Clinical Characteristics of Mild Patients with Breakthrough Infection of Omicron Variant in China after Relaxing the Dynamic Zero COVID-19 Policy
Abstract
:1. Introduction
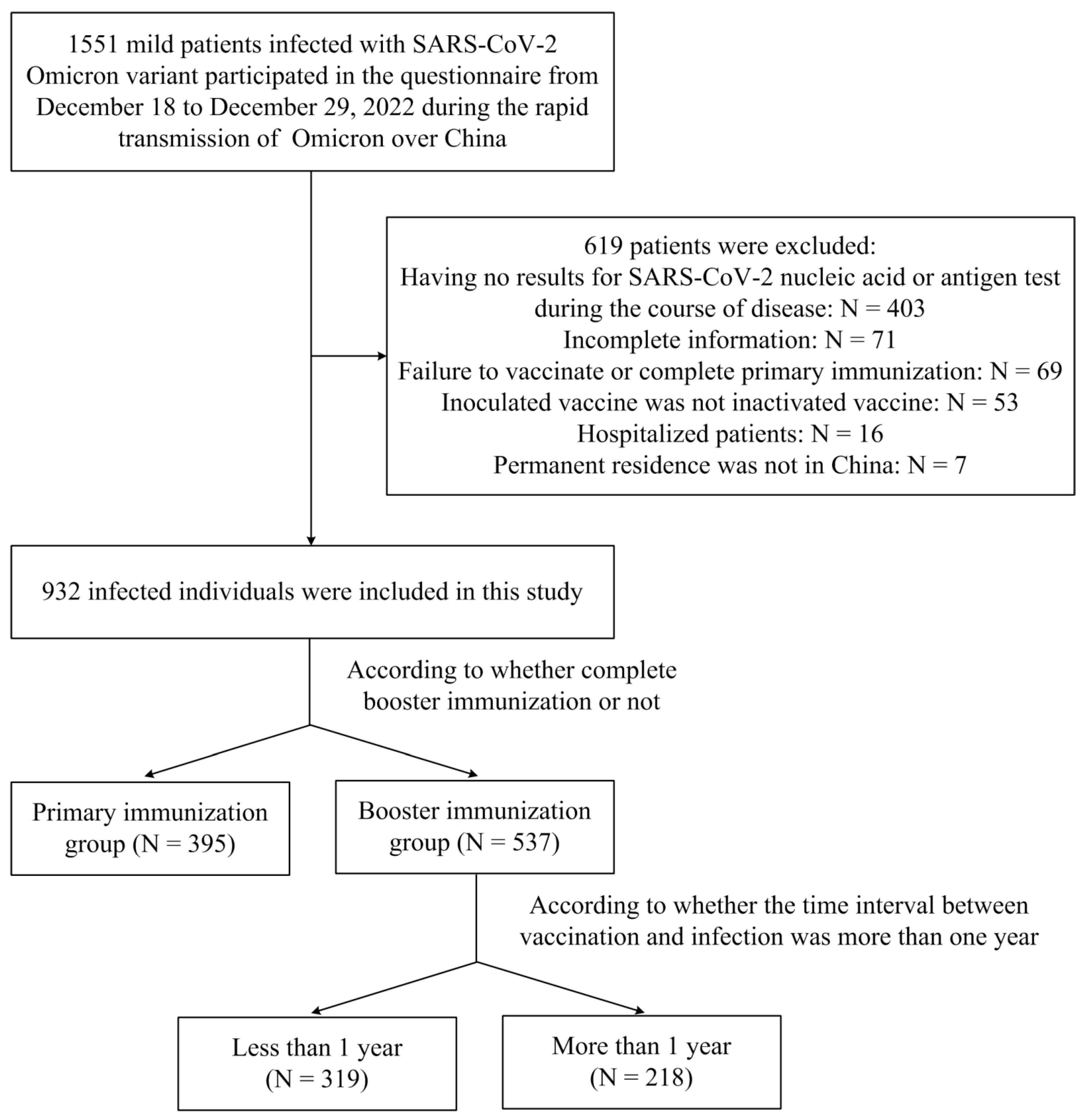
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Statistical Analyses
3. Results
3.1. Demographics and Characteristics of Patients Infected with SARS-CoV-2 Omicron Variant
3.2. Clinical Manifestations of Patients Infected with SARS-CoV-2 Omicron Variant
3.3. Clinical Manifestations of Patients with Different Booster Immunization Time Interval
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, S.T.; Kwan, A.T.; Rodriguez-Barraquer, I.; Singer, B.J.; Park, H.J.; Lewnard, J.A.; Sears, D.; Lo, N.C. Infectiousness of SARS-CoV-2 Breakthrough Infections and Reinfections During the Omicron Wave. Nat. Med. 2023, 29, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Mugisha, J.; Mpairwe, B.; Newton, R.; Cotten, M.; Phan, M.V.T. SARS-CoV-2 Omicron Ba.5 Infections in Vaccinated Persons, Rural Uganda. Emerg. Infect. Dis. 2023, 29, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R.; Owusu, D.; O’Laughlin, K.; Cohen, A.L.; Ben Hamida, A.; Patel, J.C.; Freeman, M.M.; Nsibambi, T.; Nieves, R.; Marston, B.J.; et al. SARS-CoV-2 Breakthrough Infections among Us Embassy Staff Members, Uganda, May–June 2021. Emerg. Infect. Dis. 2022, 28, 1279–1280. [Google Scholar] [CrossRef]
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of Mrna-1273 against SARS-CoV-2 Omicron and Delta Variants. Nat. Med. 2022, 28, 1063–1071. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; Zubizarreta, J.R.; Gonzalez, C.; Acevedo, J.; Pizarro, A.; Vergara, V.; Soto-Marchant, M.; Gilabert, R.; Flores, J.C.; et al. Effectiveness of Coronavac in Children 3–5 Years of Age During the SARS-CoV-2 Omicron Outbreak in Chile. Nat. Med. 2022, 28, 1377–1380. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Collie, S.; Champion, J.; Moultrie, H.; Bekker, L.G.; Gray, G. Effectiveness of Bnt162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 494–496. [Google Scholar] [CrossRef]
- Kirsebom, F.C.M.; Andrews, N.; Sachdeva, R.; Stowe, J.; Ramsay, M.; Bernal, J.L. Effectiveness of Chadox1-S COVID-19 Booster Vaccination against the Omicron and Delta Variants in England. Nat. Commun. 2022, 13, 7688. [Google Scholar] [CrossRef] [PubMed]
