Role of B Cells in Mycobacterium Tuberculosis Infection
Abstract
1. Introduction
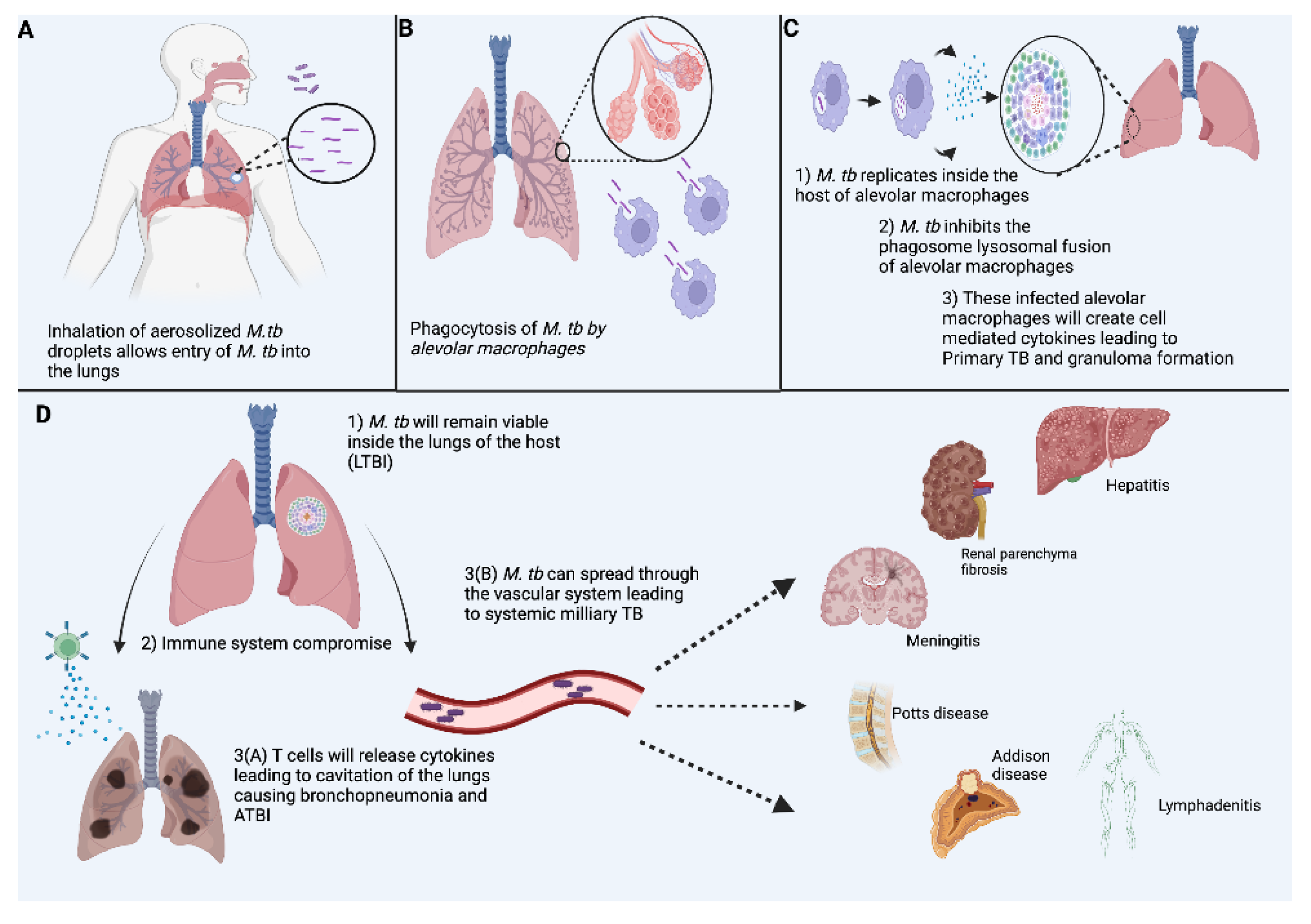
2. Pathophysiology in TB Infection
2.1. Innate Immune Response
2.2. Cell Mediated T-Cell Immune Response
2.3. Humoral B-Cell Immune Response
2.4. Germinal Centers
3. Granulomas in TB
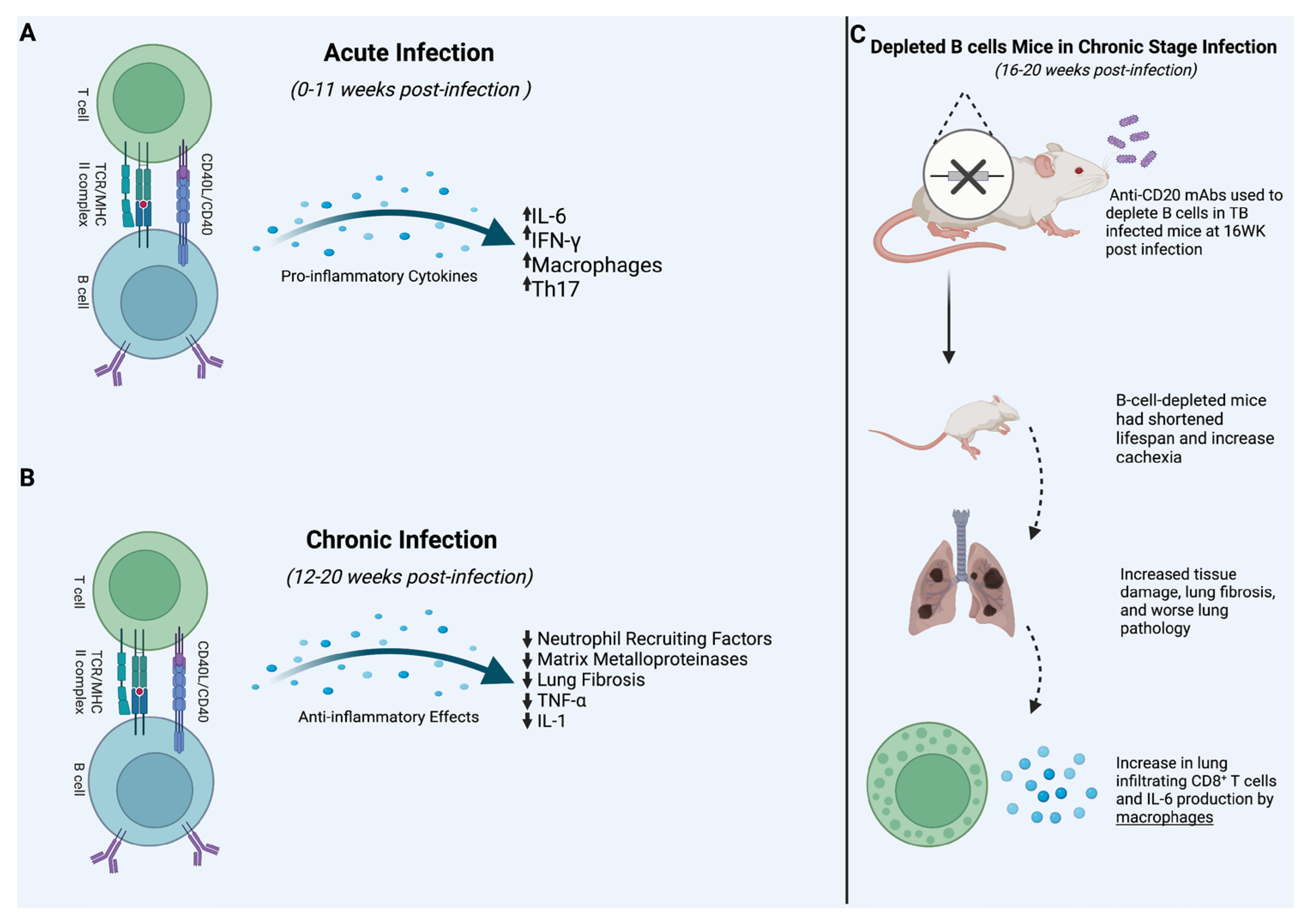
4. Acute Infection
5. Rituximab
6. Chronic Infection
7. Vaccines and B-Cell Response
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pai, M.; Behr, M.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Prim. 2016, 2, 16076. [Google Scholar] [CrossRef]
- Lyon, S.M.; Rossman, M.D. Pulmonary Tuberculosis. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Barry, C.E.; Boshoff, H.I.; Dartois, V.; Dick, T.; Ehrt, S.; Flynn, J.; Schnappinge, D.; Wilkinson, R.J.; Young, D. The spectrum of latent tuberculosis: Rethinking the biology and intervention strategies. Nat. Rev. Microbiol. 2009, 7, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Zaheen, A.; Bloom, B.R. Tuberculosis in 2020—New Approaches to a Continuing Global Health Crisis. N. Engl. J. Med. 2020, 382, e26. [Google Scholar] [CrossRef] [PubMed]
- Nagu, T.J.; Mboka, M.A. Clinical and Imaging Features of Adults with Recurrent Pulmonary Tuberculosis—A Prospective Case-Controlled Study. Elsevier 2021, 113 (Suppl. 1), S33–S39. [Google Scholar] [CrossRef]
- Hermans, S.M.; Zinyakatira, N.; Caldwell, J.; Cobelens, F.G.J.; Boulle, A.; Wood, R. High rates of recurrent TB disease: A population-level cohort study. Clin. Infect. Dis. 2020, 72, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. The influence of BCG on vaccine responses—A systematic review. Expert Rev. Vaccines 2018, 17, 547–554. [Google Scholar] [CrossRef]
- Pereira, S.M.; Dantas, O.M.S.; Ximenes, R.; Barreto, M.L. BCG vaccine against tuberculosis: Its protective effect and vaccination policies. Rev. Saúde Pública 2007, 41 (Suppl. 1), 59–66. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Zhang, G.; Chen, X. Th1, true functional signatures for protection immunity against TB? Cell. Mol. Immunol. 2018, 15, 206–215. [Google Scholar] [CrossRef]
- Turvey SE, B.D. Innate immunity. J. Allergy Clin. Immunol. 2010, 125 (Suppl. 2), S24–S32. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. 2), 49. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, L.; Phuah, J.; Flynn, J.; Chan, J. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Adv. Exp. Med. Biol. 2013, 783, 225–250. [Google Scholar] [CrossRef] [PubMed]
