Associated Factors and Immune Response to the Hepatitis B Vaccine with a Standard Schedule: A Prospective Study of People with HIV in China
Abstract
1. Introduction
2. Materials and Methods
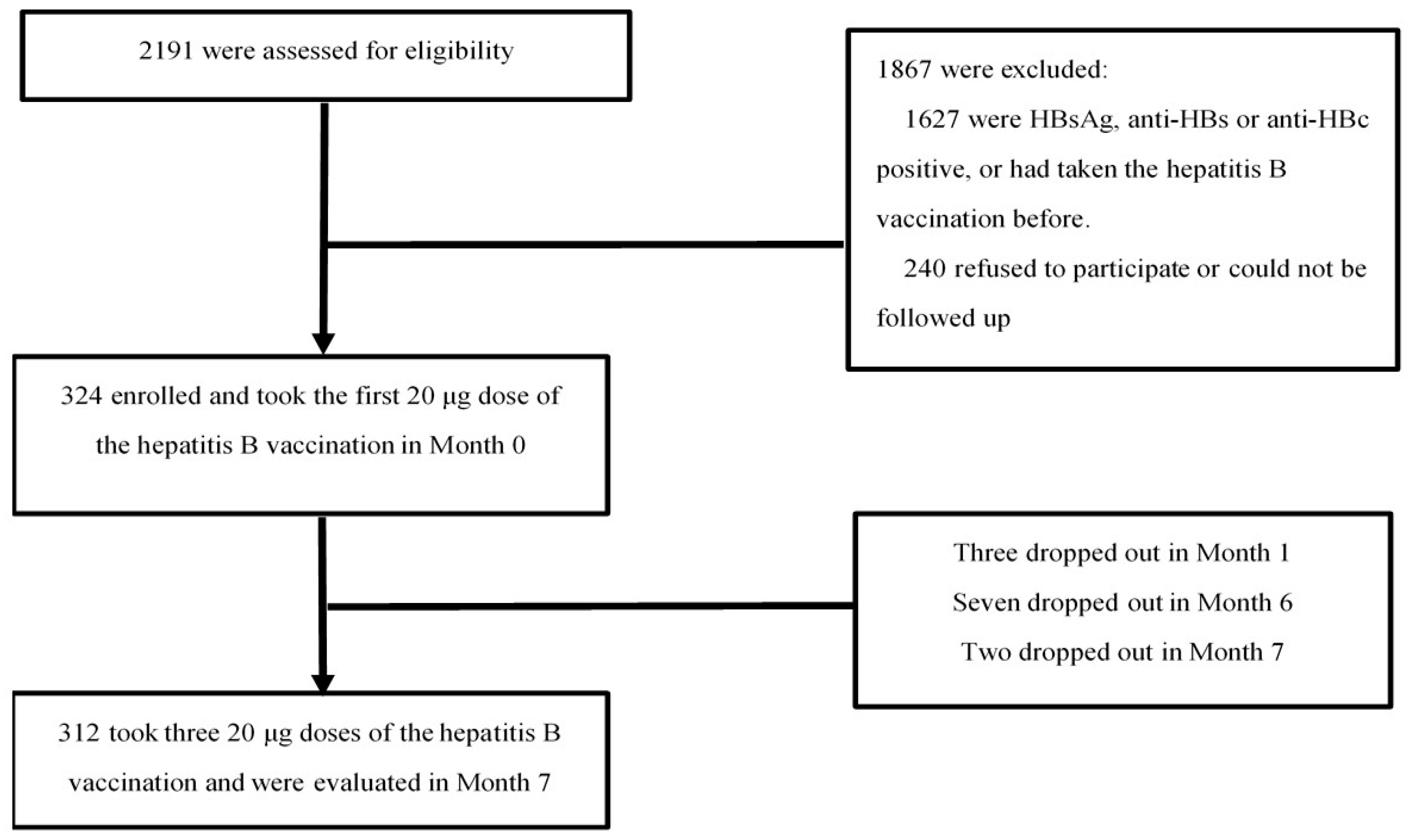
2.1. Study Design and Participants
2.2. Survey and Medical History Data Collection
2.3. Laboratory Testing
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
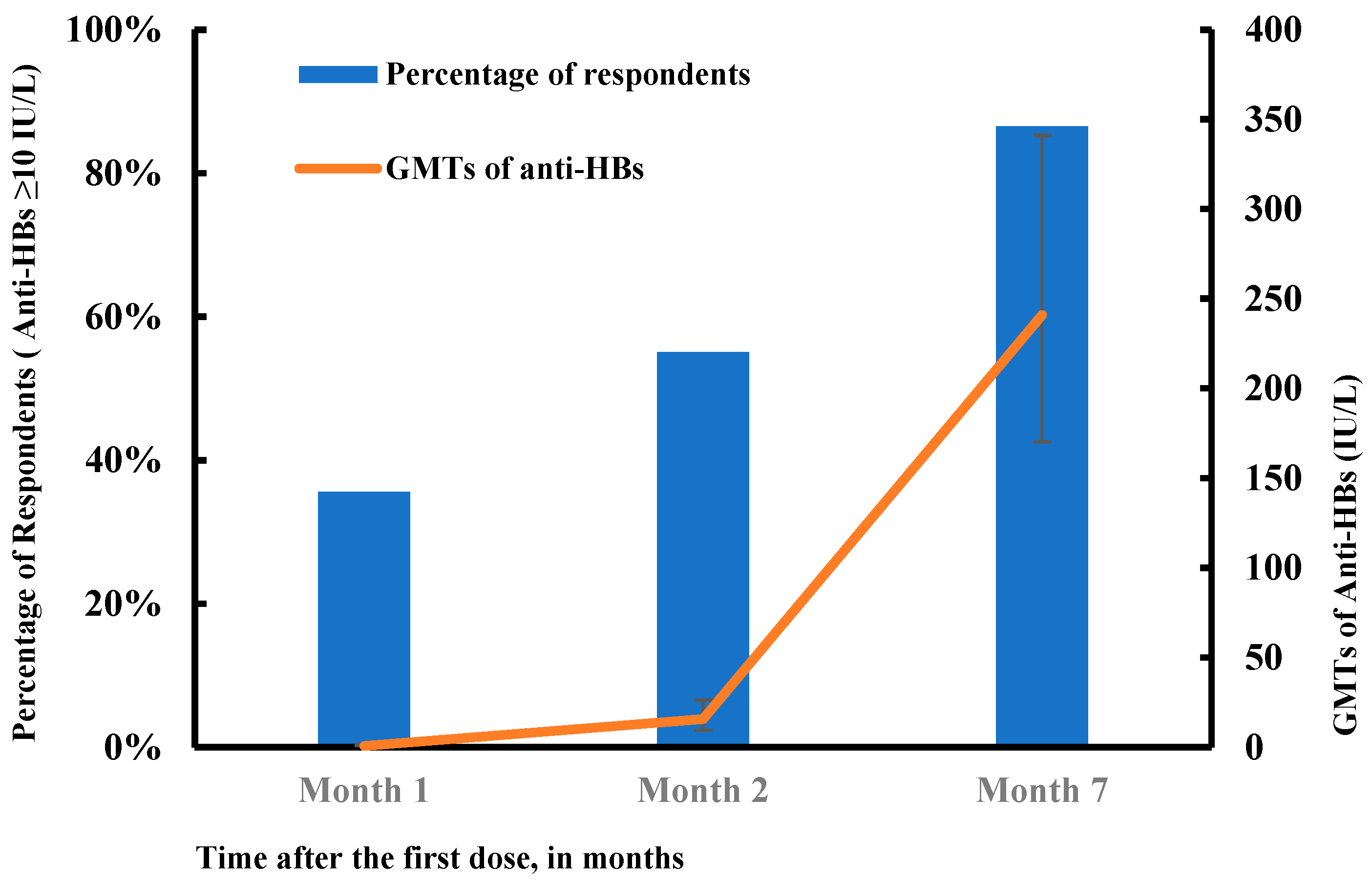
3.2. Response to HB Vaccination
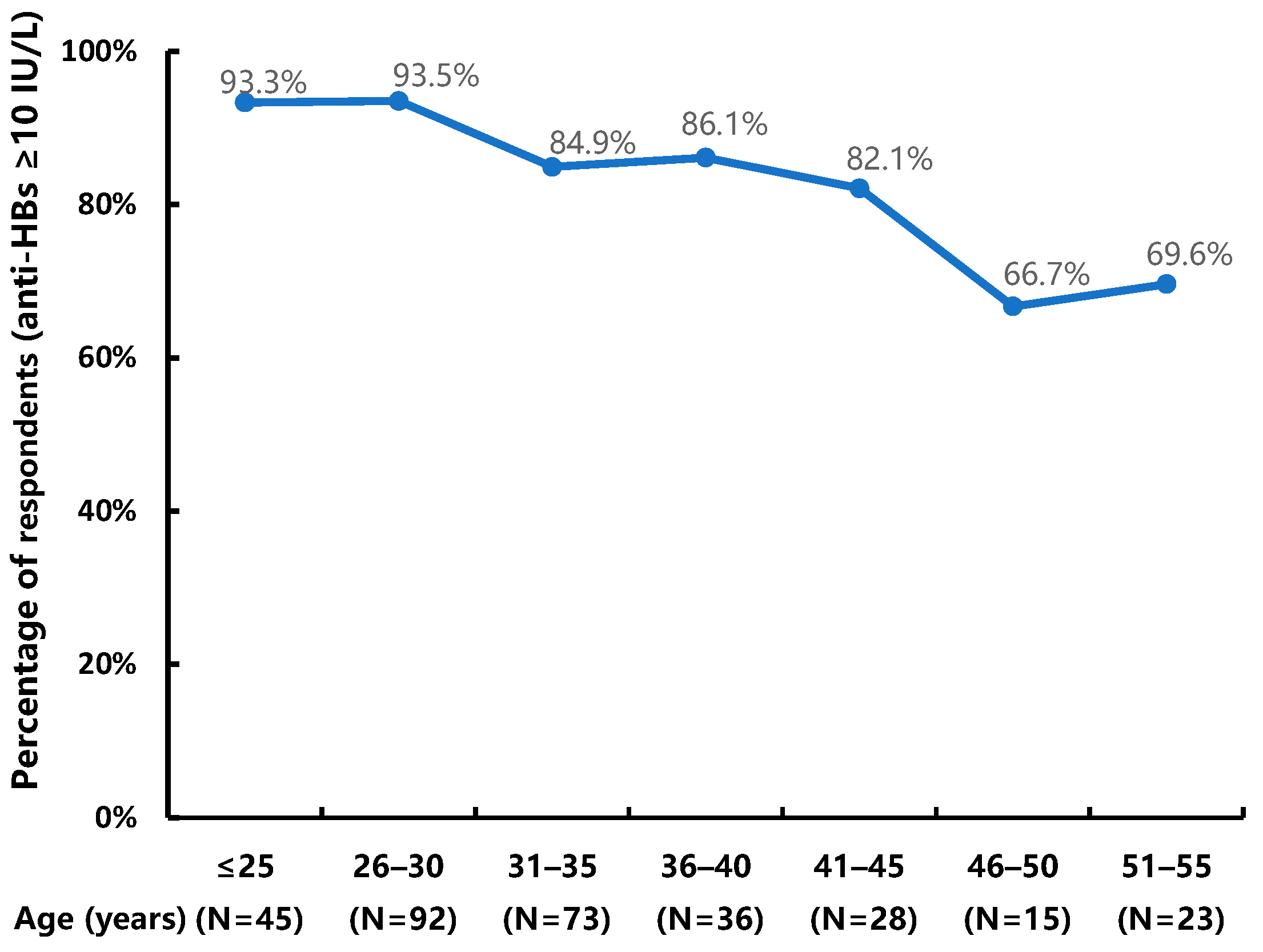
3.3. Factors Associated with Response to HB Vaccination
3.3.1. Univariate Analysis Results
3.3.2. Multivariate Analysis Results
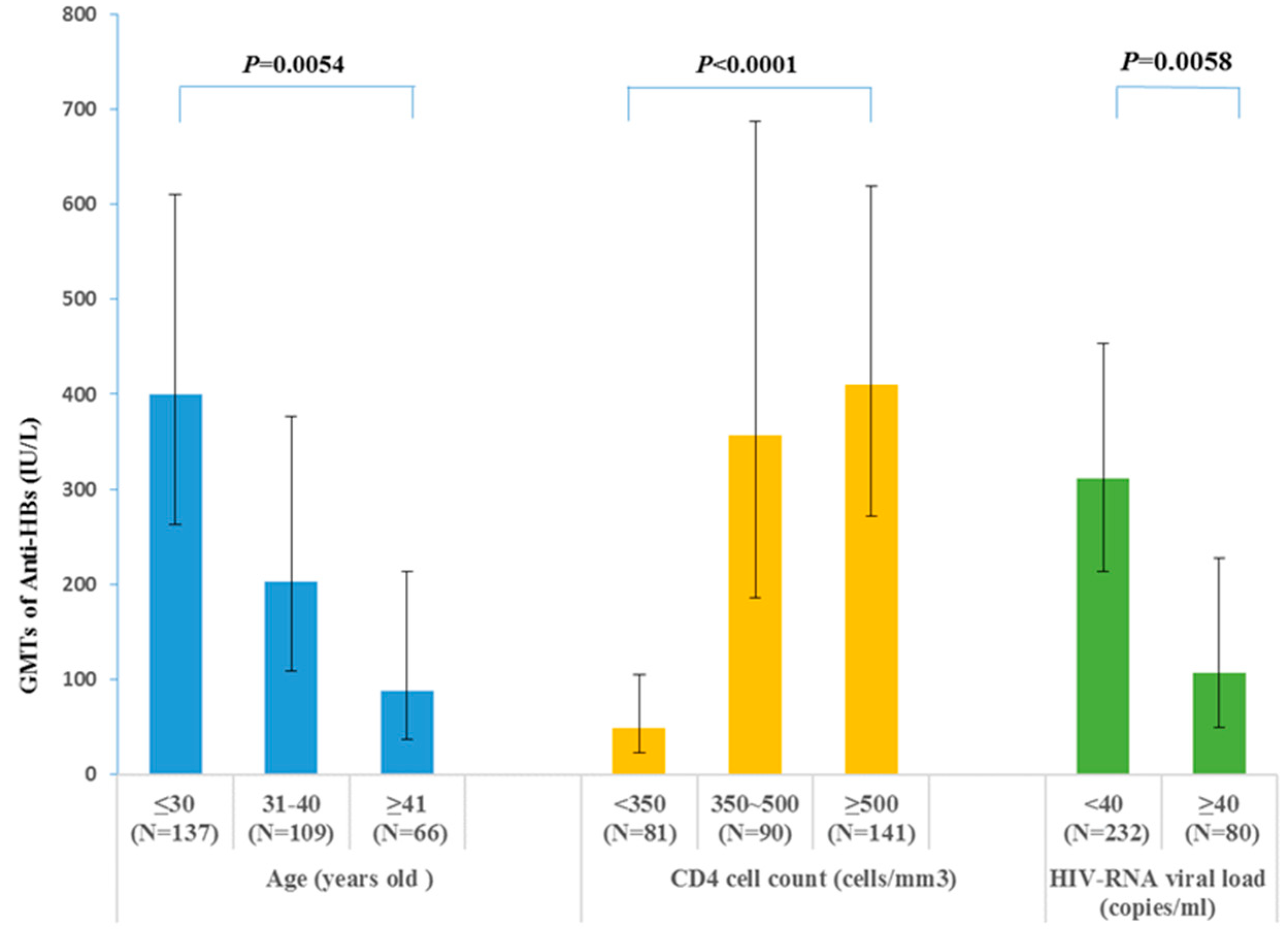
3.3.3. GMTs of the Anti-HBs in Different Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Li, J.; Wang, D.; Fung, H.; Wong, L.Y.; Zhao, L. The hepatitis B epidemic in China should receive more attention. Lancet 2018, 391, 1572. [Google Scholar] [CrossRef]
- For a hepatitis-free future. Lancet Reg. Health West. Pac. 2021, 12, 100234. [CrossRef]
- Huang, S.M.; Cai, W.P.; Hu, F.Y.; Lan, Y.; Liao, B.L.; Chen, Y.P.; Tang, X.P. Epidemiological and clinical characteristics of hepatitis B virus in HIV-infected patients in Guangdong, China. Int. J. STD AIDS 2016, 27, 890–897. [Google Scholar] [CrossRef]
- Chen, X.; He, J.M.; Ding, L.S.; Zhang, G.Q.; Zou, X.B.; Zheng, J. Prevalence of hepatitis B virus and hepatitis C virus in patients with human immunodeficiency virus infection in Central China. Arch. Virol. 2013, 158, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yan, P.; Yang, T.; Wang, Z.; Yan, Y. Epidemiological profile and risk factors of HIV and HBV/HCV co-infection in Fujian Province, southeastern China. J. Med. Virol. 2017, 89, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Chen, Q.; Li, D.; Wang, T.; Gou, Y.; Wei, B.; Tao, C. High prevalence of syphilis, HBV, and HCV co-infection, and low rate of effective vaccination against hepatitis B in HIV-infected patients in West China hospital. J. Med. Virol. 2018, 90, 101–108. