Improving the Quality of Healthcare Provision Regarding HPV Immunization for Women with CIN2+ Lesions: The Experience of the Veneto Region in Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Context
2.2. Quality Improvement Strategy
2.3. Identifying the Gap between Ideal Procedure and Real Practice
2.4. Defining Strategies to Close the Gap between Ideal Procedure and Real Practice
2.5. Quality Improvement Strategy Outcome Measures
- (number of women diagnosed with CIN2+ lesions at cervical screening from 18 Nov 2020 to 30 June 2022 who received at least one dose of HPV vaccine after said diagnosis)/(total number of women diagnosed with CIN2+ lesions at cervical screening during the same period);
- (number of women diagnosed with CIN2+ lesions at cervical screening from 1 Jan 2019 to 17 Nov 2022 who received at least one dose of HPV vaccine after said diagnosis)/(total number of women diagnosed with CIN2+ lesions at a cervical screening during the same period).
- The resulting dataset was divided into four, based on the time to the provision of care (the interval between the diagnosis of CIN2+ lesions and a first dose of HPV vaccination): less than 3 months, 3–6 months, 6–12 months, and more than 12 months.
2.6. Statistical Analysis
3. Results
- strategies to improve the training of healthcare personnel: at two LHUs, they involved webinars or face-to-face meetings held by an expert (the doctor in charge of 2nd-level screening or an oncologist); the other LHUs engaged in active training and in-house meetings (of gynecologists) to discuss the procedure; the various LHUs also arranged for the proper storage and availability of printed copies of the procedure for easy consultation;
- strategies to develop effective communications: explanatory videos and video interviews were prepared and broadcast via social channels in two cases, and television interviews were used in another (and the material was edited by both healthcare specialists and communication experts); in one case, printed information sheets were produced and distributed at most of the LHUs’ vaccination clinics;
- strategies to develop an organized and proactive patient involvement: immunization was recommended in letters notifying patients that their cervical screening had revealed CIN2+ lesions; one LHU established a routine procedure for booking a vaccination within 30 days after surgery for CIN2+ lesions and, if the patient failed to attend, vaccination was recommended again at the time of her gynecological follow-up.
4. Discussion
5. Conclusions
Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manini, I.; Montomoli, E. Epidemiology and prevention of Human Papillomavirus. Ann Ig 2018, 30, 28–32. [Google Scholar] [CrossRef]
- IARC—Cancer Over Time. Available online: https://gco.iarc.fr/overtime/en/dataviz/trends?populations=38000&sexes=1_2&types=0_1&multiple_populations=1&cancers=16 (accessed on 8 August 2022).
- Khairkhah, N.; Bolhassani, A.; Najafipour, R. Current and future direction in treatment of HPV-related cervical disease. J. Mol. Med. 2022, 100, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Xu, L. Efficacy and safety of prophylactic HPV vaccines. A Cochrane review of randomized trials. Expert Rev. Vaccines 2018, 17, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- DGRV n. 576 del 04 Maggio 2021 “Ridefinizione del Calendario di Invito a Screening Cervicale Delle Donne Venticinquenni, Vaccinate per Papilloma Virus (HPV) Nelle Campagne Vaccinali Delle 12enni, di cui alla D.G.R. n. 772 del 27/05/2014 e D.G.R. n. 760 del 14/05/2015, in Tema di Prevenzione e Diagnosi Precoce Dei Tumori” e Successiva Modifica con DGRV n. 804 del 22 Giugno. 2021. Available online: http://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDgr.aspx?id=447520 (accessed on 14 March 2023).
