Early Oral Administration of Ginseng Stem-Leaf Saponins Enhances the Peyer’s Patch-Dependent Maternal IgA Antibody Response to a PEDV Inactivated Vaccine in Mice, with Gut Microbiota Involvement
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. Adjuvant
2.3. Animal Studies
2.4. Enzyme-Linked Immunosorbent Assay (ELISA) for Antibody Quantification
2.5. Histochemistry and Immunofluorescence
2.6. Cell Isolation and Flow Cytometry
2.7. Transcriptome Analysis
2.8. Quantitative Real-Time PCR (qPCR) Analysis
2.9. Gut Microbial Analysis
2.10. Statistical Analysis
3. Results
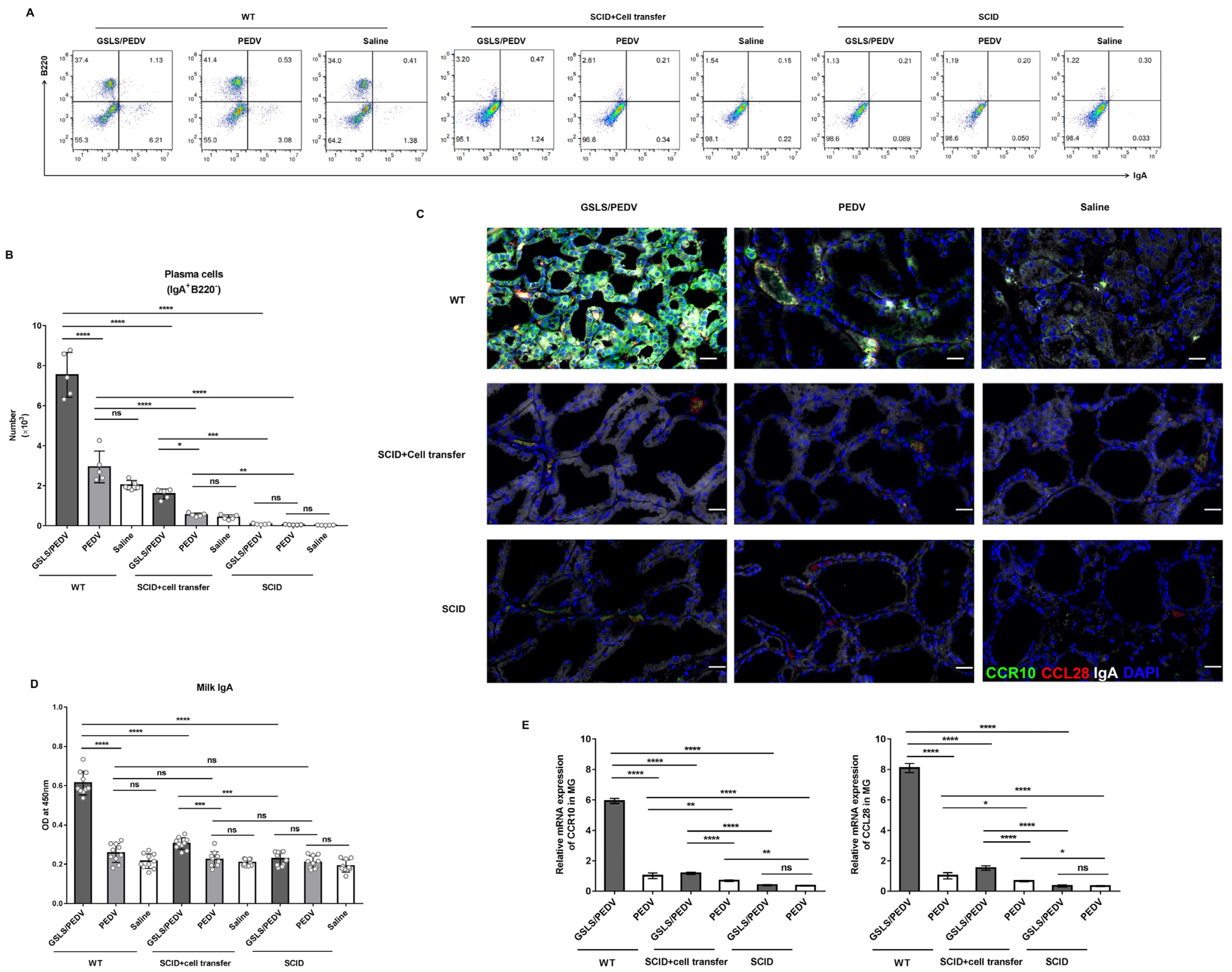
3.1. GSLS Enhance the PEDV-Specific Maternal IgA Antibody Response
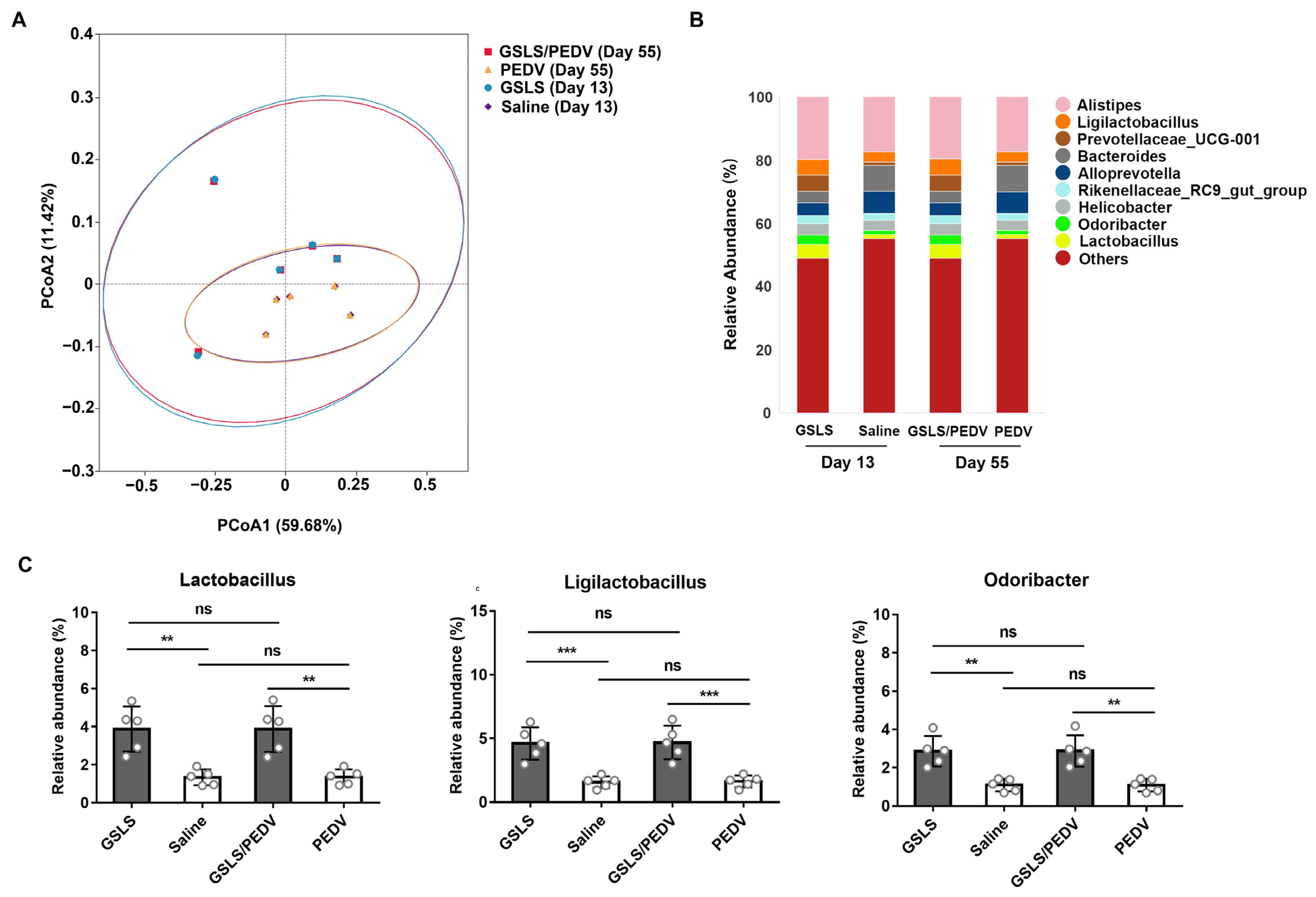
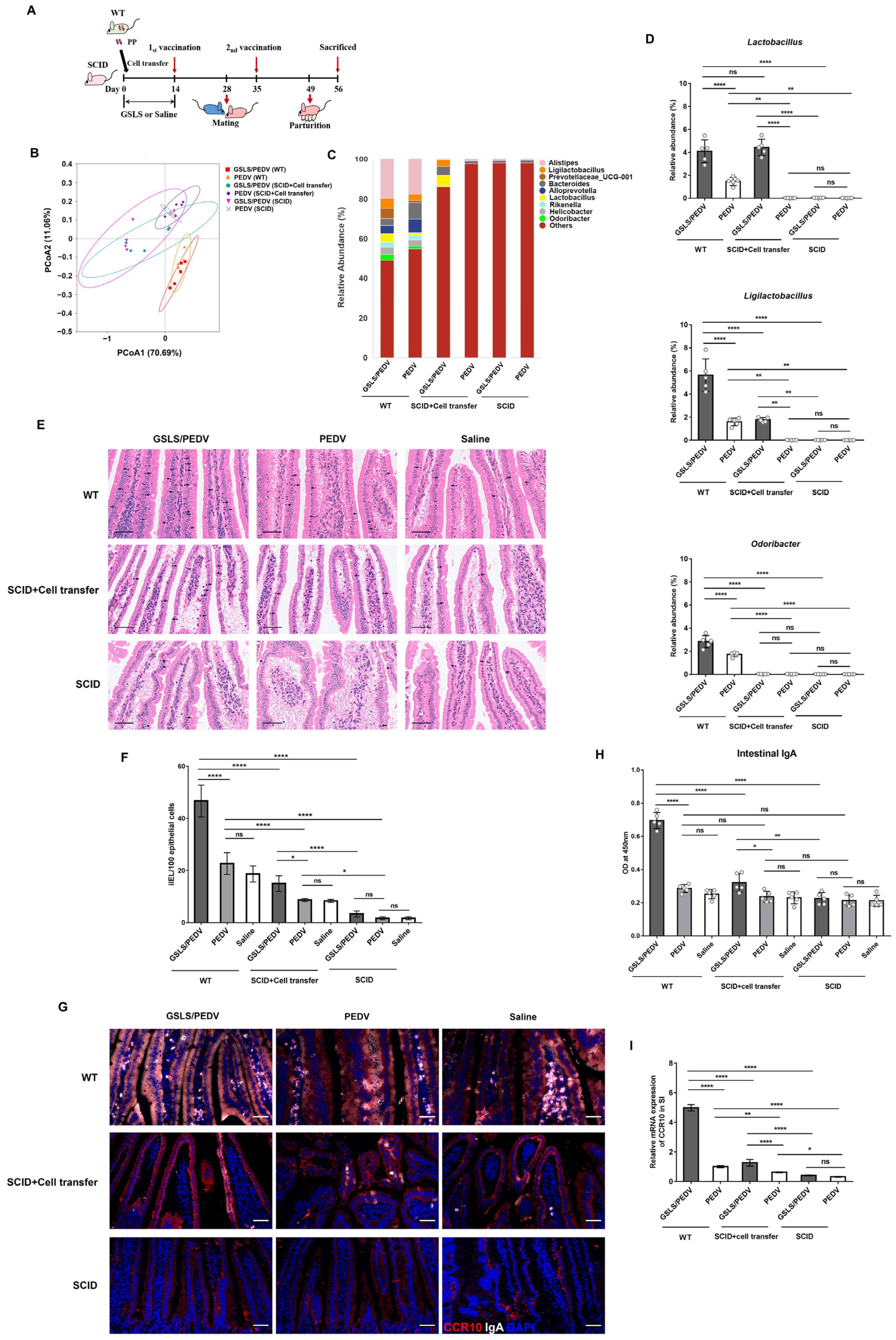
3.2. GSLS Improve the Composition of the Gut Microbiota
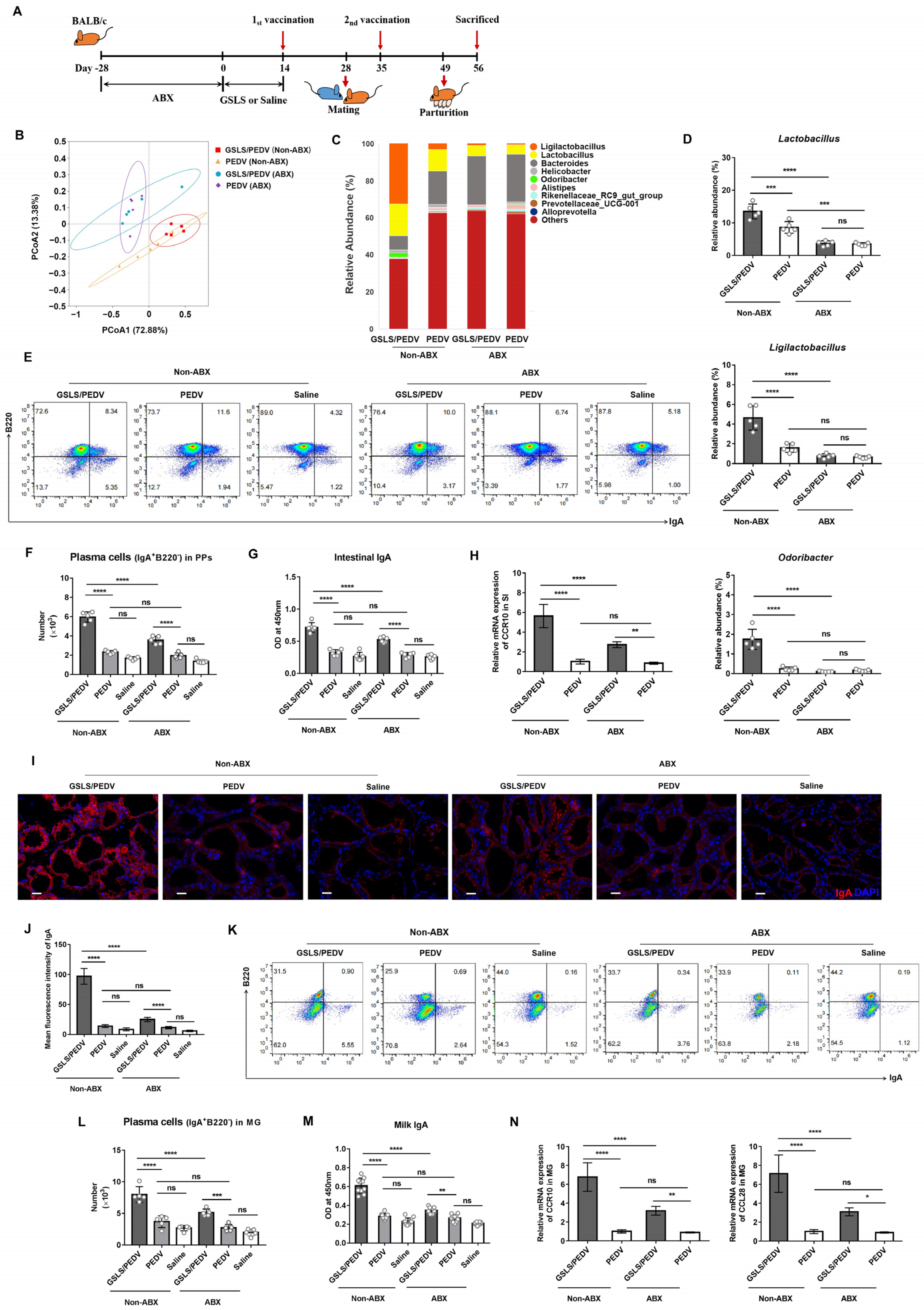
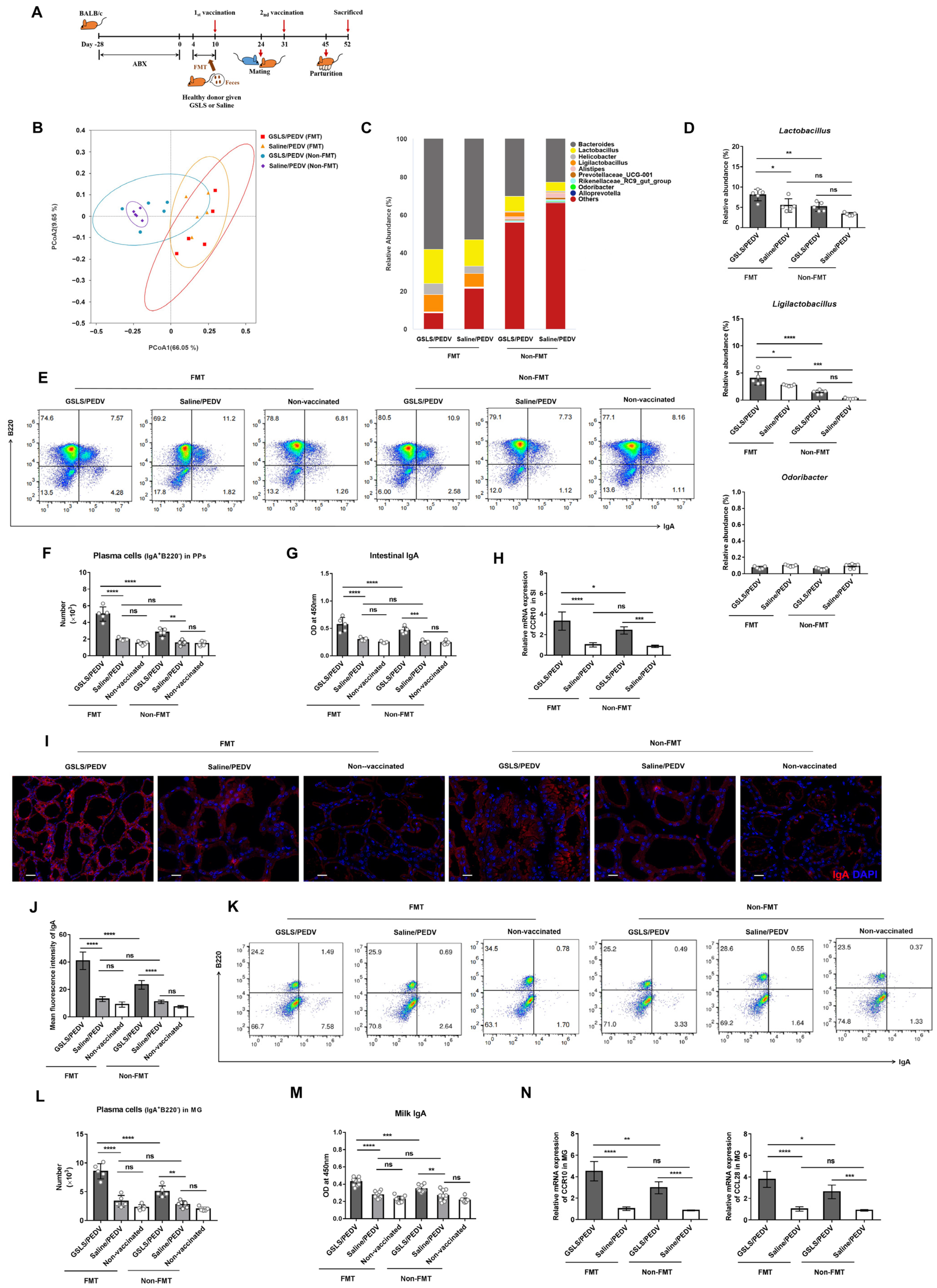
3.3. Intestinal Microflora Constituents Participate in the GSLS-Enhanced Specific Maternal IgA Response
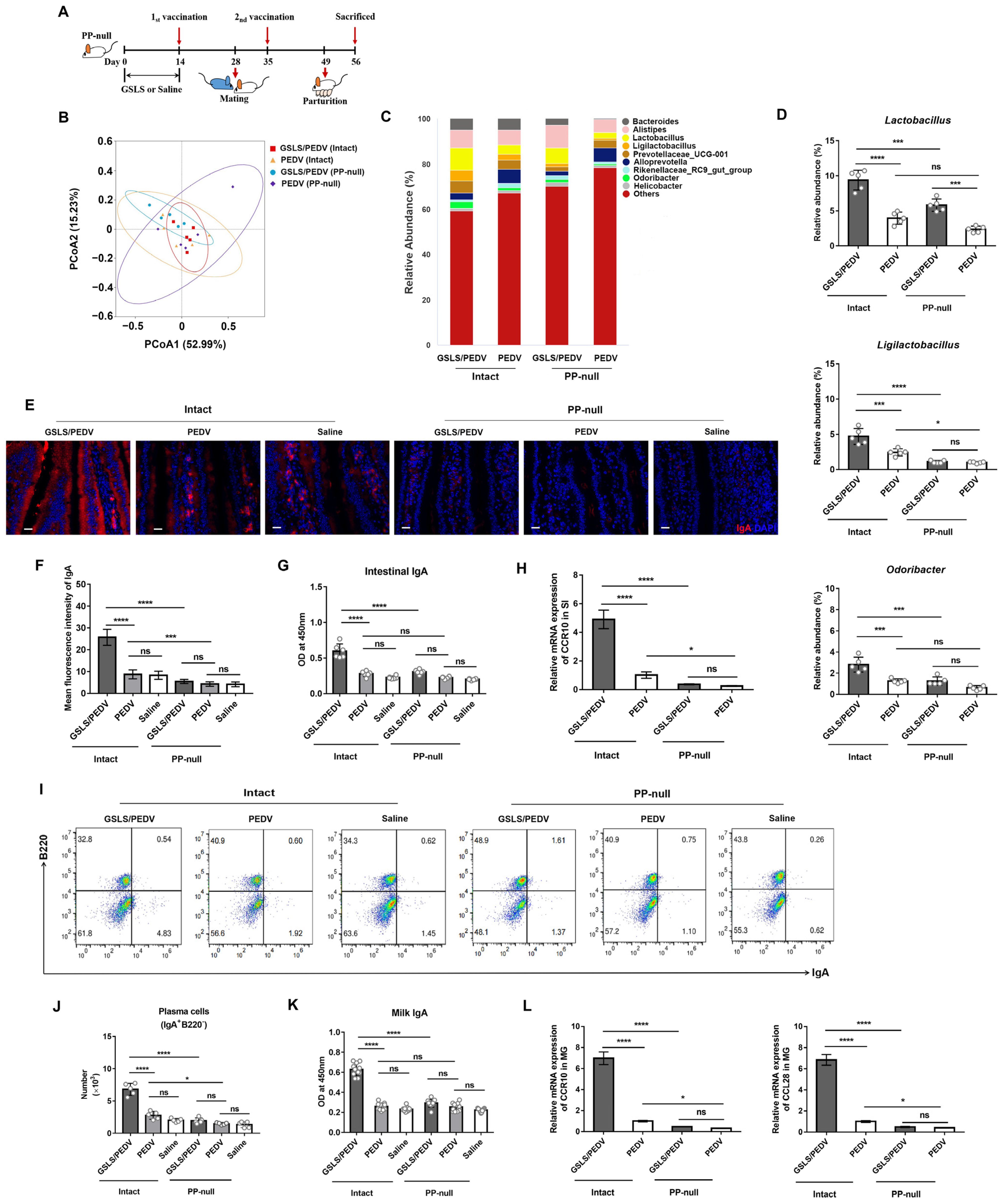
3.4. PPs Play a Key Role in GSLS Regulation of the Gut Microbiota and Enhancement of the Maternal IgA Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef] [PubMed]
- Suda, Y.; Miyazaki, A.; Miyazawa, K.; Shibahara, T.; Ohashi, S. Systemic and intestinal porcine epidemic diarrhea virus-specific antibody response and distribution of antibody-secreting cells in experimentally infected conventional pigs. Vet. Res. 2021, 52, 2. [Google Scholar] [CrossRef]