- Price, A.M.; Olson, S.M.; Newhams, M.M.; Halasa, N.B.; Boom, J.A.; Sahni, L.C.; Pannaraj, P.S.; Irby, K.; Bline, K.E.; Maddux, A.B.; et al. Bnt162b2 Protection against the Omicron Variant in Children and Adolescents. N. Engl. J. Med. 2022, 386, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Martellucci, C.A.; Flacco, M.E.; Soldato, G.; Di Martino, G.; Carota, R.; Caponetti, A.; Manzoli, L. Effectiveness of COVID-19 Vaccines in the General Population of an Italian Region before and During the Omicron Wave. Vaccines 2022, 10, 662. [Google Scholar] [CrossRef]
- McMenamin, M.E.; Nealon, J.; Lin, Y.; Wong, J.Y.; Cheung, J.K.; Lau, E.H.Y.; Wu, P.; Leung, G.M.; Cowling, B.J. Vaccine Effectiveness of One, Two, and Three Doses of Bnt162b2 and Coronavac against COVID-19 in Hong Kong: A Population-Based Observational Study. Lancet Infect. Dis. 2022, 22, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Magen, O.; Waxman, J.G.; Makov-Assif, M.; Vered, R.; Dicker, D.; Hernán, M.A.; Lipsitch, M.; Reis, B.Y.; Balicer, R.D.; Dagan, N. Fourth Dose of Bnt162b2 Mrna COVID-19 Vaccine in a Nationwide Setting. N. Engl. J. Med. 2022, 386, 1603–1614. [Google Scholar] [CrossRef]
- Zhang, Y.; He, L.; Chen, H.; Lu, S.; Xiong, Y.; Liu, J.; Zheng, Y.; Wang, S.; Liu, L. Manifestations of Blood Coagulation and Its Relation to Clinical Outcomes in Severe COVID-19 Patients: Retrospective Analysis. Int. J. Lab. Hematol. 2020, 42, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Harris, R.; Hall, J.; Woodhall, S.; Andrews, N.; Dunbar, K.; Lopez-Bernal, J.; Dabrera, G. Effects of Second Dose of SARS-CoV-2 Vaccination on Household Transmission, England. Emerg. Infect. Dis. 2023, 29, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Ferrara, F.; Troiano, V.; La Porta, R. COVID-19 Vaccines and Decreased Transmission of SARS-CoV-2. Inflammopharmacology 2021, 29, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Santos da Silva, E.; Servais, J.Y.; Kohnen, M.; Arendt, V.; Gilson, G.; Staub, T.; Seguin-Devaux, C.; Perez-Bercoff, D. Vaccine- and Bti-Elicited Pre-Omicron Immunity More Effectively Neutralizes Omicron Sublineages Ba.1, Ba.2, Ba.4 and Ba.5 Than Pre-Omicron Infection Alone. Curr. Issues Mol. Biol. 2023, 45, 1741–1761. [Google Scholar] [CrossRef]
- Higdon, M.M.; Wahl, B.; Jones, C.B.; Rosen, J.G.; Truelove, S.A.; Baidya, A.; Nande, A.A.; ShamaeiZadeh, P.A.; Walter, K.K.; Feikin, D.R.; et al. A Systematic Review of Coronavirus Disease 2019 Vaccine Efficacy and Effectiveness against Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Disease. Open Forum Infect. Dis. 2022, 9, ofac138. [Google Scholar] [CrossRef]