- Pagán, A.J.; Yang, C.-T.; Cameron, J.; Swaim, L.E.; Ellet, F.; Lieschke, G.J.; Ramakrishnan, L. Myeloid Growth Factors Promote Resistance to Mycobacterial Infection by Curtailing Granuloma Necrosis through Macrophage Replenishment. Cell Host Microbe 2015, 18, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Kumari, A.; Das, G.; Dwivedi, V.P. Tuberculosis Vaccine: A journey from BCG to present. Life Sci. 2020, 252, 117594. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, D.; Caws, M.; Marais, B.; Farrar, J. Tuberculosis in Adults and Children; Wellcome Trust–Funded Monographs and Book Chapters; Springer Nature: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Kleinnijenhuis, J.; Joosten, L.A.; van de Veerdonk, F.L.; Savage, N.; Van Crevel, R.; Jan Kullberg, B.; Van Der Ven, A.; Ottenhoff, T.H.; Dinarello, C.A.; Van Der Meer, J.W.; et al. Transcriptional and inflammasome-mediated pathways for the induction of IL-1beta production by Mycobacterium tuberculosis. Eur. J. Immunol. 2009, 39, 1914–1922. [Google Scholar] [CrossRef]
- Van Crevel, R.; Ottenhoff, T.H.; Van Der Meer, J.W. Innate immunity to Mycobacterium tuberculosis. Clin. Microbiol. Rev. 2002, 15, 294–309. [Google Scholar] [CrossRef]
- Pajuelo, D.; Gonzalez-Juarbe, N.; Tak, U.; Sun, J.; Orihuela, C.J.; Niederweis, M. NAD+ Depletion Triggers Macrophage Necroptosis, a Cell Death Pathway Exploited by Mycobacterium tuberculosis. Cell Rep. 2018, 24, 429–440. [Google Scholar] [CrossRef]
- Mirzaei, R. Adaptive Immunity. In Encyclopedia of Infection and Immunity; Elsevier: Oxford, UK, 2022; pp. 39–55. [Google Scholar]
- Bonilla, F.A.; Oettgen, H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010, 25, S33–S40. [Google Scholar] [CrossRef]
- Boom, W.H.; Scaible, U.E.; Achkar, J.M. The knowns and unknowns of latent mycobacterium tuberculosis infection. JCI 2021, 131, e136222. [Google Scholar] [CrossRef]
- Martino, M.D.; Lodi, L.; Galli, L.; Chiappini, E. Immune response to mycobacterium tuberculosis: A Narrative review. Pediatr. Immunol. 2019, 7, 350. [Google Scholar] [CrossRef]
- Pollock, K.M.; Whitworth, H.S.; Montamat-sicotte, D.J.; Grass, L.; Cooke, G.S.; Kapembwa, M.S.; Kon, O.M.; Sampson, R.D.; Taylor, G.P.; Lalvani, A. T-cell immunophenotyping distinguishes active from latent tuberculosis. Infect. Dis. 2013, 208, 952–968. [Google Scholar] [CrossRef] [PubMed]
