Vaccinomics Approach for Multi-Epitope Vaccine Design against Group A Rotavirus Using VP4 and VP7 Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rotavirus Sequence Retrieval and Multiple Alignments
2.2. T-cell Epitope Prediction
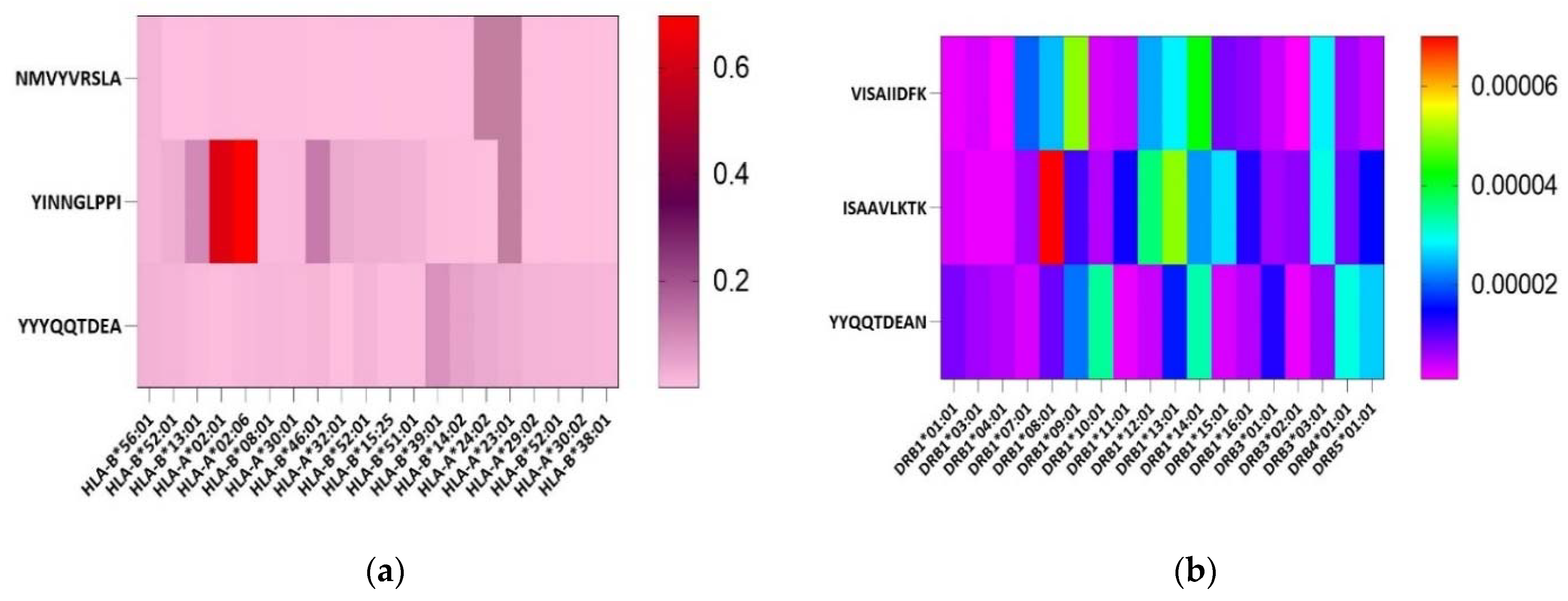
2.3. B-Cell Epitope Prediction and Common Epitope Identification
2.4. Feature Profiling of Epitopes
2.5. Development of Epitope-Based Vaccine Construct
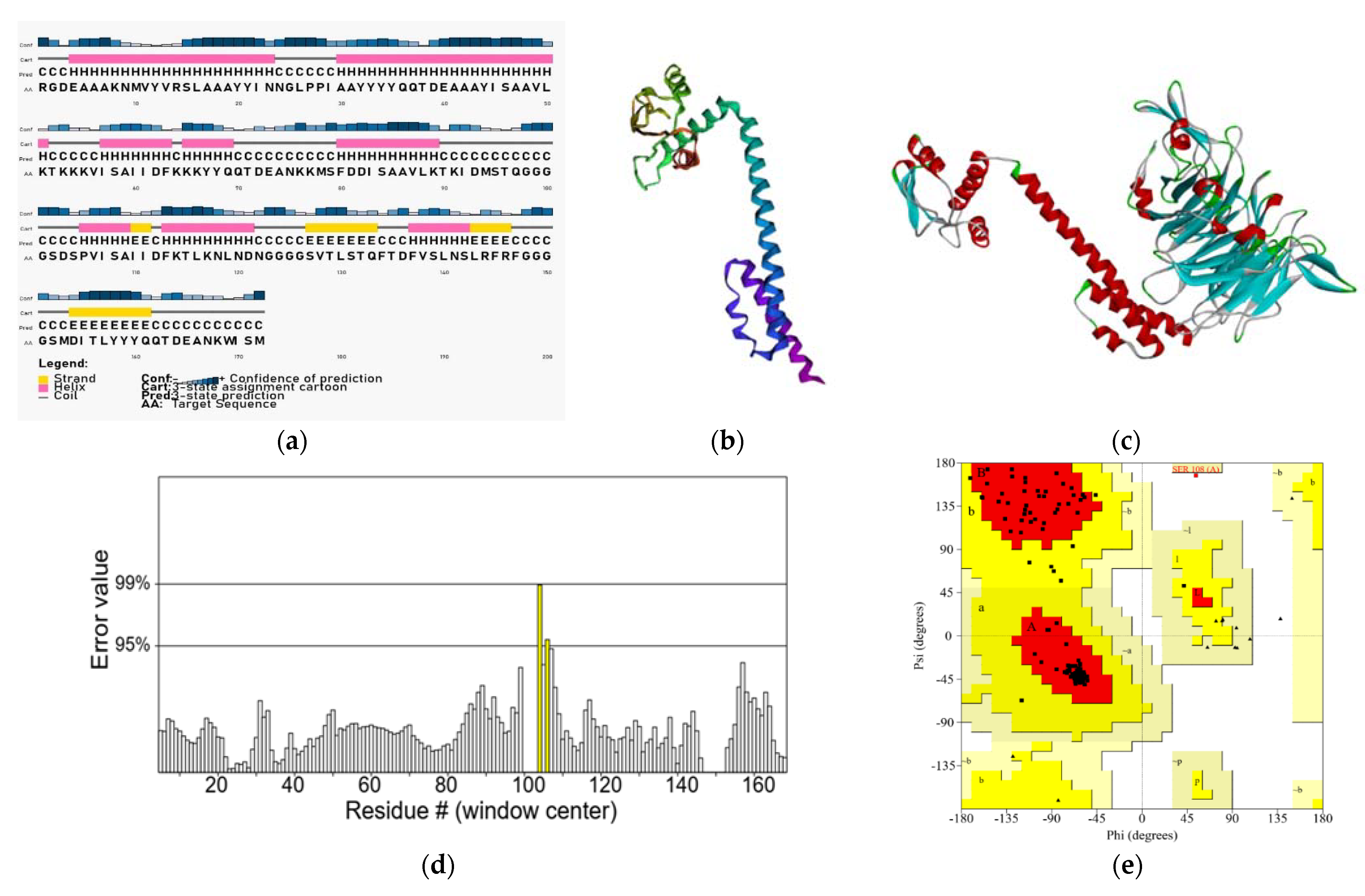