- Garbuglia, A.R.; Lapa, D.; Sias, C.; Capobianchi, M.R.; Del Porto, P. The Use of Both Therapeutic and Prophylactic Vaccines in the Therapy of Papillomavirus Disease. Front. Immunol. 2020, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, V.; Caruso, G.; Petrillo, M.; Kontopantelis, E.; Palaia, I.; Perniola, G.; Plotti, F.; Angioli, R.; Muzii, L.; Panici, P.B.; et al. Adjuvant HPV Vaccination to Prevent Recurrent Cervical Dysplasia after Surgical Treatment: A Meta-Analysis. Vaccines 2021, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Del Pino, M.; Martí, C.; Torras, I.; Henere, C.; Munmany, M.; Marimon, L.; Saco, A.; Torné, A.; Ordi, J. HPV Vaccination as Adjuvant to Conization in Women with Cervical Intraepithelial Neoplasia: A Study under Real-Life Conditions. Vaccines 2020, 8, 245. [Google Scholar] [CrossRef]
- Ghelardi, A.; Parazzini, F.; Martella, F.; Pieralli, A.; Bay, P.; Tonetti, A.; Svelato, A.; Bertacca, G.; Lombardi, S.; Joura, E.A. SPERANZA project: HPV vaccination after treatment for CIN2+. Gynecol. Oncol. 2018, 151, 229–234. [Google Scholar] [CrossRef]
- Garland, S.M.; Paavonen, J.; Jaisamrarn, U.; Naud, P.; Salmerón, J.; Chow, S.N.; Apter, D.; Castellsagué, X.; Teixeira, J.C.; Skinner, S.R.; et al. Prior human papillomavirus-16/18 AS04-adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: Post-hoc analysis from a randomized controlled trial. Int. J. Cancer 2016, 139, 2812–2826. [Google Scholar] [CrossRef]
- Joura, E.A.; Garland, S.M.; Paavonen, J.; Ferris, D.G.; Perez, G.; Ault, K.A.; Huh, W.K.; Sings, H.L.; James, M.K.; Haupt, R.M.; et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: Retrospective pooled analysis of trial data. BMJ 2012, 344, e1401. [Google Scholar] [CrossRef]
- Shirey, M.R.; Hauck, S.L.; Embree, J.; Kinner, T.J.; Schaar, G.L.; Phillips, L.A.; Ashby, S.R.; Swenty, C.F.; McCool, I.A. Showcasing Differences Between Quality Improvement, Evidence-Based Practice, and Research. J. Contin. Educ. Nurs. 2011, 42, 57–68. [Google Scholar] [CrossRef]
- Backhouse, A.; Ogunlayi, F. Quality improvement into practice. BMJ 2020, 368, m865. [Google Scholar] [CrossRef] [PubMed]
- Academy of Medical Royal Colleges. Quality Improvement—Training for Better Outcomes; Academy of Medical Royal Colleges: London, UK, 2016. [Google Scholar]
- Silver, S.A.; Harel, Z.; McQuillan, R.; Weizman, A.V.; Thomas, A.; Chertow, G.M.; Nesrallah, G.; Bell, C.M.; Chan, C.T. How to Begin a Quality Improvement Project. Clin. J. Am. Soc. Nephrol. 2016, 11, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.euro.who.int/__data/assets/pdf_file/0019/303184/The-Veneto-model-report.pdf (accessed on 10 November 2022).
- DGR n. 1858 del 29 Dicembre 2021 “Approvazione del Piano Regionale Prevenzione (PRP) 2020–2025, in Attuazione Delle Indicazioni Contenute nel Piano Nazionale Prevenzione (PNP) 2020–2025”. Available online: https://bur.regione.veneto.it/BurvServices/pubblica/DettaglioDgr.aspx?id=466964 (accessed on 14 March 2023).
- Conferenza Stato-Regioni del 19.01.2017: Piano Nazionale Prevenzione Vaccinale 2017-19. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf (accessed on 14 March 2023).
- DGRV n. 1100 del 30 luglio 2019 “Modifica del Calendario Regionale Vaccinale, di cui alla D.G.R. n. 1564 del 26/08/2014, Approvazione del Progetto “Utilizzo Dell’auto-Prelievo nel Programma di Screening per la Prevenzione del Carcinoma della Cervice Uterina con Test per Papilloma Virus (HPV) nell’AULSS 9 Scaligera” e Contestuale Autorizzazione del Finanziamento per la sua Realizzazione”. Available online: https://bur.regione.veneto.it/BurvServices/pubblica/DettaglioDgr.aspx?id=399951 (accessed on 14 March 2023).