- Du Bryan, E.; Ruzive, S.; Lindestam Arleham, C.; Sette, A.; Sher, A.; Barber, D.L.; Wilkinson, R.J.; Riou, C. Mycobacterium tuberculosis-specific CD4 T cells expressing CD153 inversely associate with bacterial load and disease severity in human tuberculosis. Mucosal. Immunol. 2021, 14, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Ling Lin, P.; Plessner, H.L.; Voitenok, N.N.; Flynn, J.L. Tumor Necrosis Factor and Tuberculosis. J. Investig. Dermatol. Symp. Proc. 2007, 12, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Canaday, D.H.; Wilkinson, R.J.; Li, Q.; Harding, C.V.; Silver, R.F.; Henry Boom, W. CD4+ and CD8+ T Cells Kill Intracellular Mycobacterium tuberculosis by a Perforin and Fas/Fas Ligand-Independent Mechanism. J. Immunol. 2001, 167, 2734–2742. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.L.; Flynn, J.L. CD8 T cells and Mycobacterium tuberculosis infection. Semin. Immunopathol. 2015, 37, 239–249. [Google Scholar] [CrossRef]
- Van Pinxteren, L.A.; Cassidy, J.; Smedegaard, B.; Agger, E.; Andersen, P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur. J. Immunol. 2000, 30, 3689–3698. [Google Scholar] [CrossRef]
- De Souza Silva, B.D.; Martins Trentini, M.; Castro da Costa, A.; Kipnis, A.; Paula Junqueira-Kipnis, A. Different phenotypes of CD8+ T cells associated with bacterial load in active tuberculosis. Immunol. Lett. 2014, 160, 23–32. [Google Scholar] [CrossRef]
- Pal, A.; Chakrvarty, A.K. Genetics and Breeding for Disease Resistance of Livestock; Academic Press: Cambridge, MA, USA, 2020; pp. 119–125. [Google Scholar]
- Forthal, D.N. Functions of antibodies. Microbiol. Spectr. 2014, 2, 1–17. [Google Scholar] [CrossRef]
- Dogan, I.; Bertocci, B.; Vilmont, V.; Delbos, F.; Mégret, J.; Storck, S.; Reynaud, C.-A.; Weill, J.-C. Multiple layers of B cell memory with different effector functions. Nat. Immunol. 2009, 10, 1292–1299. [Google Scholar] [CrossRef]
- Lin, K.; Chen, S.; Chen, G. Role of memory T cells and Perspectives for Intervention in Organ Transplant. Front. Immunol. 2015, 6, 473. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Chung, A.W.; Rosebrock, T.R.; Ghebremichael, M.; Yu, W.H.; Grace, P.S.; Schoen, M.K.; Tafesse, F.; Martin, C.; Leung, V.; et al. A functional role for antibodies in tuberculosis. Cell 2016, 167, 433–443. [Google Scholar] [CrossRef]