- Langel, S.N.; Paim, F.C.; Lager, K.M.; Vlasova, A.N.; Saif, L.J. Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): Historical and current concepts. Virus Res. 2016, 226, 93–107. [Google Scholar] [CrossRef]
- Gopalakrishna, K.P.; Macadangdang, B.R.; Rogers, M.B.; Tometich, J.T.; Firek, B.A.; Baker, R.; Ji, J.; Burr, A.H.P.; Ma, C.; Good, M.; et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 2019, 25, 1110–1115. [Google Scholar] [CrossRef]
- Fleming, A.; Castro-Dopico, T.; Clatworthy, M.R. B cell class switching in intestinal immunity in health and disease. Scand. J. Immunol. 2022, 95, e13139. [Google Scholar] [CrossRef] [PubMed]
- Bohl, E.H.; Gupta, R.K.; Olquin, M.V.; Saif, L.J. Antibody responses in serum, colostrum, and milk of swine after infection or vaccination with transmissible gastroenteritis virus. Infect. Immun. 1972, 6, 289–301. [Google Scholar] [CrossRef]
- Chattha, K.S.; Roth, J.A.; Saif, L.J. Strategies for design and application of enteric viral vaccines. Annu. Rev. Anim. Biosci. 2015, 3, 375–395. [Google Scholar] [CrossRef]
- Butcher, E.C.; Picker, L.J. Lymphocyte homing and homeostasis. Science 1996, 272, 60–66. [Google Scholar] [CrossRef]
- Hernandez-Ruiz, M.; Zlotnik, A. Mucosal Chemokines. J. Interferon Cytokine Res. 2017, 37, 62–70. [Google Scholar] [CrossRef]