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, H.; Wu, Y.; Dou, Z.; Zhang, Y.; Kleinman, N.; Bulterys, M.; Wu, Z.; Ma, Y.; Zhao, D.; et al. HIV, hepatitis B virus, and hepatitis C virus co-infection in patients in the China National Free Antiretroviral Treatment Program, 2010–2012: A retrospective observational cohort study. Lancet Infect. Dis. 2014, 14, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; O’Grady, J.; Dieterich, D.; Gazzard, B.; Agarwal, K. Increasing burden of liver disease in patients with HIV infection. Lancet 2011, 377, 1198–1209. [Google Scholar] [CrossRef]
- Mena, G.; García-Basteiro, A.L.; Bayas, J.M. Hepatitis B and A vaccination in HIV-infected adults: A review. Hum. Vaccin. Immunother. 2015, 11, 2582–2598. [Google Scholar] [CrossRef]
- Tian, Y.; Hua, W.; Wu, Y.; Zhang, T.; Wang, W.; Wu, H.; Guo, C.; Huang, X. Immune Response to Hepatitis B Virus Vaccine Among People Living With HIV: A Meta-Analysis. Front. Immunol. 2021, 12, 745541. [Google Scholar] [CrossRef]
- Farooq, P.D.; Sherman, K.E. Hepatitis B Vaccination and Waning Hepatitis B Immunity in Persons Living with HIV. Curr. HIV AIDS Rep. 2019, 16, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, J.A.; Rouphael, N.G.; Edupuganti, S.; Lai, L.; Mulligan, M.J. Strategies to increase responsiveness to hepatitis B vaccination in adults with HIV-1. Lancet. Infect. Dis. 2012, 12, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yao, T.; Chang, Y.; Gao, L.; Shao, Z.; Dong, S.; Wu, Y.; Shi, X.; Shi, J.; Feng, D.; et al. Immunogenicity and persistence of high-dose recombinant hepatitis B vaccine in adults infected with human immunodeficiency virus in China: A randomized, double-blind, parallel controlled trial. Vaccine 2021, 39, 3582–3589. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, M.; Greco, G.; Serapide, F.; Scaglione, V.; Morrone, H.; Marascio, N.; Giancotti, A.; Liberto, M.; Matera, G.; Trecarichi, E.; et al. Outcome of HBV screening and vaccination in a migrant population in southern Italy. Infez. Med. 2021, 29, 236–241. [Google Scholar] [PubMed]