- Mesin, L.; Ersching, J.; Victora, G.D. Germinal Center B Cell Dynamics. Immunity 2016, 45, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Ruddle, N.H.; Akiran, E.M. Secondary Lymphoid Organs: Responding to Genetic and Environmental Cues in Ontogeny and the Immune Response. J. Immunol. 2009, 183, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gracia-Ibanez, L.; Toellner, K.-M. Regulation of germinal center B-cell differention. Immunol. Rev. 2016, 270, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Victoria, G.D.; Schwickert, T.A.; Fooksman, D.R.; Kamphorst, A.O.; Meyer-Hermann, M.; Dustin, M.L.; Nussenzweig, M.C. Germinal Center Dynamics Revealed by Multiphoton Microscopy Using a Photoactivatable Fluorescent Reporter. Cell 2010, 143, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, R.; Pedro Calado, D. Positive Selection in the Light Zone of Germinal Centers. Front. Immunol. 2021, 12, 661678. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bharrhan, S.; Xu, J.; Sharma, T.; Wang, Y.; Salgame, P.; Zhang, J.; Nargan, K.; Steyn, A.; Maglione, P.; et al. B cells promote granulomatous inflammation during chronic Mycobacterium tuberculosis infection in mice. PLoS Pathog. 2023, 19, e1011187. [Google Scholar] [CrossRef]
- Flynn, J.; Chan, J.; Lin, P. Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal. Immunol. 2011, 4, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, S.; Schaible, U. The granuloma in tuberculosis: Dynamics of a host-pathogen collusion. Front. Immunol. 2013, 3, 411. [Google Scholar] [CrossRef] [PubMed]
- Maglione, P.; Chan, J. How B cells shape the immune response against Mycobacterium tuberculosis. Eur. J. Immunol. 2009, 39, 676–686. [Google Scholar] [CrossRef]
- Joosten, S.A.; van Meijgaarden, K.E.; Del Nonno, F.; Baiocchini, A.; Petrone, L.; Vanini, V.; Smits, H.H.; Palmieri, F.; Goletti, D.; Ottenhoff, T.H.M. Patients with Tuberculosis Have a Dysfunctional Circulating B-Cell Compartment, Which Normalizes following Successful Treatment. PLoS Pathog. 2016, 12, e1005687. [Google Scholar] [CrossRef] [PubMed]
- Loxton, A.G. Bcells and their regulatory functions during Tuberculosis: Latency and active disease. Mol. Immunol. 2019, 111, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, H.; Teles, R.; Chen, Y.; Wu, A.; Lu, J.; Chen, Z.; Ma, F.; Pellegrini, M.; Modlin 6, R.L. Cellular, Molecular, and Immunological Characteristics of Langhans Multinucleated Giant Cells Programmed by IL-15. J. Investig. Derm. 2020, 140, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Phuah, J.Y.; Matilla, J.T.; Lin, P.L.; Flynn, J.L. Activated B Cells in the Granulomas of Nonhuman Primates Infected with Mycobacterium tuberculosis. Am. J. Pathol. 2012, 181, 508–514. [Google Scholar] [CrossRef]