- DGRV n. 1557 del 17 Novembre 2020 “Approvazione di Procedure Operative Regionali Nell’ambito dei Programmi di Screening Oncologici per il Carcinoma Della Cervice Uterina e per il Tumore del Colon-Retto”. Available online: https://bur.regione.veneto.it/BurvServices/Pubblica/DettaglioDgr.aspx?id=434569 (accessed on 14 March 2023).
- Laboratorio Mes Istituto di Management Scuola Superiore di Studi Universitari e di Perfezionamento Sant’Anna. ComuniCARE Sanità—Strumenti Online per i Servizi ai Cittadini; Laboratorio Mes Istituto di Management Scuola Superiore di Studi Universitari e di Perfezionamento Sant’Anna: Pisa, Italy, 2016. [Google Scholar]
- Nuti, S.; Caputo, A.; Cerasuolo, D.; D’Orio, G.; Noci, A.; Parenti, A.; Vainieri, M.; Vola, F. Scuola Superiore Sant’Anna Istituto di Management Laboratorio Management e Sanità, Il Sistema di Valutazione della Performance dei Sistemi Sanitari Regionali—I Risultati Delle Aziende Ospedaliero-Universitarie a Confronto. Report 2020. Available online: https://www.santannapisa.it/sites/default/files/u23658/report_aou2020_web.pdf (accessed on 14 March 2023).
- Ministero della Salute e Sapienza Università di Roma. Linee Guida per la Comunicazione Online in Tema di Tutela e Promozione Della Salute; Ministero della Salute e Sapienza Università di Roma: Roma, Italy, 2010. [Google Scholar]
- Devine, T.; Broderick, J.; Harris, L.M.; Wu, H.; Hilfiker, S.W. Making Quality Health Websites a National Public Health Priority: Toward Quality Standards. J. Med. Internet Res. 2016, 18, e211. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, G.E.; Carini, E.; Favaretti, C.; Bonanni, P.; De Vincenzo, R.; Ghelardi, A.; Chiccheti, A.; Basile, M.; Tafuri, S.; Conversano, M. Report di approfondimento e valutazione, con metodologia hta (health technology assessment), della vaccinazione anti-hpv nelle donne trattate per lesioni hpv-correlate. QIJPH 2019, 8, 7. [Google Scholar]
- Available online: https://www.aslroma1.it/news/al-santanna-avviata-la-vaccinazione-anti-hpv-per-le-donne-sottoposte-a-intervento-di-conizzazione#:~:text=La%20ASL%20Roma%201%20promuove,a%20intervento%20di%20conizzazione%20cervicale (accessed on 13 March 2023).
- Available online: https://www.vaccinarsintoscana.org/notizie/2021/02/vaccino-hpv-a-firenze-somministrato-direttamente-in-sala-operatoria (accessed on 13 March 2023).