- Ghazy, R.M.; Ashmawy, R.; Hamdy, N.A.; Elhadi, Y.A.M.; Reyad, O.A.; Elmalawany, D.; Almaghraby, A.; Shaaban, R.; Taha, S.H.N. Efficacy and Effectiveness of SARS-CoV-2 Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 350. [Google Scholar] [CrossRef]
- Kislaya, I.; Rodrigues, E.F.; Borges, V.; Gomes, J.P.; Sousa, C.; Almeida, J.P.; Peralta-Santos, A.; Nunes, B. Comparative Effectiveness of Coronavirus Vaccine in Preventing Breakthrough Infections among Vaccinated Persons Infected with Delta and Alpha Variants. Emerg. Infect. Dis. 2022, 28, 331–337. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Moustsen-Helms, I.R.; Schelde, A.B.; Gram, M.A.; Emborg, H.D.; Nielsen, J.; Hansen, C.H.; Andersen, M.A.; Meaidi, M.; Wohlfahrt, J.; et al. Vaccine Effectiveness against SARS-CoV-2 Reinfection During Periods of Alpha, Delta, or Omicron Dominance: A Danish Nationwide Study. PLoS Med. 2022, 19, e1004037. [Google Scholar] [CrossRef]
- Kissler, S.M.; Fauver, J.R.; Mack, C.; Tai, C.G.; Breban, M.I.; Watkins, A.E.; Samant, R.M.; Anderson, D.J.; Metti, J.; Khullar, G.; et al. Viral Dynamics of SARS-CoV-2 Variants in Vaccinated and Unvaccinated Persons. N. Engl. J. Med. 2021, 385, 2489–2491. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association between 3 Doses of Mrna COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. Jama 2022, 327, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Fleming-Dutra, K.E.; Britton, A.; Shang, N.; Derado, G.; Link-Gelles, R.; Accorsi, E.K.; Smith, Z.R.; Miller, J.; Verani, J.R.; Schrag, S.J. Association of Prior Bnt162b2 COVID-19 Vaccination with Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance. Jama 2022, 327, 2210–2219. [Google Scholar] [CrossRef] [PubMed]
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Devilliers, H.; Ghillani, P.; et al. Iga Dominates the Early Neutralizing Antibody Response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef] [PubMed]
- Wisnewski, A.V.; Luna, J.C.; Redlich, C.A. Human Igg and Iga Responses to COVID-19 Mrna Vaccines. PLoS ONE 2021, 16, e0249499. [Google Scholar] [CrossRef] [PubMed]
- Ng, O.T.; Marimuthu, K.; Lim, N.; Lim, Z.Q.; Thevasagayam, N.M.; Koh, V.; Chiew, C.J.; Ma, S.; Koh, M.; Low, P.Y.; et al. Analysis of COVID-19 Incidence and Severity among Adults Vaccinated with 2-Dose Mrna COVID-19 or Inactivated SARS-CoV-2 Vaccines with and without Boosters in Singapore. JAMA Netw. Open 2022, 5, e2228900. [Google Scholar] [CrossRef]