- Hunter, L.; Hingley-Wilson, S.; Stewart, G.R.; Sharpe, S.A.; Javier Salguero, F. Dynamics of Macrophage, T and B Cell Infiltration Within Pulmonary Granulomas Induced by Mycobacterium tuberculosis in Two Non-Human Primate Models of Aerosol Infection. Front. Immunol. 2022, 12, 776913. [Google Scholar] [CrossRef]
- Linge, I.; Tsareva, A.; Kondratieva, E.; Dyatlov, A.; Hidalgo, J.; Zvartsev, R.; Apt, A. Pleiotropic Effect of IL-6 Produced by B-Lymphocytes During Early Phases of Adaptive Immune Responses Against TB Infection. Front. Immunol. 2022, 13, 750068. [Google Scholar] [CrossRef] [PubMed]
- Linge, I.; Kondratieva, E.; Apt, A. Prolonged B-Lymphocyte-Mediated Immune and Inflammatory Responses to Tuberculosis Infection in the Lungs of TB-Resistant Mice. Int. J. Mol. Sci. 2023, 24, 1140. [Google Scholar] [CrossRef]
- Brancati, S.; Gozzo, L.; Longo, L.; Vitale, D.C.; Drago, F. Rituximab in Multiple Sclerosis: Are We Ready for Regulatory Approval? Front. Immunol. 2021, 12, 661882. [Google Scholar] [CrossRef] [PubMed]
- Leandro, M.; Isenberg, D.A. Rituximab—The first twenty years. Lupus 2021, 30, 371–377. [Google Scholar] [CrossRef]
- Monson, N.L.; Cravens, P.; Hussain, R.; Harp, C.T.; Cummings, M.; de Pilar Martin, M.; Ben, L.-H.; Do, J.; Lyons, J.-A.; Lovette-Racke, A.; et al. Rituximab therapy reduces organ-specific T cell responses and ameliorates experimental autoimmune encephalomyelitis. PLoS ONE 2011, 6, e17103. [Google Scholar] [CrossRef]
- Cerny, T.; Borisch, B.; Introna, M.; Johnson, P.; Rose, A.L. Mechanism of action of rituximab. Anticancer Drugs 2002, 13, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Phuah, J.; Wong, E.A.; Gideon, H.P.; Maiello, P.; Coleman, M.T.; Hendricks, M.R.; Ruden, R.; Cirrincione, L.R.; Chan, J.; Ling Lin, P.; et al. Effects of B Cell Depletion on Early Mycobacterium tuberculosis Infection in Cynomolgus Macaques. Infect. Immun. 2016, 84, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Cantini, F.; Niccoli, L.; Goletti, D. Tuberculosis Risk in Patients Treated with Non-Anti-Tumor Necrosis Factor-α (TNF-α) Targeted Biologics and Recently Licensed TNF-α Inhibitors: Data from Clinical Trials and National Registries. J. Rheumatol. 