- Wilson, E.; Butcher, E.C. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J. Exp. Med. 2004, 200, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Morteau, O.; Gerard, C.; Lu, B.; Ghiran, S.; Rits, M.; Fujiwara, Y.; Law, Y.; Distelhorst, K.; Nielsen, E.M.; Hill, E.D.; et al. An indispensable role for the chemokine receptor CCR10 in IgA antibody-secreting cell accumulation. J. Immunol. 2008, 181, 6309–6315. [Google Scholar] [CrossRef]
- Langel, S.N.; Wang, Q.; Vlasova, A.N.; Saif, L.J. Host Factors Affecting Generation of Immunity Against Porcine Epidemic Diarrhea Virus in Pregnant and Lactating Swine and Passive Protection of Neonates. Pathogens 2020, 9, 130. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, P.; Liu, P.; Li, Y.; Li, J.; Zhang, E.; Jin, Y.; Yang, Q. A Novel Pathway for Porcine Epidemic Diarrhea Virus Transmission from Sows to Neonatal Piglets Mediated by Colostrum. J. Virol. 2022, 96, e0047722. [Google Scholar] [CrossRef]
- Su, F.; Xu, L.; Xue, Y.; Xu, W.; Li, J.; Yu, B.; Ye, S.; Yuan, X. Immune Enhancement of Nanoparticle-Encapsulated Ginseng Stem-Leaf Saponins on Porcine Epidemic Diarrhea Virus Vaccine in Mice. Vaccines 2022, 10, 1810. [Google Scholar] [CrossRef]
- Zhai, L.; Wang, Y.; Yu, J.; Hu, S. Enhanced immune responses of chickens to oral vaccination against infectious bursal disease by ginseng stem-leaf saponins. Poult. Sci. 2014, 93, 2473–2481. [Google Scholar] [CrossRef]
- Li, R.; Ma, Y.; Zhai, L.; Lu, Y.; Chi, X.; Wu, J.; Hu, S. Enhanced immune response to foot-and-mouth disease vaccine by oral administration of ginseng stem-leaf saponins. Immunopharmacol. Immunotoxicol. 2016, 38, 257–263. [Google Scholar] [CrossRef]