- Pereira, V.C.; Silva, S.N.; Carvalho, V.K.S.; Zanghelini, F.; Barreto, J.O.M. Strategies for the implementation of clinical practice guidelines in public health: An overview of systematic reviews. Health Res. Policy Syst. 2022, 20, 1–21. [Google Scholar] [CrossRef] [PubMed]
- McSherry, L.A.; O’Leary, E.; Dombrowski, S.U.; Francis, J.J.; Martin, C.M.; O’Leary, J.J.; Sharp, L. on behalf of the ATHENS (A Trial of HPV Education and Support) Group Which primary care practitioners have poor human papillomavirus (HPV) knowledge? A step towards informing the development of professional education initiatives. PLoS ONE 2018, 13, e0208482. [Google Scholar] [CrossRef]
- Hurley, L.P.; Bridges, C.B.; Harpaz, R.; Allison, M.A.; Leary, S.T.O.; Crane, L.A.; Brtnikova, M.; Stokley, S.; Beaty, B.L.; Jimenez-Zambrano, A.; et al. Physician Attitudes toward Adult Vaccines and other Preventive Practices, United States, 2012. Public Health Rep. 2016, 131, 320–330. [Google Scholar] [CrossRef]
- Pavlovic, D.; Sahoo, P.; Larson, H.J.; Karafillakis, E. Factors influencing healthcare professionals’ confidence in vaccination in Europe: A literature review. Hum. Vaccines Immunother. 2022, 18, 2041360. [Google Scholar] [CrossRef]
- Ariza-Heredia, E.; Gulbis, A.; Stolar, K.; Kebriaei, P.; Shah, D.; McConn, K.; Champlin, R.; Chemaly, R. Vaccination guidelines after hematopoietic stem cell transplantation: Practitioners’ knowledge, attitudes, and gap between guidelines and clinical practice. Transpl. Infect. Dis. 2014, 16, 878–886. [Google Scholar] [CrossRef]
- Costantino, C.; Casuccio, A.; Caracci, F.; Bono, S.; Calamusa, G.; Ventura, G.; Maida, C.M.; Vitale, F.; Restivo, V. Impact of Communicative and Informative Strategies on Influenza Vaccination Adherence and Absenteeism from Work of Health Care Professionals Working at the University Hospital of Palermo, Italy: A Quasi-Experimental Field Trial on Twelve Influenza Seasons. Vaccines 2019, 8, 5. [Google Scholar] [CrossRef]
- Benis, A.; Khodos, A.; Ran, S.; Levner, E.; Ashkenazi, S. Social Media Engagement and Influenza Vaccination During the COVID-19 Pandemic: Cross-sectional Survey Study. J. Med. Internet Res. 2021, 23, e25977. [Google Scholar] [CrossRef] [PubMed]
- Piltch-Loeb, R.; Savoia, E.; Goldberg, B.; Hughes, B.; Verhey, T.; Kayyem, J.; Miller-Idriss, C.; Testa, M. Examining the effect of information channel on COVID-19 vaccine acceptance. PLoS ONE 2021, 16, e0251095. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention, Standards for Adult Immunization Practice, Page Last Reviewed: 2 May 2016. Available online: https://www.cdc.gov/vaccines/hcp/adults/for-practice/standards/index.html (accessed on 20 August 2022).