- Cao, W.; Hsieh, E.; Li, T. Optimizing Treatment for Adults with HIV/AIDS in China: Successes over Two Decades and Remaining Challenges. Curr. HIV/AIDS Rep. 2020, 17, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S.; Marston, H.D. Ending the HIV–AIDS Pandemic—Follow the Science. N. Engl. J. Med. 2015, 373, 2197–2199. [Google Scholar] [CrossRef] [PubMed]
- Nauta, J. Statistics in Clinical Vaccine Trials; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- He, N. Research Progress in the Epidemiology of HIV/AIDS in China. China CDC Wkly. 2021, 3, 1022–1030. [Google Scholar] [CrossRef]
- Beijing Municipal Health Commission. Beijing HIV/AIDS Epidemic Bulletin 2021; Beijing Municipal Health Commission: Beijing, China, 2022. [Google Scholar]
- Launay, O.; van der Vliet, D.; Rosenberg, A.R.; Michel, M.-L.; Piroth, L.; Rey, D.; Colin de Verdière, N.; Slama, L.; Martin, K.; Lortholary, O.; et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: A randomized controlled trial. JAMA 2011, 305, 1432–1440. [Google Scholar] [CrossRef]
- de Vries-Sluijs, T.E.M.S.; Hansen, B.E.; van Doornum, G.J.J.; Kauffmann, R.H.; Leyten, E.M.S.; Mudrikova, T.; Brinkman, K.; den Hollander, J.G.; Kroon, F.P.; Janssen, H.L.A.; et al. A randomized controlled study of accelerated versus standard hepatitis B vaccination in HIV-positive patients. J. Infect. Dis. 2011, 203, 984–991. [Google Scholar] [CrossRef]
- Fuster, F.; Vargas, J.I.; Jensen, D.; Sarmiento, V.; Acuña, P.; Peirano, F.; Fuster, F.; Arab, J.P.; Martínez, F. CD4/CD8 ratio as a predictor of the response to HBV vaccination in HIV-positive patients: A prospective cohort study. Vaccine 2016, 34, 1889–1895. [Google Scholar] [CrossRef]
- Chaiklang, K.; Wipasa, J.; Chaiwarith, R.; Praparattanapan, J.; Supparatpinyo, K. Comparison of immunogenicity and safety of four doses and four double doses vs. standard doses of hepatitis B vaccination in HIV-infected adults: A randomized, controlled trial. PLoS ONE 2013, 8, e80409. [Google Scholar] [CrossRef] [PubMed]