- Šmíd, M.; Berec, L.; Přibylová, L.; Májek, O.; Pavlík, T.; Jarkovský, J.; Weiner, J.; Barusová, T.; Trnka, J. Protection by Vaccines and Previous Infection against the Omicron Variant of Severe Acute Respiratory Syndrome Coronavirus 2. J. Infect. Dis. 2022, 226, 1385–1390. [Google Scholar] [CrossRef]
- Külper-Schiek, W.; Piechotta, V.; Pilic, A.; Batke, M.; Dreveton, L.S.; Geurts, B.; Koch, J.; Köppe, S.; Treskova, M.; Vygen-Bonnet, S.; et al. Facing the Omicron Variant-How Well Do Vaccines Protect against Mild and Severe COVID-19? Third Interim Analysis of a Living Systematic Review. Front. Immunol. 2022, 13, 940562. [Google Scholar] [CrossRef]
- Castaldo, M.; Waliszewska-Prosół, M.; Koutsokera, M.; Robotti, M.; Straburzyński, M.; Apostolakopoulou, L.; Capizzi, M.; Çibuku, O.; Ambat, F.D.F.; Frattale, I.; et al. Headache onset after vaccination against SARS-CoV-2: A systematic literature review and meta-analysis. J. Headache Pain 2022, 23, 41. [Google Scholar] [CrossRef]
| Characteristics | All Patients (N = 932) | Primary Immunization (N = 395) | Booster Immunization (N = 537) | p Value |
|---|---|---|---|---|
| Age groups (years) | ||||
| <14 | 5 (0.5) | 5 (1.2) | 0 (0.0) | 0.088 |
| 14–29 | 392 (42.1) | 165 (41.8) | 227 (42.3) | |
| 30–45 | 442 (47.4) | 188 (47.6) | 254 (47.3) | |
| 46–59 | 84 (9.0) | 32 (8.1) | 52 (9.7) | |
| >59 | 9 (1.0) | 5 (1.3) | 4 (0.7) | |
| Sex | ||||
| Male | 362 (38.8) | 141 (35.7) | 221 (41.2) | 0.091 |
| Female | 570 (61.2) | 254 (64.3) | 316 (58.8) | |
| Occupation | ||||
| Teacher ** | 49 (5.3) | 11 (2.8) | 38 (7.1) | 0.019 * |
| Student | 110 (11.8) | 46 (11.6) | 64 (11.9) | |
| Self-employed | 44 (4.7) | 22 (5.6) | 22 (4.0) | |
| Company Staff ** | 216 (23.2) | 106 (26.8) | 110 (20.5) | |
| Public servants | 47 (5.0) | 17 (4.3) | 30 (5.6) | |
| Healthcare workers | 201 (21.6) | 89 (22.5) | 112 (20.9) | |
| Others | 265 (28.4) | 104 (26.4) | 161 (30.0) | |
| Coexisting disorders | ||||
| Hypertension | 39 (4.2) | 21 (5.3) | 18 (3.4) | 0.139 |
| Diabetes | 13 (1.4) | 7 (1.8) | 6 (1.1) | 0.400 |
| Respiratory diseases | 15 (1.6) | 8 (2.8) | 7 (1.3) | 0.387 |
| Cardiovascular and cerebrovascular diseases | 9 (1.0) | 5 (1.3) | 4 (0.7) | 0.422 |
| Tumor | 5 (0.5) | 4 (1.0) | 1 (0.2) | 0.088 |
| Past history of COVID-19 | ||||
| Yes | 21 (2.3) | 12 (3.0) | 9 (1.7) | 0.166 |
| No | 911 (97.7) | 383 (97.0) | 528 (98.3) | |
| Medical treatment | ||||
| Untreated | 141 (15.1) | 57 (14.4) | 84 (15.6) | 0.871 |
| Self-purchased medicine | 753 (80.8) | 322 (81.5) | 431 (80.3) | |