- National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory Committee: Standards for Adult Immunization Practice. Public Health Rep. 2014, 129, 115–123. [Google Scholar] [CrossRef] [PubMed]

| Training | ||
|---|---|---|
| Are there dedicated training courses for healthcare personnel involved in cervical screening and HPV vaccination pathways (recommendations, good practices, counseling techniques, co-administration, etc.)? | Questions 1, 2 | |
| How LHUs communicate with the population | ||
| Social networks | Are there specific campaigns or messages promoting HPV vaccination? | Question 3 |
| Website | Is there a dedicated web page? If so, does it provide basic information on vaccination, who to contact, and booking methods? | Question 4 |
| Printed matter | Is there any printed information material available at the screening facility? | Question 5 |
| Logistics and organization | ||
| Definition of pathways, logistics, and personnel involved | Are there established pathways for offering and organizing vaccinations? Which staff are involved? | Questions 6, 7, 8, 9, 10, 14, 15, 16, 17, 18, 19, 20 |
| Access modalities | How can the population access vaccination (active call, booking, gynecological counseling,…)? | Questions 10, 11, 12, 13 |
| Evaluation | ||
| Process | Are process indicators used in the implementation of the pathway? | Question 21 |
| Quality | Have any quality improvement activities or interventions been carried out (internal audits, focus groups, etc.)? | Question 22 |
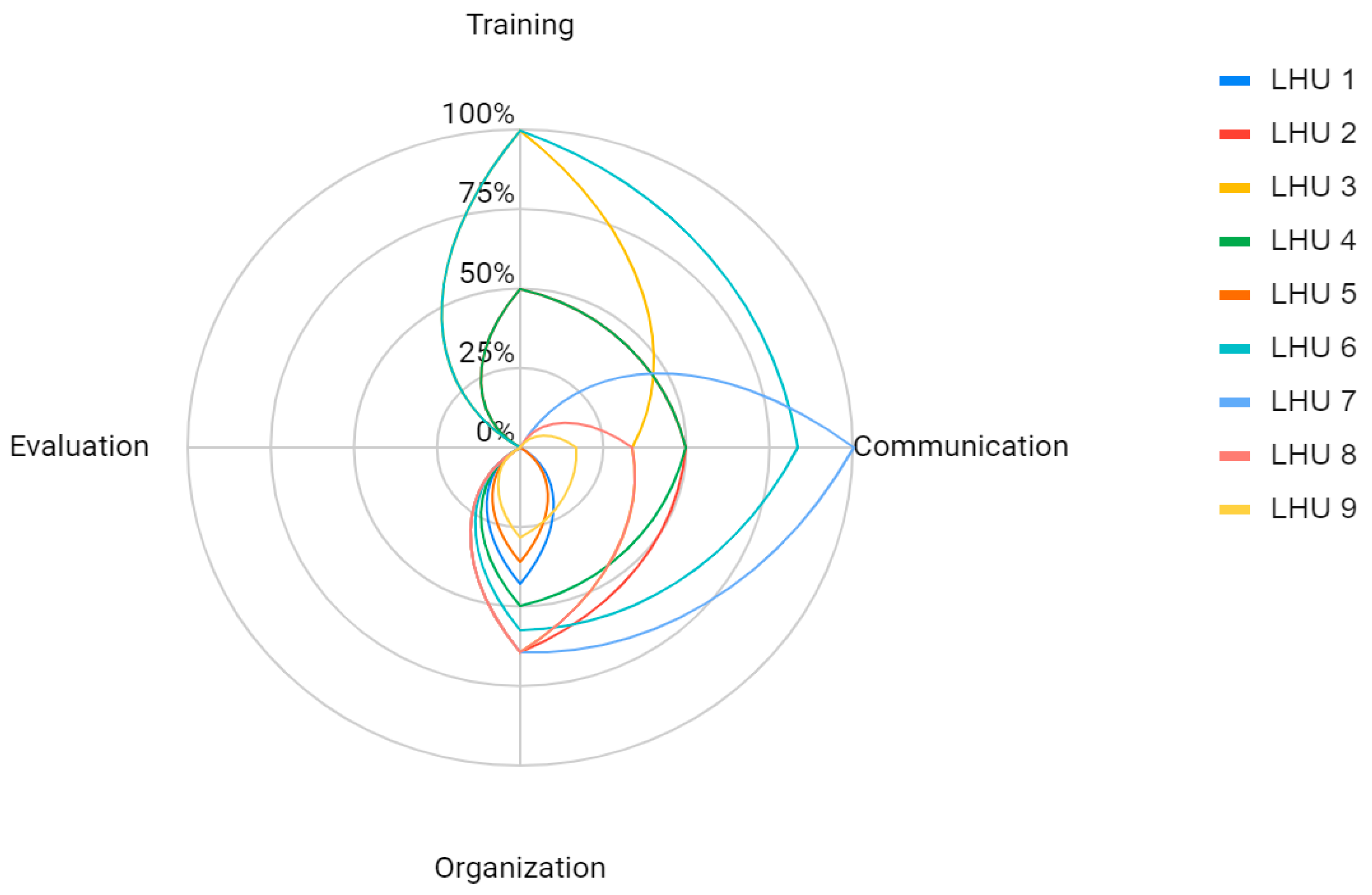
| Dedicated Web Page | Web Page Accessibility | Text: Sentence Length | Text: Word Complexity | Text: Layout | Vaccination Booking Information | |
|---|---|---|---|---|---|---|
| LHU 1 | 1 | 3 | 3 | 3 | 4 | 2 |
| LHU 2 | 1 | 3 | 1 | 1 | 1 | 1 |
| LHU 3 | 3 | 2 | 5 | 5 | 3 | 4 |
| LHU 4 | 1 | 2 | 1 | 1 | 1 | 3 |
| LHU 5 | 2 | 3 | 3 | 1 | 5 | 5 |
| LHU 6 | 4 | 3 | 5 | 5 | 5 | 4 |
| LHU 7 | 5 | 2 | 5 | 3 | 4 | 3 |
| LHU 8 | 5 | 1 | 5 | 3 | 4 | 5 |
| LHU 9 | 1 | 3 | 1 | 1 | 1 | 1 |
| Training | |
|---|---|
| General practitioners, gynecologists |
|
| How LHUs communicate with the population | |
| Social networks |
|
| Website |
|
| Printed matter |
|
| Logistics and organization | |
| Definition of pathways, logistics, and personnel involved |
|
| Access modalities |
|
| Evaluation | |
| Process |
|
| Quality |
|