- Seremba, E.; Ocama, P.; Ssekitoleko, R.; Mayanja-Kizza, H.; Adams, S.V.; Orem, J.; Katabira, E.; Reynolds, S.J.; Nabatanzi, R.; Casper, C.; et al. Immune response to the hepatitis B vaccine among HIV-infected adults in Uganda. Vaccine 2021, 39, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Gesemann, M.; Scheiermann, N. Quantification of hepatitis B vaccine-induced antibodies as a predictor of anti-HBs persistence. Vaccine 1995, 13, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.B.; Hassing, R.J.; de Vries-Sluijs, T.E.M.S.; El Barzouhi, A.; Hansen, B.E.; Schutten, M.; de Man, R.A.; van der Ende, M.E. Long-term response rates of successful hepatitis B vaccination in HIV-infected patients. Vaccine 2013, 31, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cai, B.; Rao, D.; Liu, M.; Li, Y.; Liang, X.; Cui, F.; Zhang, G.; Wang, F.; Pang, X.; et al. Rapid immunization effects of a new type of 60 μg hepatitis B vaccine compared with traditional 20 μg hepatitis B vaccines in adults. Hum. Vaccin. Immunother. 2016, 12, 2921–2926. [Google Scholar] [CrossRef]
- Bi, X.; Suzuki, Y.; Gatanaga, H.; Oka, S. High frequency and proliferation of CD4+ FOXP3+ Treg in HIV-1-infected patients with low CD4 counts. Eur. J. Immunol. 2009, 39, 301–309. [Google Scholar] [CrossRef]
- Goncalves, L.; Albarran, B.; Salmen, S.; Borges, L.; Fields, H.; Montes, H.; Soyano, A.; Diaz, Y.; Berrueta, L. The nonresponse to hepatitis B vaccination is associated with impaired lymphocyte activation. Virology 2004, 326, 20–28. [Google Scholar] [CrossRef]
- Pasricha, N.; Datta, U.; Chawla, Y.; Singh, S.; Arora, S.K.; Sud, A.; Minz, R.W.; Saikia, B.; Singh, H.; James, I.; et al. Immune responses in patients with HIV infection after vaccination with recombinant Hepatitis B virus vaccine. BMC Infect. Dis. 2006, 6, 65. [Google Scholar] [CrossRef]
- Bekele, Y.; Lemma, M.; Bobosha, K.; Yibeltal, D.; Nasi, A.; Gebre, M.; Nilsson, A.; Aseffa, A.; Howe, R.; Chiodi, F. Homing defects of B cells in HIV-1 infected children impair vaccination responses. Vaccine 2019, 37, 2348–2355. [Google Scholar] [CrossRef]