- Marciani, D.J. Vaccine adjuvants: Role and mechanisms of action in vaccine immunogenicity. Drug Discov. Today. 2003, 8, 934–943. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Moore, D.J.; Shen, X.; Peek, R.M.; Acra, S.A.; Li, H.; Ren, X.; Polk, D.B.; Yan, F. An LGG-derived protein promotes IgA production through upregulation of APRIL expression in intestinal epithelial cells. Mucosal Immunol. 2017, 10, 373–384. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Tres, A.; Massot-Cladera, M.; Franch, A.; Castell, M.; Guardiola, F.; Perez-Cano, F.J.; Rodriguez-Lagunas, M.J. Lactobacillus fermentum CECT5716 Supplementation in Rats during Pregnancy and Lactation Impacts Maternal and Offspring Lipid Profile, Immune System and Microbiota. Cells 2020, 9, 575. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Gao, Z.Y.; Liang, W.; Sun, Y.C. Ameliorative effect of ginsenoside Rg1 on dextran sulfate sodium-induced colitis: Involvement of intestinal barrier remodeling in mice. Ann. Transl. Med. 2022, 10, 1328. [Google Scholar] [CrossRef]
- Zhou, R.; He, D.; Zhang, H.; Xie, J.; Zhang, S.; Tian, X.; Zeng, H.; Qin, Y.; Huang, L. Ginsenoside Rb1 protects against diabetes-associated metabolic disorders in Kkay mice by reshaping gut microbiota and fecal metabolic profiles. J. Ethnopharmacol. 2023, 303, 115997. [Google Scholar] [CrossRef]