| Adoption of Regional Procedure (Nov 2020) | Vaccinated only after a Diagnosis of CIN2+ Lesions [n (%)] | Already Vaccinated [n (%)] | Never Vaccinated [n (%)] | Total [n (%)] |
|---|---|---|---|---|
| Before (1 Jan 2019 to 17 Nov 2020) | 1381 (46.97) | 316 (10.75) | 1243 (42.28) | 2940 (100) |
| After (18 Nov 2020 to 30 June 2022) | 1249 (50.65) | 313 (12.69) | 904 (36.66) | 2466 (100) |
| Adoption of Regional Procedure (Nov 2020) | Vaccinated only after a Diagnosis of CIN2+ Lesions [n (%)] | Up to 3 Months n (%) | 3–6 Months n (%) | 6–12 Months n (%) | More than 1 Year n (%) |
|---|---|---|---|---|---|
| Before (1 Jan 2019 to 17 Nov 2020) | 1381 (100) | 426 (30.85) | 318 (23.03) | 287 (20.78) | 350 (25.34) |
| After (18 Nov 2020 to 30 June 2022) | 1249 (100) | 625 (50.04) | 332 (26.58) | 232 (18.57) | 60 (4.80) |
| Regional Procedure (Nov 2020) | Median (days) | 5th Percentile (days) | 25th Percentile (days) | 75th Percentile (days) | 95th Percentile (days) |
|---|---|---|---|---|---|
| Before (1 Jan 2019 to 17 Nov 2020) | 158 | 23 | 78 | 367 | 857 |
| After (18 Nov 2020 to 30 June 2022) | 90 | 22 | 55 | 170 | 362 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Polo, A.; Tonon, M.; Da Re, F.; Rosafio, S.; Narne, E.; Gentili, D.; Cocchio, S.; Baldo, V.; Russo, F.; Buja, A. Improving the Quality of Healthcare Provision Regarding HPV Immunization for Women with CIN2+ Lesions: The Experience of the Veneto Region in Italy. Vaccines 2023, 11, 757. https://doi.org/10.3390/vaccines11040757
De Polo A, Tonon M, Da Re F, Rosafio S, Narne E, Gentili D, Cocchio S, Baldo V, Russo F, Buja A. Improving the Quality of Healthcare Provision Regarding HPV Immunization for Women with CIN2+ Lesions: The Experience of the Veneto Region in Italy. Vaccines. 2023; 11(4):757. https://doi.org/10.3390/vaccines11040757
Chicago/Turabian StyleDe Polo, Anna, Michele Tonon, Filippo Da Re, Sara Rosafio, Elena Narne, Davide Gentili, Silvia Cocchio, Vincenzo Baldo, Francesca Russo, and Alessandra Buja. 2023. "Improving the Quality of Healthcare Provision Regarding HPV Immunization for Women with CIN2+ Lesions: The Experience of the Veneto Region in Italy" Vaccines 11, no. 4: 757. https://doi.org/10.3390/vaccines11040757
APA StyleDe Polo, A., Tonon, M., Da Re, F., Rosafio, S., Narne, E., Gentili, D., Cocchio, S., Baldo, V., Russo, F., & Buja, A. (2023). Improving the Quality of Healthcare Provision Regarding HPV Immunization for Women with CIN2+ Lesions: The Experience of the Veneto Region in Italy. Vaccines, 11(4), 757. https://doi.org/10.3390/vaccines11040757