- Nicolini, L.A.; Magne, F.; Signori, A.; Di Biagio, A.; Sticchi, L.; Paganino, C.; Durando, P.; Viscoli, C. Hepatitis B Virus Vaccination in HIV: Immunogenicity and Persistence of Seroprotection up to 7 Years Following a Primary Immunization Course. AIDS Res. Hum. Retrovir. 2018, 34, 922–928. [Google Scholar] [CrossRef]
- Pettit, N.N.; DePestel, D.D.; Malani, P.N.; Riddell, J.T. Factors associated with seroconversion after standard dose hepatitis B vaccination and high-dose revaccination among HIV-infected patients. HIV Clin. Trials 2010, 11, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Van Den Ende, C.; Marano, C.; Van Ahee, A.; Bunge, E.M.; De Moerlooze, L. The immunogenicity and safety of GSK’s recombinant hepatitis B vaccine in adults: A systematic review of 30 years of experience. Expert. Rev. Vaccines 2017, 16, 811–832. [Google Scholar] [CrossRef]
- Yang, S.; Tian, G.; Cui, Y.; Ding, C.; Deng, M.; Yu, C.; Xu, K.; Ren, J.; Yao, J.; Li, Y.; et al. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci. Rep. 2016, 6, 27251. [Google Scholar] [CrossRef]
- Psevdos, G.; Kim, J.H.; Groce, V.; Sharp, V. Efficacy of double-dose hepatitis B rescue vaccination in HIV-infected patients. AIDS Patient Care STDS 2010, 24, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.; de Barra, E.; Sadlier, C.; Kelly, S.; Dowling, C.; McNally, C.; Bergin, C. Impact of a new vaccine clinic on hepatitis B vaccine completion and immunological response rates in an HIV-positive cohort. J. Infect. Public Health 2013, 6, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Chonwattana, W.; Raengsakulrach, B.; Holtz, T.H.; Wasinrapee, P.; Tongtoyai, J.; Chaikummao, S.; Pattanasin, S.; McNicholl, J.M.; van Griensven, F.; Curlin, M.E. Hepatitis B vaccination uptake and correlates of serologic response among HIV-infected and uninfected men who have sex with men (MSM) in Bangkok, Thailand. Vaccine 2016, 34, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.Q.; Moorman, J.P. Immune exhaustion and immune senescence: Two distinct pathways for HBV vaccine failure during HCV and/or HIV infection. Arch. Immunol. Ther. Exp. 2013, 61, 193–201. [Google Scholar] [CrossRef]