| Fever outpatient treatment | 38 (4.1) | 16 (4.1) | 22 (4.1) | |
| Drugs use | ||||
| Antipyretics and analgesics | 666 (84.2) | 282 (83.4) | 384 (84.8) | 0.610 |
| Antiviral drugs | 202 (25.5) | 80 (23.7) | 122 (26.9) | 0.298 |
| Antibiotics | 127 (16.1) | 55 (16.3) | 72 (15.9) | 0.886 |
| Chinese medicine | 251 (31.7) | 81 (24.0) | 170 (37.5) | <0.001* |
| Fluid administration | 15 (1.9) | 5 (1.5) | 10 (2.2) | 0.458 |
| Most likely infection mode | ||||
| Family | 257 (27.6) | 107 (27.1) | 150 (27.9) | <0.001 * |
| Colleagues ** | 277 (29.7) | 102 (25.8) | 175 (32.6) | |
| Confronting and treating patients | 174 (18.7) | 70 (17.7) | 104 (19.4) | |
| Public places ** | 207 (22.2) | 109 (27.6) | 98 (18.2) | |
| Takeaway delivery | 17 (1.8) | 7 (1.8) | 10 (1.9) |
| Characteristics | All Patients (N = 932) | Primary Immunization (N = 395) | Booster Immunization (N = 537) | p Value |
|---|---|---|---|---|
| Initial symptom | ||||
| Fever | 337 (36.2) | 129 (32.7) | 208 (38.7) | 0.160 |
| Pharyngalgia | 216 (23.2) | 85 (21.4) | 131 (24.4) | 0.304 |
| Myalgia | 111 (11.9) | 53 (13.4) | 58 (10.8) | 0.223 |
| Headache and dizziness | 92 (9.8) | 47 (11.9) | 45 (8.4) | 0.075 |
| Cough and expectoration | 65 (7.0) | 37 (9.4) | 28 (5.2) | 0.014 * |
| Cardinal symptoms | ||||
| Fever | 844 (90.6) | 355 (89.9) | 489 (91.1) | 0.540 |
| Cough | 786 (84.3) | 336 (85.1) | 450 (83.8) | 0.600 |
| Weakness | 721 (77.4) | 297 (75.2) | 424 (79.0) | 0.174 |
| Dizziness and headache | 709 (76.1) | 300 (75..9) | 409 (76.2) | 0.940 |
| Myalgia | 689 (73.9) | 291 (73.7) | 398 (74.1) | 0.897 |
| Pharyngalgia | 647 (69.4) | 256 (64.8) | 391 (72.8) | 0.009 * |
| Expectoration | 626 (67.2) | 264 (66.8) | 362 (67.4) | 0.853 |
| Poor appetite | 494 (53.0) | 215 (54.4) | 279 (52.0) | 0.454 |
| Hoarseness | 412 (44.2) | 172 (43.5) | 240 (44.7) | 0.727 |
| Ageusia | 328 (35.2) | 162 (41.0) | 166 (30.9) | 0.001 * |
| Anosmia | 276 (29.6) | 123 (31.1) | 153 (28.5) | 0.382 |
| Nausea | 252 (27.0) | 92 (23.3) | 160 (29.8) | 0.027 * |
| Diarrhea | 205 (22.0) | 82 (20.8) | 123 (22.9) | 0.435 |
| Vomiting | 172 (18.5) | 72 (18.2) | 100 (18.6) | 0.878 |
| Dyspnea | 116 (12.4) | 57 (14.4) | 59 (11.0) | 0.116 |
| Symptom duration (d) | ||||
| 1–3 | 216 (23.3) | 100 (25.3) | 116 (21.6) | 0.150 |
| 4–6 | 372 (39.8) | 141 (35.7) | 231 (43.0) | |
| 7–10 | 245 (26.2) | 105 (26.6) | 140 (26.1) | |
| 11–14 | 49 (5.3) | 23 (5.8) | 26 (4.8) | |
| ≥15 | 50 (5.4) | 26 (6.6) | 24 (4.5) | |
| Maximum body temperature (°C) | ||||