- Xia, T.; Fang, B.; Kang, C.; Zhao, Y.; Qiang, X.; Zhang, X.; Wang, Y.; Zhong, T.; Xiao, J.; Wang, M. Hepatoprotective Mechanism of Ginsenoside Rg1 against Alcoholic Liver Damage Based on Gut Microbiota and Network Pharmacology. Oxid. Med. Cell Longev. 2022, 2022, 5025237. [Google Scholar] [CrossRef] [PubMed]
- Usami, K.; Niimi, K.; Matsuo, A.; Suyama, Y.; Sakai, Y.; Sato, S.; Fujihashi, K.; Kiyono, H.; Uchino, S.; Furukawa, M.; et al. The gut microbiota induces Peyer’s-patch-dependent secretion of maternal IgA into milk. Cell Rep. 2021, 36, 109655. [Google Scholar] [CrossRef]
- Hashizume, T.; Togawa, A.; Nochi, T.; Igarashi, O.; Kweon, M.N.; Kiyono, H.; Yamamoto, M. Peyer’s patches are required for intestinal immunoglobulin A responses to Salmonella spp. Infect. Immun. 2008, 76, 927–934. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Langel, S.N.; Paim, F.C.; Alhamo, M.A.; Buckley, A.; Van Geelen, A.; Lager, K.M.; Vlasova, A.N.; Saif, L.J. Stage of Gestation at Porcine Epidemic Diarrhea Virus Infection of Pregnant Swine Impacts Maternal Immunity and Lactogenic Immune Protection of Neonatal Suckling Piglets. Front. Immunol. 2019, 10, 727. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Notas, G.; Stathopoulos, E.N.; Tsapis, A.; Castanas, E. The TNFSF Members APRIL and BAFF and Their Receptors TACI, BCMA, and BAFFR in Oncology, With a Special Focus in Breast Cancer. Front. Oncol. 2020, 10, 827. [Google Scholar] [CrossRef] [PubMed]
- Reboldi, A.; Arnon, T.I.; Rodda, L.B.; Atakilit, A.; Sheppard, D.; Cyster, J.G. IgA production requires B cell interaction with subepithelial dendritic cells in Peyer’s patches. Science 2016, 352, aaf4822. [Google Scholar] [CrossRef]
