Salivary Antibody Responses to Two COVID-19 Vaccines following Different Vaccination Regimens
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. SARS-CoV-2 Antibody Detection by ELISA
2.3. Commercial Kit Validation
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
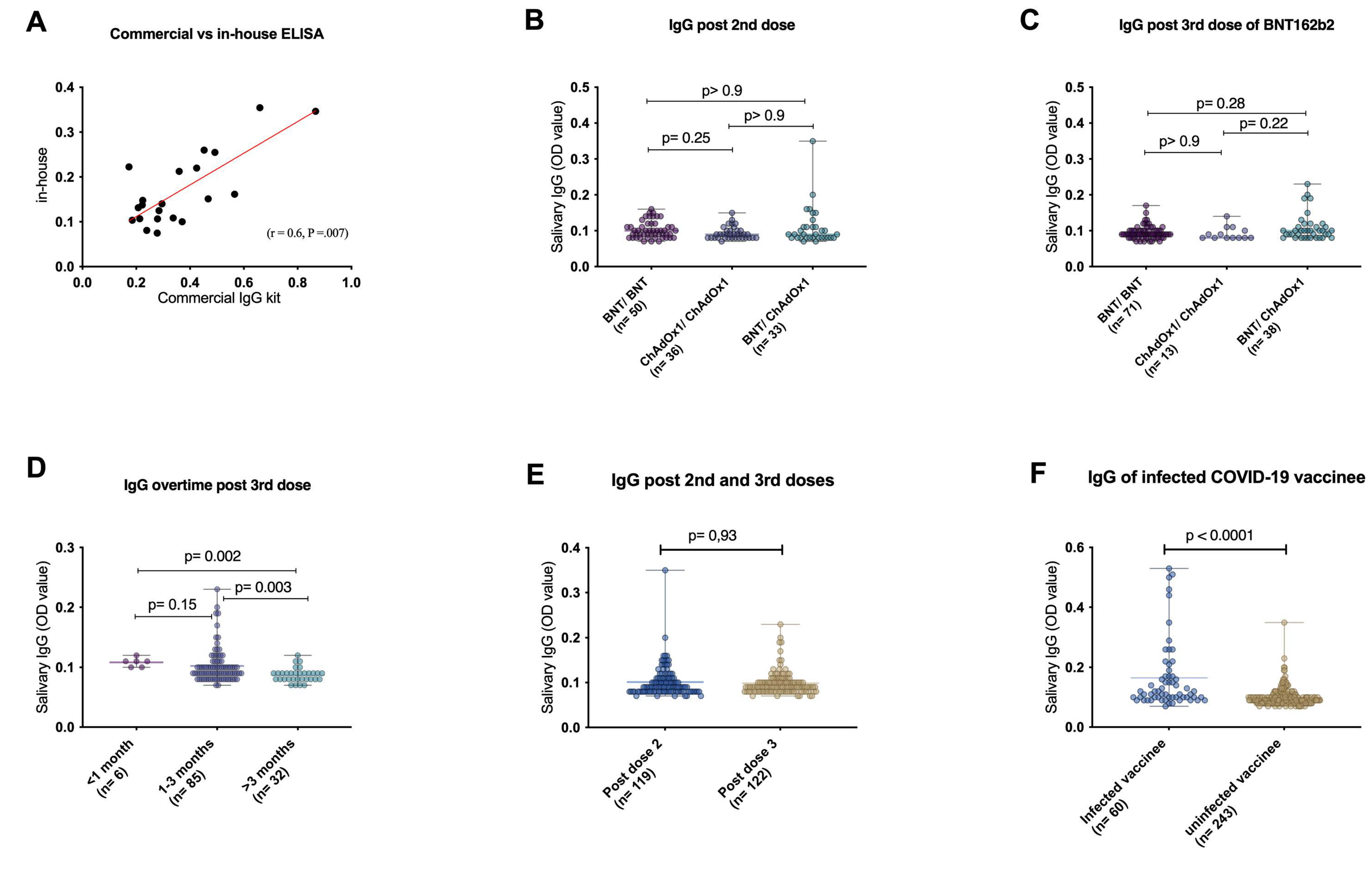
3.2. Validation of the In-House ELISA
3.3. Different Vaccine Types and Regimens Elicit Similar Salivary Anti-SARS-CoV-2 IgG
3.4. Waning of Salivary IgG Levels after BNT162b2 Booster
3.5. Impact of Previous COVID-19 Infection on the Levels of Vaccine-Induced Salivary IgG
3.6. Persistence Patterns of Salivary IgG Levels following Different Vaccination Regimens
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- University of Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 18 January 2023).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and Effectiveness of MRNA BNT162b2 Vaccine against SARS-CoV-2 Infections and COVID-19 Cases, Hospitalisations, and Deaths Following a Nationwide Vaccination Campaign in Israel: An Observational Study Using National Surveillance Data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 NCoV-19) Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.D.; Kitchin, N.; Xu, X.; Dychter, S.S.; Lockhart, S.; Gurtman, A.; Perez, J.L.; Zerbini, C.; Dever, M.E.; Jennings, T.W.; et al. Safety and Efficacy of a Third Dose of BNT162b2 Covid-19 Vaccine. N. Engl. J. Med. 2022, 386, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 Vaccine Coverage in Health-Care Workers in England and Effectiveness of BNT162b2 MRNA Vaccine against Infection (SIREN): A Prospective, Multicentre, Cohort Study. Lancet 2021, 397, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Fischinger, S.; Boudreau, C.M.; Butler, A.L.; Streeck, H.; Alter, G. Sex Differences in Vaccine-Induced Humoral Immunity. Semin. Immunopathol. 2019, 41, 239–249. [Google Scholar] [CrossRef]
- Healy, K.; Pin, E.; Chen, P.; Söderdahl, G.; Nowak, P.; Mielke, S.; Hansson, L.; Bergman, P.; Smith, C.I.E.; Ljungman, P.; et al. Salivary IgG to SARS-CoV-2 Indicates Seroconversion and Correlates to Serum Neutralization in MRNA-Vaccinated Immunocompromised Individuals. Med 2022, 3, 137–153.e3. [Google Scholar] [CrossRef]
- Bekliz, M.; Adea, K.; Vetter, P.; Eberhardt, C.S.; Hosszu-fellous, K.; Vu, D.; Puhach, O.; Essaidi-laziosi, M.; Waldvogel-abramowski, S.; Stephan, C.; et al. Neutralization Capacity of Antibodies Elicited through Homologous or Heterologous Infection or Vaccination against SARS-CoV-2 VOCs. Nat. Commun. 2022, 13, 3840. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A.; Araki, K.; Ahmed, R. From Vaccines to Memory and Back. Immunity 2010, 33, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Muecksch, F.; Schaefer-Babajew, D.; Wang, Z.; Finkin, S.; Gaebler, C.; Ramos, V.; Cipolla, M.; Mendoza, P.; Agudelo, M.; et al. Anti-SARS-CoV-2 Receptor-Binding Domain Antibody Evolution after MRNA Vaccination. Nature 2021, 600, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Morillas Ramos, G.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune Responses against SARS-CoV-2 Variants after Heterologous and Homologous ChAdOx1 NCoV-19/BNT162b2 Vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Borobia, A.M.; Carcas, A.J.; Pérez-Olmeda, M.; Castaño, L.; Bertran, M.J.; García-Pérez, J.; Campins, M.; Portolés, A.; González-Pérez, M.; García Morales, M.T.; et al. Immunogenicity and Reactogenicity of BNT162b2 Booster in ChAdOx1-S-Primed Participants (CombiVacS): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct Antibody and Memory B Cell Responses in SARSCoV-2 Naïve and Recovered Individuals Following MRNA Vaccination. Sci. Immunol. 2021, 6, 1–19. [Google Scholar] [CrossRef]
- Painter, M.M.; Mathew, D.; Goel, R.R.; Apostolidis, S.A.; Pattekar, A.; Kuthuru, O.; Baxter, A.E.; Herati, R.S.; Oldridge, D.A.; Gouma, S.; et al. Rapid Induction of Antigen-Specific CD4+ T Cells Is Associated with Coordinated Humoral and Cellular Immunity to SARS-CoV-2 MRNA Vaccination. Immunity 2021, 54, 2133–2142.e3. [Google Scholar] [CrossRef]
- Havervall, S.; Ng, H.; Jernbom Falk, A.; Greilert-Norin, N.; Månberg, A.; Marking, U.; Laurén, I.; Gabrielsson, L.; Salomonsson, A.C.; Aguilera, K.; et al. Robust Humoral and Cellular Immune Responses and Low Risk for Reinfection at Least 8 Months Following Asymptomatic to Mild COVID-19. J. Intern. Med. 2022, 291, 72–80. [Google Scholar] [CrossRef]
- Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Viant, C.; Gaebler, C.; Cipolla, M.; Hoffmann, H.-H.; Oliveira, T.Y.; Oren, D.A.; et al. Enhanced SARS-CoV-2 Neutralization by Dimeric IgA. Sci. Transl. Med. 2021, 13, eabf1555. [Google Scholar] [CrossRef]
- Huang, N.; Pérez, P.; Kato, T.; Mikami, Y.; Okuda, K.; Gilmore, R.C.; Conde, C.D.; Gasmi, B.; Stein, S.; Beach, M.; et al. SARS-CoV-2 Infection of the Oral Cavity and Saliva. Nat. Med. 2021, 27, 892–903. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 Entry Factors Are Highly Expressed in Nasal Epithelial Cells Together with Innate Immune Genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef]
- Alkharaan, H.; Bayati, S.; Hellström, C.; Aleman, S.; Olsson, A.; Lindahl, K.; Bogdanovic, G.; Healy, K.; Tsilingaridis, G.; de Palma, P.; et al. Persisting Salivary IgG against SARS-CoV-2 at 9 Months after Mild COVID-19: A Complementary Approach to Population Surveys. J. Infect. Dis. 2021, 224, 407–414. [Google Scholar] [CrossRef]