2014, 91, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Choreño-Parra, J.A.; Bobba, S.; Rangel-Moreno, J.; Ahmed, M.; Mehra, S.; Rosa, B.; Martin, J.; Mitreva, M.; Kaushal, D.; Zúñiga, J.; et al. Mycobacterium tuberculosis HN878 Infection Induces Human-Like B-Cell Follicles in Mice. J. Infect. Dis. 2020, 221, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Mehta, S.; Chen, Y.; Achkar, J.; Casadevall, A.; Flynn, J. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Semin. Immunol. 2014, 26, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Delogu, G.; Fadda, G. The biology of mycobacterium tuberculosis infection. Mediterr. J. Hematol. Infect. Dis. 2013, 5, e2013070. [Google Scholar] [CrossRef]
- Achkar, J.M.; Chan, J.; Casadevall, A. B cells and antibodies in the defense against Mycobacterium tuberculosis infection. Immunol. Rev. 2015, 264, 167–181. [Google Scholar] [CrossRef]
- Thau, L.; Asuka, E.; Mahajan, K. Physiology, Opsonization; StatPearls Publishing: Tampa, FL, USA, 2023. [Google Scholar]
- Rao, M.; Valentini, D.; Poiret, T.; Dodoo, E.; Parida, S.; Zumla, A.; Brighenti, S.; Maeurer, M. B in TB: B Cells as Mediators of Clinically. Clin. Infect. Dis. 2015, 61, S225–S234. [Google Scholar] [CrossRef]
- Rijnink, W.F.; Ottenhoff, T.H.M.; Joosten, S.A. B-Cells and Antibodies as Contributors to Effector Immune Responses in Tuberculosis. Front. Immunol. 2021, 12, 640168. [Google Scholar] [CrossRef]
- Jacobs, A.J.; Mongkolsapaya, J.; Screaton, G.R.; McShane, H.; Wilkinson, R.J. Antibodies and tuberculosis. Tuberculosis 2016, 101, 102–113. [Google Scholar] [CrossRef]
- Olivares, N.; Marquina, B.; Mata-Espinoza, D.; Zatarain-Barron, Z.L.; Pinzón, E.; Estrada, I.; Parada, C.; Collin, M.; Rook, G.; Hernandez-Pando, R. The protective effect of immunoglobulin in murine tuberculosis is dependent on IgG glycosylation. Pathog. Dis. 2013, 69, 176–183. [Google Scholar] [CrossRef]
- Lyaschenko, K.P.; Vordermeier, H.M.; Waters, W.R. Memory B cells and tuberculosis. Vet. Immunol. Immunopathol. 2020, 221, 110016. [Google Scholar] [CrossRef]
- Melkie, S.T.; Arias, L.; Farroni, C.; Makek, M.J.; Goletti, D.; Vilaplana, C. The role of antibodies in tuberculosis diagnosis, prophylaxis and therapy: A review from the ESGMYC study group. Eur. Respir. Rev. 2022, 31, 210218. [Google Scholar] [CrossRef]