- Nashibi, R.; Alavi, S.M.; Yousefi, F.; Salmanzadeh, S.; Moogahi, S.; Ahmadi, F.; Farashahinejad, M. Post-vaccination immunity against hepatitis B virus and predictors for non-responders among medical staff. Jundishapur J. Microbiol. 2015, 8, e19579. [Google Scholar] [CrossRef]
- Tohme, R.A.; Awosika-Olumo, D.; Nielsen, C.; Khuwaja, S.; Scott, J.; Xing, J.; Drobeniuc, J.; Hu, D.J.; Turner, C.; Wafeeg, T.; et al. Evaluation of hepatitis B vaccine immunogenicity among older adults during an outbreak response in assisted living facilities. Vaccine 2011, 29, 9316–9320. [Google Scholar] [CrossRef]
- Williams, R.E.; Sena, A.C.; Moorman, A.C.; Moore, Z.S.; Sharapov, U.M.; Drobenuic, J.; Hu, D.J.; Wood, H.W.; Xing, J.; Spradling, P.R. Hepatitis B vaccination of susceptible elderly residents of long term care facilities during a hepatitis B outbreak. Vaccine 2012, 30, 3147–3150. [Google Scholar] [CrossRef]

| Characteristic | Total Sample (N = 312) | Respondents (N = 270) | Non-Respondents (N = 42) |
|---|---|---|---|
| Age at enrollment, years †** | 34.0 (9.8) (18–73) | 33.3 (9.3) (18–73) | 38.7 (11.1) (18–61) |
| Sex, N (%) | |||
| Male | 299 (95.8) | 259 (95.9) | 40 (95.2) |
| Female | 13 (4.2) | 11 (4.1) | 2 (4.8) |
| Transgender | 0 (0) | 0 (0) | 0 (0) |
| Place of birth, N (%) | |||
| Beijing | 58 (18.6) | 47 (17.4) | 11(26.2) |
| Other provinces | 254 (81.4) | 223 (82.6) | 31 (73.8) |
| Marriage, N (%) * | |||
| Never married | 209 (67.0) | 188 (69.6) | 21 (50.0) |
| Married | 103 (33.0) | 82 (30.4) | 21 (50.0) |
| Education, N (%) ** | |||
| High school diploma or less | 130 (41.7) | 105 (38.9) | 25 (59.5) |
| College degree or higher | 182 (58.3) | 165 (61.1) | 17 (40.5) |
| Transmission route, N (%) | |||
| MSM | 275 (88.1) | 238 (88.2) | 37 (88.1) |
| MSWM and others | 37 (11.9) | 32 (11.8) | 5 (11.8) |
| Occupation, N (%) | |||
| Fixed | 265 (84.9) | 231 (85.6) | 34 (81.0) |