- Isho, B.; Abe, K.T.; Zuo, M.; Jamal, A.J.; Rathod, B.; Wang, J.H.; Li, Z.; Chao, G.; Rojas, O.L.; Bang, Y.M.; et al. Persistence of Serum and Saliva Antibody Responses to SARS-CoV-2 Spike Antigens in COVID-19 Patients. Sci. Immunol. 2020, 5, eabe5511. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Sheikh-Mohamed, S.; Isho, B.; Chao, G.Y.C.; Zuo, M.; Cohen, C.; Lustig, Y.; Nahass, G.R.; Salomon-Shulman, R.E.; Blacker, G.; Fazel-Zarandi, M.; et al. Systemic and Mucosal IgA Responses Are Variably Induced in Response to SARS-CoV-2 MRNA Vaccination and Are Associated with Protection against Subsequent Infection. Mucosal Immunol. 2022, 15, 799–808. [Google Scholar] [CrossRef]
- Sano, K.; Bhavsar, D.; Singh, G.; Floda, D.; Srivastava, K.; Gleason, C.; Amoako, A.A.; Andre, D.; Beach, K.F.; Bermúdez-González, M.C.; et al. SARS-CoV-2 Vaccination Induces Mucosal Antibody Responses in Previously Infected Individuals. Nat. Commun. 2022, 13, 5135. [Google Scholar] [CrossRef] [PubMed]
- Azzi, L.; Dalla Gasperina, D.; Veronesi, G.; Shallak, M.; Ietto, G.; Iovino, D.; Baj, A.; Gianfagna, F.; Maurino, V.; Focosi, D.; et al. Mucosal Immune Response in BNT162b2 COVID-19 Vaccine Recipients. EBioMedicine 2022, 75, 103788. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.K.; Alghnam, S.; Algaissi, A.; Albalawi, H.; Alenazi, M.W.; Albargawi, A.M.; Alharbi, A.G.; Alhazmi, A.; Al Qarni, A.; Alfarhan, A.; et al. Nationwide Seroprevalence of SARS-CoV-2 in Saudi Arabia. J. Infect. Public Health 2021, 14, 832–838. [Google Scholar] [CrossRef]
- Alharbi, N.K.; Al-Tawfiq, J.A.; Alwehaibe, A.; Alenazi, M.W.; Almasoud, A.; Algaisi, A.; Alhumaydhi, F.A.; Hashem, A.M.; Bosaeed, M.; Alsagaby, S.A. Persistence of Anti-SARS-CoV-2 Spike IgG Antibodies Following COVID-19 Vaccines. Infect. Drug Resist. 2022, 15, 4127–4136. [Google Scholar] [CrossRef]
- Lipsitch, M.; Krammer, F.; Regev-Yochay, G.; Lustig, Y.; Balicer, R.D. SARS-CoV-2 Breakthrough Infections in Vaccinated Individuals: Measurement, Causes and Impact. Nat. Rev. Immunol. 2022, 22, 57–65. [Google Scholar] [CrossRef]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. Community Transmission and Viral Load Kinetics of the SARS-CoV-2 Delta (B.1.617.2) Variant in Vaccinated and Unvaccinated Individuals in the UK: A Prospective, Longitudinal, Cohort Study. Lancet Infect. Dis. 2022, 22, 183–195. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, R.; Baos, S.; Cappel-Porter, H.; Carson-Stevens, A.; Clout, M.; Culliford, L.; Emmett, S.R.; Garstang, J.; Gbadamoshi, L.; Hallis, B.; et al. Safety and Immunogenicity of Concomitant Administration of COVID-19 Vaccines (ChAdOx1 or BNT162b2) with Seasonal Influenza Vaccines in Adults in the UK (ComFluCOV): A Multicentre, Randomised, Controlled, Phase 4 Trial. Lancet 2021, 398, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and Reactogenicity of Heterologous ChAdOx1 NCoV-19/MRNA Vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, A.; Almutairi, S.; Al-Dhabbah, A.; Aldabas, S.; Bhat, R.; Alqoufail, M.M.; Abdel-maksoud, M.A.; Almanaa, T.N.; Farrag, M.A. Durability of SARS-CoV-2 Specific IgG Antibody Responses Following Two Doses of Match and Mixed COVID-19 Vaccines Regimens in Saudi Population. Infect. Drug Resist. 