- Bonnardel, J.; Da Silva, C.; Henri, S.; Tamoutounour, S.; Chasson, L.; Montanana-Sanchis, F.; Gorvel, J.P.; Lelouard, H. Innate and adaptive immune functions of peyer’s patch monocyte-derived cells. Cell Rep. 2015, 11, 770–784. [Google Scholar] [CrossRef]
- Tezuka, H.; Ohteki, T. Regulation of IgA Production by Intestinal Dendritic Cells and Related Cells. Front. Immunol. 2019, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hu, S.; Davila, M.L.; Yang, J.; Lin, Y.D.; Albanese, J.M.; Lo, Y.; Wang, Y.; Kennett, M.J.; Liu, Q.; et al. Coordinated co-migration of CCR10+ antibody-producing B cells with helper T cells for colonic homeostatic regulation. Mucosal Immunol. 2021, 14, 420–430. [Google Scholar] [CrossRef]
- Biram, A.; Stromberg, A.; Winter, E.; Stoler-Barak, L.; Salomon, R.; Addadi, Y.; Dahan, R.; Yaari, G.; Bemark, M.; Shulman, Z. BCR affinity differentially regulates colonization of the subepithelial dome and infiltration into germinal centers within Peyer’s patches. Nat. Immunol. 2019, 20, 482–492. [Google Scholar] [CrossRef]
- Zhao, Q.; Elson, C.O. Adaptive immune education by gut microbiota antigens. Immunology 2018, 154, 28–37. [Google Scholar] [CrossRef]
- Hapfelmeier, S.; Lawson, M.A.; Slack, E.; Kirundi, J.K.; Stoel, M.; Heikenwalder, M.; Cahenzli, J.; Velykoredko, Y.; Balmer, M.L.; Endt, K.; et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 2010, 328, 1705–1709. [Google Scholar] [CrossRef]