| <37.0 | 49 (5.3) | 18 (4.6) | 31 (5.8) | 0.202 |
| 37.0–38.5 | 335 (35.9) | 150 (38.0) | 185 (34.5) | |
| 38.6–39.9 | 520 (55.8) | 211 (53.4) | 309 (57.5) | |
| ≥40.0 | 28 (3.0) | 16 (4.0) | 12 (2.2) | |
| Fever duration (d) | ||||
| 0–2 | 542 (61.4) | 242 (64.2) | 300 (59.3) | 0.500 |
| 3–4 | 310 (35.1) | 122 (32.4) | 188 (37.2) | |
| 5–6 | 28 (3.2) | 12 (3.2) | 16 (3.2) | |
| ≥7 | 3 (0.3) | 1 (0.2) | 2 (0.3) | |
| Days of antigen/nucleic acid positive after symptom onset (d) | ||||
| 0 | 123 (13.2) | 40 (10.1) | 83 (15.5) | 0.191 |
| 1 | 287 (30.8) | 124 (31.4) | 163 (30.4) | |
| 2 | 285 (30.6) | 128 (32.4) | 157 (29.2) | |
| 3 | 150 (16.1) | 68 (17.2) | 82 (15.3) | |
| 4 | 38 (4.1) | 13 (3.3) | 25 (4.7) | |
| ≥5 | 49 (5.3) | 22 (5.6) | 27 (5.0) | |
| Days of antigen/nucleic acid negative after symptom onset (d) | ||||
| 1–3 | 65 (7.0) | 26 (6.6) | 39 (7.3) | 0.974 |
| 4–6 | 211 (22.6) | 87 (22.0) | 124 (23.1) | |
| 7–10 | 538 (57.7) | 230 (58.2) | 308 (57.4) | |
| 11–14 | 78 (8.4) | 35 (8.9) | 43 (8.0) | |
| ≥15 | 40 (4.3) | 17 (4.3) | 23 (4.3) |
| Characteristics | All Patients (N = 537) | ≤1 Year (N = 319) | >1 Year (N = 218) | p Value |
|---|---|---|---|---|
| Initial symptom | ||||
| Fever | 209 (38.9) | 121 (37.9) | 88 (40.4) | 0.570 |
| Pharyngalgia | 131 (24.4) | 75 (23.5) | 56 (25.7) | 0.564 |
| Myalgia | 58 (10.8) | 40 (12.5) | 18 (8.3) | 0.116 |
| Headache and dizziness | 45 (8.4) | 25 (7.8) | 20 (9.2) | 0.583 |
| Cough and expectoration | 28 (5.2) | 15 (4.8) | 13 (6.0) | 0.519 |
| Cardinal symptoms | ||||
| Fever | 489 (91.1) | 290 (90.9) | 199 (91.3) | 0.881 |
| Cough | 450 (83.8) | 272 (85.3) | 178 (81.7) | 0.264 |
| Weakness | 424 (79.0) | 256 (80.3) | 168 (77.1) | 0.374 |
| Dizziness and headache | 409 (76.2) | 244 (76.5) | 165 (75.7) | 0.831 |
| Myalgia | 398 (74.1) | 231 (72.4) | 167 (76.6) | 0.276 |
| Pharyngalgia | 391 (72.8) | 235 (73.7) | 156 (71.6) | 0.590 |
| Expectoration | 362 (67.4) | 221 (69.3) | 141 (64.7) | 0.264 |
| Poor appetite | 279 (52.0) | 169 (53.0) | 110 (50.5) | 0.566 |
| Hoarseness | 240 (44.7) | 144 (45.1) | 96 (44.0) | 0.800 |
| Ageusia | 166 (30.9) | 105 (32.9) | 61 (28.0) | 0.224 |
| Anosmia | 153 (28.5) | 101 (31.7) | 52 (23.9) | 0.050 |
| Nausea | 160 (29.8) | 101 (31.7) | 59 (27.1) | 0.253 |
| Diarrhea | 123 (22.9) | 75 (23.5) | 48 (22.0) | 0.686 |
| Vomiting | 100 (18.6) | 62 (19.4) | 38 (17.4) | 0.558 |
| Dyspnea | 59 (11.0) | 38 (11.9) | 21 (9.6) | 0.407 |
| Symptom duration (d) | ||||
| 1–3 | 116 (21.6) | 72 (22.6) | 44 (20.2) | 0.728 |