2022, 15, 3791–3800. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.S.; Ash, N.; Alroy-Preis, S.; Huppert, A.; Milo, R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl. J. Med. 2022, 386, 2201–2212. [Google Scholar] [CrossRef]
- Wang, Z.; Muecksch, F.; Schaefer-Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally Enhanced Neutralizing Breadth against SARS-CoV-2 One Year after Infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of Antibody Immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Chen, Y.; Tong, P.; Whiteman, N.B.; Moghaddam, A.S.; Zuiani, A.; Habibi, S.; Gautam, A.; Xiao, T.; Cai, Y.; Chen, B.; et al. Differential Antibody Dynamics to SARS-CoV-2 Infection and Vaccination. bioRxiv 2021. [Google Scholar] [CrossRef]
- Tejedor Vaquero, S.; de Campos-Mata, L.; Ramada, J.M.; Díaz, P.; Navarro-Barriuso, J.; Ribas-Llaurado, C.; Rodrigo Melero, N.; Carolis, C.; Cerutti, A.; Gimeno, R.; et al. The MRNA-1273 Vaccine Induces Cross-Variant Antibody Responses to SARS-CoV-2 With Distinct Profiles in Individuals with or Without Pre-Existing Immunity. Front. Immunol. 2021, 12, 737083. [Google Scholar] [CrossRef] [PubMed]
- Stamatatos, L.; Czartoski, J.; Wan, Y.H.; Homad, L.J.; Rubin, V.; Glantz, H.; Neradilek, M.; Seydoux, E.; Jennewein, M.F.; MacCamy, A.J.; et al. MRNA Vaccination Boosts Cross-Variant Neutralizing Antibodies Elicited by SARS-CoV-2 Infection. Science 2021, 372, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Wall, E.C.; Wu, M.; Harvey, R.; Kelly, G.; Warchal, S.; Sawyer, C.; Daniels, R.; Hobson, P.; Hatipoglu, E.; Ngai, Y.; et al. Neutralising Antibody Activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 Vaccination. Lancet 2021, 397, 2331–2333. [Google Scholar] [CrossRef] [PubMed]
- Langel, S.N.; Johnson, S.; Martinez, C.I.; Tedjakusuma, S.N.; Peinovich, N.; Dora, E.G.; Kuehl, P.J.; Irshad, H.; Barrett, E.G.; Werts, A.D.; et al. Adenovirus Type 5 SARS-CoV-2 Vaccines Delivered Orally or Intranasally Reduced Disease Severity and Transmission in a Hamster Model. Sci. Transl. Med. 2022, 14, eabn6868. [Google Scholar] [CrossRef]
- Afkhami, S.; D’Agostino, M.R.; Zhang, A.; Stacey, H.D.; Marzok, A.; Kang, A.; Singh, R.; Bavananthasivam, J.; Ye, G.; Luo, X.; et al. Respiratory Mucosal Delivery of Next-Generation COVID-19 Vaccine Provides Robust Protection against Both Ancestral and Variant Strains of SARS-CoV-2. Cell 2022, 185, 896–915.e19. [Google Scholar] [CrossRef]