- Rose-John, S.; Winthrop, K.; Calabrese, L. The Role of IL–6 in Host Defence Against Infections: Immunobiology and Clinical Implications. Nat. Rev. Rheumatol. 2017, 13, 399–409. [Google Scholar] [CrossRef]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The Role of Interleukin 6 During Viral Infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Parihar, S.P.; Ozturk, M.; Höft, M.A.; Chia, J.E.; Guler, R.; Keeton, R.; Van Rensburg, I.C.; Loxton, A.G.; Brombacher, F. IL-4-Responsive B Cells Are Detrimental During Chronic Tuberculosis Infection in Mice. Front. Immunol. 2021, 12, 611673. [Google Scholar] [CrossRef]
- Ulrichs, T.; Kosmiadi, G.A.; Trusov, V.; Jörg, S.; Pradl, L.; Titukhina, M.; Mishenko, V.; Gushina, N.; Kaufmann, S.H.E. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J. Pathol. 2004, 204, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Yin, Q. B Cell Response to Vaccination. Immunol. Investig. 2021, 50, 780–801. [Google Scholar] [CrossRef] [PubMed]
- Buisman, A.M.; Rond, C.G.H.D.; Ten Hulscher, H.I.; Van Binnendijk, R.S. Long-term presence of memory B-cells specific for different vaccine components. Vaccine 2009, 28, 179–186. [Google Scholar] [CrossRef]
- Luca, S.; Mihaescu, T. History of BCG Vaccine. Maedica J. Clin. Med. 2013, 8, 43–58. [Google Scholar]
- Achkar, J.M.; Chan, J.; Casadevall, A. Role of B cells and antibodies in acquired immunity against Mycobacterium tuberculosis. Cold Spring Harb. Perspect. Med. 2014, 5, a018432. [Google Scholar] [CrossRef] [PubMed]
- Esser, M.T.; Marchese, R.D.; Kierstead, L.S.; Tussey, L.G.; Wang, F.; Chirmule, N.; Washabaugh, M.W. Memory T cells and vaccines. Vaccine 2003, 21, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Lanzavecchia, A.; Araki, K.; Ahmed, R. From Vaccines to Memory and Back. Immunity 2010, 33, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Aguilo, N.; Uranga, S.; Mata, E.; Tarancon, R.; Gomez, A.B.; Marinova, D.; Otal, I.; Monzón, M.; Badiola, J.; Montenegro, D.; et al. Respiratory Immunization With a Whole Cell Inactivated Vaccine Induces Functional Mucosal Immunoglobulins Against Tuberculosis in Mice and Non-human Primates. Front. Microbiol. 2020, 11, 1339. [Google Scholar] [CrossRef] [PubMed]
- Prados-Rosales, R.; Carreño, L.; Cheng, T.; Blanc, C.; Weinrick, B.; Malek, A.; Lowary, T.; Baena, A.; Joe, M.; Bai, Y.; et al. Enhanced control of Mycobacterium tuberculosis extrapulmonary dissemination in mice by an arabinomannan-protein conjugate vaccine. PLoS Pathog. 2017, 13, e1006250. [Google Scholar] [CrossRef]
- Dijkman, K.; Sombroek, C.; Vervenne, R.; Hofman, S.; Boot, C.; Remarque, E.; Kocken, C.; Ottenhoff, T.; Kondova, I.; Khayum, M.; et al. Prevention of tuberculosis infection and disease by local BCG in repeatedly exposed rhesus macaques. Nat. Med. 2019, 25, 255–262. [Google Scholar] [CrossRef]