- Lecuyer, E.; Rakotobe, S.; Lengline-Garnier, H.; Lebreton, C.; Picard, M.; Juste, C.; Fritzen, R.; Eberl, G.; McCoy, K.D.; Macpherson, A.J.; et al. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity 2014, 40, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Wang, Z.; Sui, L.; Xu, Y.; Wang, L.; Qiao, X.; Cui, W.; Jiang, Y.; Zhou, H.; Tang, L.; et al. Lactobacillus johnsonii activates porcine monocyte derived dendritic cells maturation to modulate Th cellular immune response. Cytokine 2021, 144, 155581. [Google Scholar] [CrossRef]
- Guerrero Sanchez, M.; Passot, S.; Campoy, S.; Olivares, M.; Fonseca, F. Ligilactobacillus salivarius functionalities, applications, and manufacturing challenges. Appl. Microbiol. Biotechnol. 2022, 106, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Huber-Ruano, I.; Calvo, E.; Mayneris-Perxachs, J.; Rodriguez-Pena, M.M.; Ceperuelo-Mallafre, V.; Cedo, L.; Nunez-Roa, C.; Miro-Blanch, J.; Arnoriaga-Rodriguez, M.; Balvay, A.; et al. Orally administered Odoribacter laneus improves glucose control and inflammatory profile in obese mice by depleting circulating succinate. Microbiome 2022, 10, 135. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The gut microbiome as a modulator of healthy ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565–584. [Google Scholar] [CrossRef]