| Cohort 1 (n = 145) | ||||
|---|---|---|---|---|
| BNT/BNT (n = 65) | ChAd/ChAd (n = 38) | BNT/ChAd (n = 42) | ||
| Parameters | ||||
| Gender (F:M) | 46:19 | 17:21 *Ω | 22:19 | |
| Age (years) median (range) | 23 (15–54) | 28 (16–52) | 25 (14–55) | |
| BMI (kg/m2) median (range) | 24 (16.8–37) | 25 (15.6–42) | 23 (14–44.5) | |
| Smoking (%) | 17.5 | 29.7 | 32.5 | |
| Antibiotic (%) (<3 month) | 19 | 17 | 23.8 | |
| Previous infection with SARS-CoV-2 (%) | 23 | 5 * Ω | 19.5 | |
| Days since 2nd dose median (range) § | 137 (53–289) | 154 (35–311) | 129.5 (36–242) | |
| Cohort 2 (n = 156) | ||||
|---|---|---|---|---|
| BNT/BNT/BNT (n = 88) | ChAd/ChAd/BNT (n = 19) | BNT/ChAd/BNT (n = 49) | ||
| Parameters | ||||
| Gender (F:M) | 34:54 | 7:12 | 23:26 | |
| Age (years) median (range) | 22 (18–38) * Φ | 23 (16–52) | 23 (17–49) * Ω | |
| BMI (kg/m2) median (range) | 24.7 (16.7–40.9) | 24.5 (15.8–36.3) | 24.4 (16–34.5) | |
| Smoking (%) | 21.8 | 44.5 | 20.4 | |
| Antibiotic (%) (<3 month) | 12.2 | 22.2 | 12.5 | |
| Previous infection with SARS-CoV-2 (%) | 18.6 | 27.8 | 22.5 | |
| Days since 3rd dose median (range) § | 81 (14–122) | 75 (19–99) | 72 (42–124) | |
| Variables | ß | 95% Cl | p Value |
|---|---|---|---|
| Previous infection with SARS-CoV-2 (yes) | 0.065 | 0.048–0.082 | <0.0001 |
| Age | 0.001 | –0.001–0.002 | 0.2504 |
| Gender (male) | 0.005 | –0.021–0.011 | 0.5257 |
| BMI (kg/m2) | 0.0003 | –0.001–0.002 | 0.5892 |
| Smoking (yes) | 0.0062 | –0.012–0.024 | 0.4995 |
| Days since 2nd dose | –0.0001 | –0.0002–0.0001 | 0.2947 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkharaan, H.; Al-Qarni, H.; Aldosari, M.A.; Alsaloum, M.; Aldakheel, G.; Alenazi, M.W.; Alharbi, N.K. Salivary Antibody Responses to Two COVID-19 Vaccines following Different Vaccination Regimens. Vaccines 2023, 11, 744. https://doi.org/10.3390/vaccines11040744
Alkharaan H, Al-Qarni H, Aldosari MA, Alsaloum M, Aldakheel G, Alenazi MW, Alharbi NK. Salivary Antibody Responses to Two COVID-19 Vaccines following Different Vaccination Regimens. Vaccines. 2023; 11(4):744. https://doi.org/10.3390/vaccines11040744
Chicago/Turabian StyleAlkharaan, Hassan, Hatem Al-Qarni, Muath A. Aldosari, Mohammed Alsaloum, Ghada Aldakheel, Mohammed W. Alenazi, and Naif Khalaf Alharbi. 2023. "Salivary Antibody Responses to Two COVID-19 Vaccines following Different Vaccination Regimens" Vaccines 11, no. 4: 744. https://doi.org/10.3390/vaccines11040744
APA StyleAlkharaan, H., Al-Qarni, H., Aldosari, M. A., Alsaloum, M., Aldakheel, G., Alenazi, M. W., & Alharbi, N. K. (2023). Salivary Antibody Responses to Two COVID-19 Vaccines following Different Vaccination Regimens. Vaccines, 11(4), 744. https://doi.org/10.3390/vaccines11040744