| Non-fixed | 47 (15.1) | 39 (14.4) | 8 (19.1) |
| BMI † | 22.2 (3.2) (15.7–36.3) | 22.2 (3.2) (15.2–36.3) | 22.6 (3.2) (18.0–33.1) |
| Age at HIV diagnosis, years †** | 31.4 (9.7) (7–68) | 30.5 (9.0) (17–68) | 36.8 (12.4) (7–60) |
| Time since HIV diagnosis, years §** | 2.0 (4.0) (0–17) | 2.0 (3.0) (0–17) | 1.0 (2.0) (0–17) |
| Time using ART, years §** | 2.0 (3.0) (0–17) | 2.0 (3.0) (0–17) | 1.0 (1.0) (0–17) |
| CD4 cell count at enrollment, cells/mm3 §** | 482.8 (308) (30.1–1338.0) | 494.4 (276.7) (40.7–1338.0) | 325.6 (305.5) (30.1–851.3) |
| CD4/CD8 ratio §** | 0.54 (0.46) (0.02–2.64) | 0.56 (0.44) (0.02–2.64) | 0.38 (0.45) (0.05–1.11) |
| HIV-RNA viral load < 40 copies/mL, N (%) ** | 232(74.4) | 210 (77.8) | 22 (52.4) |
| Concomitant diseases, N (%) ** | 20 (6.4) | 13 (4.8) | 7 (16.7) |
| Smoked in the past 6 months, N (%) | 100 (32.1) | 84 (31.1) | 16 (38.1) |
| Time Points | Response | Characteristic | Multivariate OR (95% CI) | p Value |
|---|---|---|---|---|
| Month 1 | Strong response | Age (years) | ||
| ≤30 | Reference | |||
| 31–40, ≥41 | 0.46 (0.25–0.87) | 0.0162 | ||
| Moderate response | Age (years) | |||
| ≤30 | Reference | |||
| 31–40, ≥41 | 0.43 (0.27–0.71) | 0.0007 | ||
| Education | ||||
| High school diploma or less | Reference | |||
| College degree or higher | 2.33 (1.15–4.73) | 0.0192 | ||
| Weak response | Age (years) | |||
| ≤30 | Reference | |||
| 31–40, ≥41 | 0.38 (0.21–0.68) | 0.0012 | ||
| Education | ||||
| High school diploma or less | Reference | |||
| College degree or higher | 4.28 (1.69–10.86) | 0.0022 | ||
| Month 2 | Strong response | Age (years) | ||
| ≤30 | Reference | |||
| 31–40, ≥41 | 0.33 (0.20–0.54) | <0.0001 | ||
| Moderate response | Age (years) | |||
| ≤30 | Reference | |||
| 31–40, ≥41 | 0.31 (0.21–0.48) | <0.0001 | ||
| Weak response | Age (years) | |||
| ≤30 | Reference | |||
| 31–40, ≥41 | 0.51 (0.33–0.79) | 0.0024 | ||
| Month 7 | Strong response | Age (years) | ||
| ≤30 | Reference | |||
| 31–40, ≥41 | 0.41 (0.24–0.69) | 0.0007 | ||
| HIV-RNA viral load (copies/mL) | ||||
