2023. "Experiences, Enablers, and Challenges in Service Delivery and Integration of COVID-19 Vaccines: A Rapid Systematic Review" Vaccines 11, no. 5: 974. https://doi.org/10.3390/vaccines11050974
APA StyleNabia, S., Wonodi, C. B., Vilajeliu, A., Sussman, S., Olson, K., Cooke, R., Udayakumar, K., Twose, C., Ezeanya, N., Adefarrell, A. A., & Lindstrand, A. (2023). Experiences, Enablers, and Challenges in Service Delivery and Integration of COVID-19 Vaccines: A Rapid Systematic Review. Vaccines, 11(5), 974. https://doi.org/10.3390/vaccines11050974