| 4–6 | 231 (43.0) | 140 (43.9) | 91 (41.7) | |
| 7–10 | 140 (26.1) | 79 (24.8) | 61 (28.0) | |
| 11–14 | 26 (4.8) | 13 (4.0) | 13 (6.0) | |
| ≥15 | 24 (4.5) | 15 (4.7) | 9 (4.1) | |
| Maximum body temperature (°C) | ||||
| <37.0 | 31 (5.8) | 20 (6.3) | 11 (5.0) | 0.762 |
| 37.0–38.5 | 185 (34.5) | 113 (35.4) | 72 (33.0) | |
| 38.6–39.9 | 309 (57.5) | 180 (56.4) | 129 (59.2) | |
| ≥40.0 | 12 (2.2) | 6 (1.9) | 6 (2.8) | |
| Fever duration (d) | ||||
| 0–2 | 300 (59.2) | 173 (57.9) | 127 (61.4) | 0.771 |
| 3–4 | 188 (37.2) | 114 (38.1) | 74 (35.7) | |
| 5–6 | 16 (3.2) | 11 (3.7) | 5 (2.4) | |
| ≥7 | 2 (0.4) | 1 (0.3) | 1 (0.5) | |
| Days of antigen/nucleic acid positive after symptom onset (d) | ||||
| 0 | 123 (13.2) | 40 (10.1) | 83 (15.5) | 0.410 |
| 1 | 287 (30.8) | 124 (31.4) | 163 (30.4) | |
| 2 | 285 (30.6) | 128 (32.4) | 157 (29.2) | |
| 3 | 150 (16.1) | 68 (17.2) | 82 (15.3) | |
| 4 | 38 (4.1) | 13 (3.3) | 25 (4.7) | |
| ≥5 | 49 (5.3) | 22 (5.6) | 27 (5.0) | |
| Days of antigen/nucleic acid negative after symptom onset (d) | ||||
| 1–3 | 44 (8.2) | 28 (8.8) | 16 (7.3) | 0.162 |
| 4–6 | 119 (22.2) | 75 (23.5) | 44 (20.2) | |
| 7–10 | 305 (56.8) | 183 (57.4) | 122 (56.0) | |
| 11–14 | 41 (7.6) | 17 (5.3) | 24 (11.0) | |
| ≥15 | 28 (5.2) | 16 (5.0) | 12 (5.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Zhang, F.; Liu, Y.; Xiong, Z.; Zheng, S.; Liu, W.; Liu, L. Clinical Characteristics of Mild Patients with Breakthrough Infection of Omicron Variant in China after Relaxing the Dynamic Zero COVID-19 Policy. Vaccines 2023, 11, 968. https://doi.org/10.3390/vaccines11050968
He Y, Zhang F, Liu Y, Xiong Z, Zheng S, Liu W, Liu L. Clinical Characteristics of Mild Patients with Breakthrough Infection of Omicron Variant in China after Relaxing the Dynamic Zero COVID-19 Policy. Vaccines. 2023; 11(5):968. https://doi.org/10.3390/vaccines11050968
Chicago/Turabian StyleHe, Yingyu, Fang Zhang, Yan Liu, Zhou Xiong, Shangen Zheng, Wanbing Liu, and Lei Liu. 2023. "Clinical Characteristics of Mild Patients with Breakthrough Infection of Omicron Variant in China after Relaxing the Dynamic Zero COVID-19 Policy" Vaccines 11, no. 5: 968. https://doi.org/10.3390/vaccines11050968
APA StyleHe, Y., Zhang, F., Liu, Y., Xiong, Z., Zheng, S., Liu, W., & Liu, L. (2023). Clinical Characteristics of Mild Patients with Breakthrough Infection of Omicron Variant in China after Relaxing the Dynamic Zero COVID-19 Policy. Vaccines, 11(5), 968. https://doi.org/10.3390/vaccines11050968