- Murphy, K.; Curley, D.; O’Callaghan, T.F.; O’Shea, C.A.; Dempsey, E.M.; O’Toole, P.W.; Ross, R.P.; Ryan, C.A.; Stanton, C. The Composition of Human Milk and Infant Faecal Microbiota Over the First Three Months of Life: A Pilot Study. Sci. Rep. 2017, 7, 40597. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, F.; Li, J.; Xue, Y.; Yu, B.; Ye, S.; Xu, L.; Fu, Y.; Yuan, X. Early Oral Administration of Ginseng Stem-Leaf Saponins Enhances the Peyer’s Patch-Dependent Maternal IgA Antibody Response to a PEDV Inactivated Vaccine in Mice, with Gut Microbiota Involvement. Vaccines 2023, 11, 830. https://doi.org/10.3390/vaccines11040830
Su F, Li J, Xue Y, Yu B, Ye S, Xu L, Fu Y, Yuan X. Early Oral Administration of Ginseng Stem-Leaf Saponins Enhances the Peyer’s Patch-Dependent Maternal IgA Antibody Response to a PEDV Inactivated Vaccine in Mice, with Gut Microbiota Involvement. Vaccines. 2023; 11(4):830. https://doi.org/10.3390/vaccines11040830
Chicago/Turabian StyleSu, Fei, Junxing Li, Yin Xue, Bin Yu, Shiyi Ye, Lihua Xu, Yuan Fu, and Xiufang Yuan. 2023. "Early Oral Administration of Ginseng Stem-Leaf Saponins Enhances the Peyer’s Patch-Dependent Maternal IgA Antibody Response to a PEDV Inactivated Vaccine in Mice, with Gut Microbiota Involvement" Vaccines 11, no. 4: 830. https://doi.org/10.3390/vaccines11040830
APA StyleSu, F., Li, J., Xue, Y., Yu, B., Ye, S., Xu, L., Fu, Y., & Yuan, X. (2023). Early Oral Administration of Ginseng Stem-Leaf Saponins Enhances the Peyer’s Patch-Dependent Maternal IgA Antibody Response to a PEDV Inactivated Vaccine in Mice, with Gut Microbiota Involvement. Vaccines, 11(4), 830. https://doi.org/10.3390/vaccines11040830