| Topic | Summary | References |
|---|---|---|
| Granuloma | Phuah et al., 2012 showed the similarities between granulomas and GC in NHP. Their research shows that both structures house B cells in the periphery to create a follicle-like structure. However, unlike GC, only a few B cells within the granuloma were shown to be actively proliferating. | [47,48] |
| Acute Infection | Linge et al., 2022 revealed that knocking out B cell production of IL-6 in mice led to a significantly lower proportion of IFN-γ and Th-17 differentiation. The study was limited, however, due to not providing evidence of IL-6 from B cells after the acute phase of the infection. | [49] |
| Chronic Infection | Linge et al., 2023 used CD-20 monoclonal Abs to KO B cells in mice. Results showed chronic infection of TB in B-cell-depleted mice lead to shorter life spans and increased mRNA expression of neutrophil recruiting factors. These results suggest that B cells may have a protective functionality in chronic stage infection. | [50] |
| Rituximab | Phuah et al., 2016 showed that rituximab-treated NHP had a decrease in IL-6 and IL-10 in their granuloma. However, the results were not statistically significant. Furthermore, a study by Cantini et al. showed that Pt taking rituximab had no increased incidence of TB. | [51,52,53,54,55,56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stewart, P.; Patel, S.; Comer, A.; Muneer, S.; Nawaz, U.; Quann, V.; Bansal, M.; Venketaraman, V. Role of B Cells in Mycobacterium Tuberculosis Infection. Vaccines 2023, 11, 955. https://doi.org/10.3390/vaccines11050955
Stewart P, Patel S, Comer A, Muneer S, Nawaz U, Quann V, Bansal M, Venketaraman V. Role of B Cells in Mycobacterium Tuberculosis Infection. Vaccines. 2023; 11(5):955. https://doi.org/10.3390/vaccines11050955
Chicago/Turabian StyleStewart, Paul, Shivani Patel, Andrew Comer, Shafi Muneer, Uzma Nawaz, Violet Quann, Mira Bansal, and Vishwanath Venketaraman. 2023. "Role of B Cells in Mycobacterium Tuberculosis Infection" Vaccines 11, no. 5: 955. https://doi.org/10.3390/vaccines11050955
APA StyleStewart, P., Patel, S., Comer, A., Muneer, S., Nawaz, U., Quann, V., Bansal, M., & Venketaraman, V. (2023). Role of B Cells in Mycobacterium Tuberculosis Infection. Vaccines, 11(5), 955. https://doi.org/10.3390/vaccines11050955