| <40 | Reference | |||
| ≥40 | 0.35 (0.15–0.83) | 0.0170 | ||
| CD4 cell count (cells/mm3) | ||||
| <350 | Reference | |||
| 350~500, ≥500 | 2.54 (1.54–4.19) | 0.0003 | ||
| Moderate response | Age (years) | |||
| ≤30 | Reference | |||
| 31–40, ≥41 | 0.42 (0.26–0.69) | 0.0006 | ||
| HIV-RNA viral load (copies/mL) | ||||
| <40 | Reference | |||
| ≥40 | 0.37 (0.17–0.82) | 0.0150 | ||
| CD4 cell count (cells/mm3) | ||||
| <350 | Reference | |||
| 350~500, ≥500 | 2.11 (1.31–3.39) | 0.0020 | ||
| Weak response | Age (years) | |||
| ≤30 | Reference | |||
| 31–40, ≥41 | 0.54 (0.32–0.92) | 0.0241 | ||
| HIV-RNA viral load (copies/mL) | ||||
| <40 | Reference | |||
| ≥40 | 0.37 (0.15–0.90) | 0.0275 | ||
| CD4 cell count (cells/mm3) | ||||
| <350 | Reference | |||
| 350–500, ≥500 | 1.70 (1.02–2.84) | 0.0436 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, L.; Hua, W.; Liu, X.; Pang, X.; Guo, C.; Zhang, W.; Tian, Y.; Qiu, Q. Associated Factors and Immune Response to the Hepatitis B Vaccine with a Standard Schedule: A Prospective Study of People with HIV in China. Vaccines 2023, 11, 921. https://doi.org/10.3390/vaccines11050921
Nie L, Hua W, Liu X, Pang X, Guo C, Zhang W, Tian Y, Qiu Q. Associated Factors and Immune Response to the Hepatitis B Vaccine with a Standard Schedule: A Prospective Study of People with HIV in China. Vaccines. 2023; 11(5):921. https://doi.org/10.3390/vaccines11050921
Chicago/Turabian StyleNie, Li, Wei Hua, Xiuying Liu, Xinghuo Pang, Caiping Guo, Wei Zhang, Yakun Tian, and Qian Qiu. 2023. "Associated Factors and Immune Response to the Hepatitis B Vaccine with a Standard Schedule: A Prospective Study of People with HIV in China" Vaccines 11, no. 5: 921. https://doi.org/10.3390/vaccines11050921
APA StyleNie, L., Hua, W., Liu, X., Pang, X., Guo, C., Zhang, W., Tian, Y., & Qiu, Q. (2023). Associated Factors and Immune Response to the Hepatitis B Vaccine with a Standard Schedule: A Prospective Study of People with HIV in China. Vaccines, 11(5), 921. https://doi.org/10.3390/vaccines11050921