2.6. Secondary and Tertiary Structure Prediction and Validation of the Designed Construct
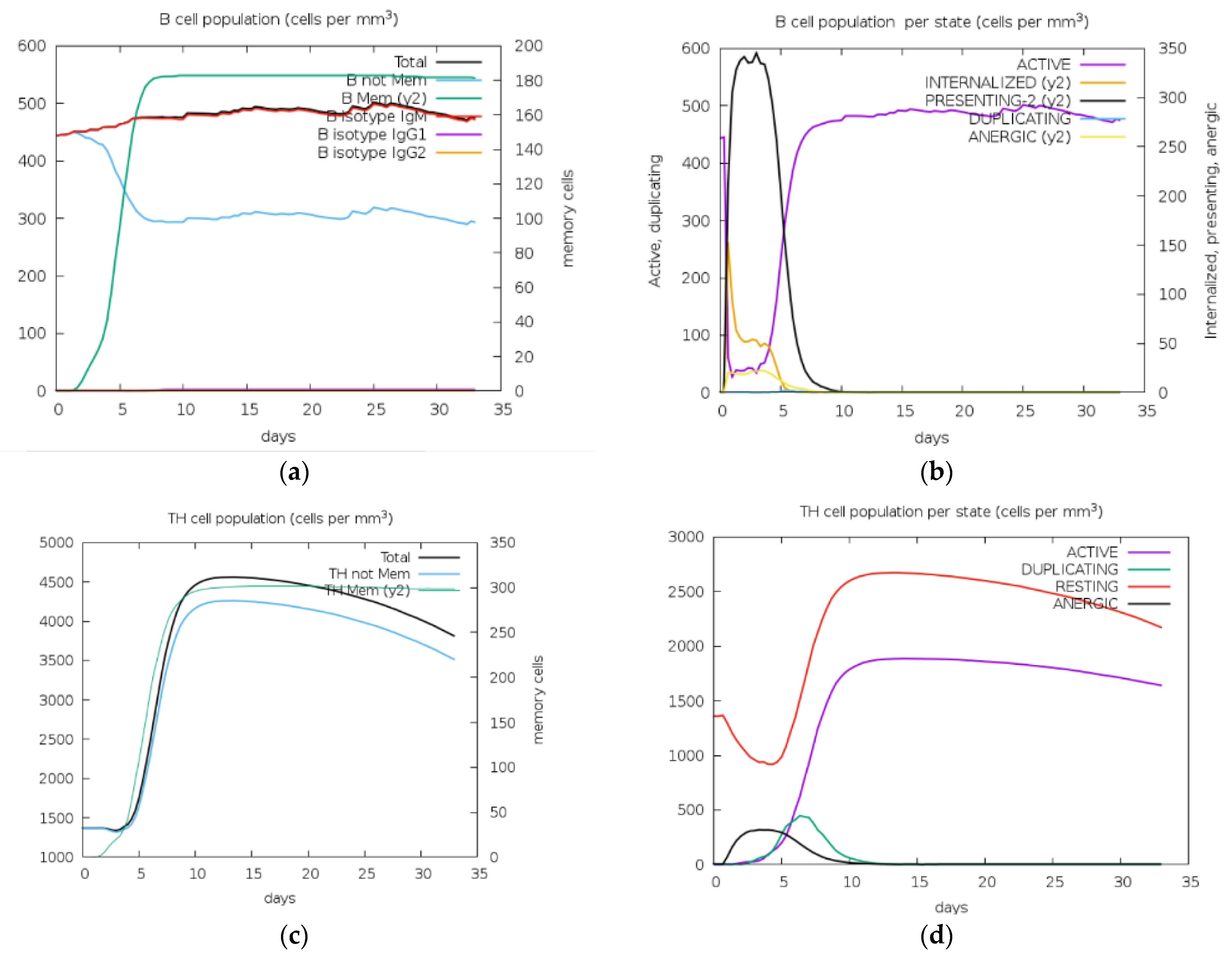
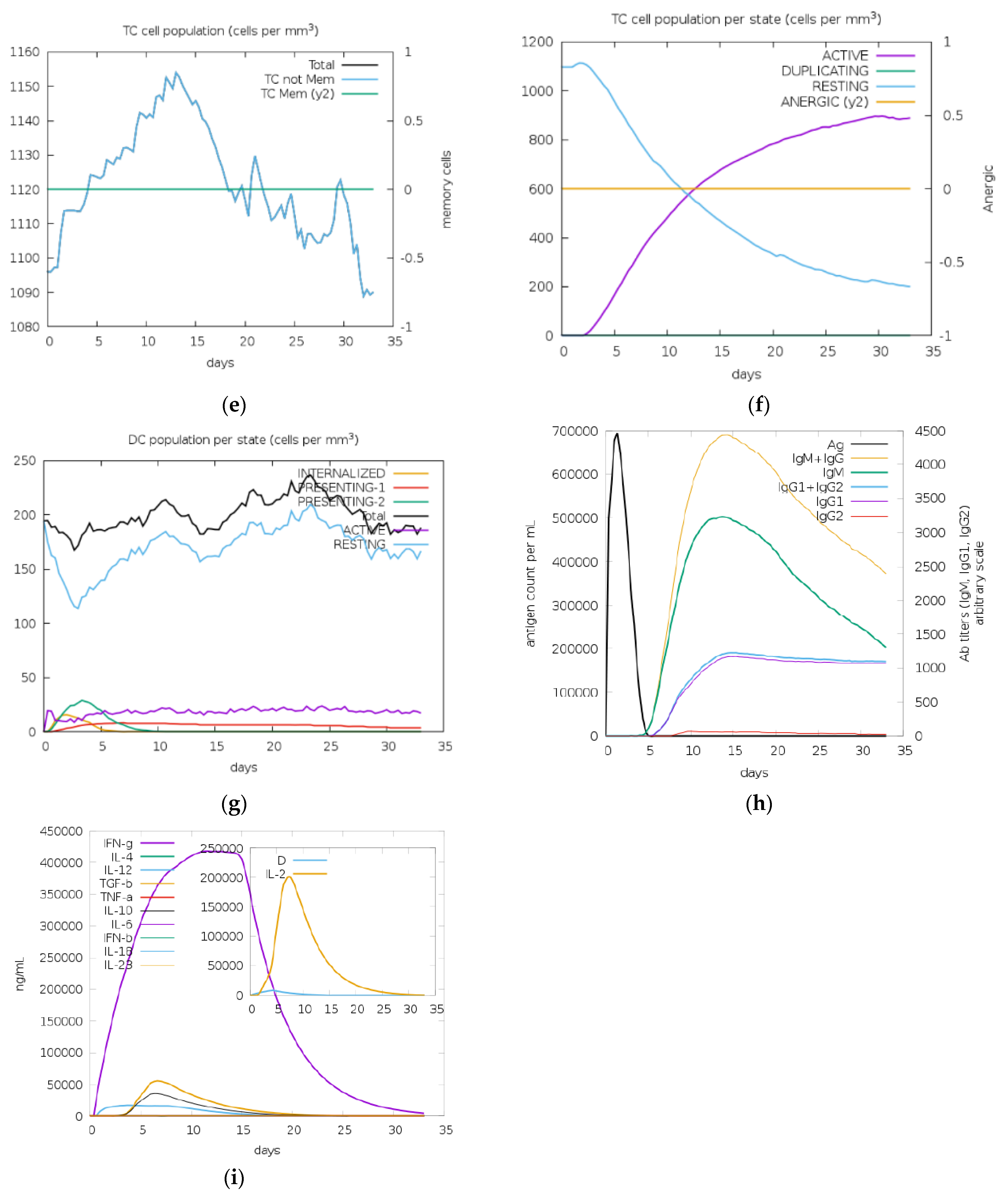
2.7. Immune Simulation
2.8. Codon Optimization and In Silico Cloning
2.9. Post-Translational Modification (PTM)
2.10. Molecular Docking
2.11. Molecular Dynamics (MD) Simulation
3. Results
3.1. Rotavirus Sequence Retrieval and Selection of Epitopes for Multivalent Construct
3.2. Epitopes Population Coverage across Asia, Worldwide and in Africa
3.3. Secondary Structure Prediction
3.4. Physicochemical Properties Prediction
3.5. Molecular Docking with Integrin Subunit
3.6. Immune Stimulation for Vaccine Construct
3.7. Codon Optimization and In-Silico Cloning
3.8. Post-Translational Modification (PTM)
3.9. Molecular Dynamics Simulation for Validation of the Tertiary Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.; Cortese, M.M. 216—Rotaviruses, in Principles and Practice of Pediatric Infectious Diseases, 4th ed.; Long, S.S., Ed.; Elsevier: London, UK, 2012; pp. 1094–1097. [Google Scholar]
- Angel, J.; Franco, M.A.; Greenberg, H.B. Rotaviruses, in Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Oxford, UK, 2008; pp. 507–513. [Google Scholar]
- Esona, M.D.; Gautam, R. Rotavirus. Clin. Lab. Med. 2015, 35, 363–391. [Google Scholar] [CrossRef] [PubMed]
- Meštrović, T. Rotavirus Structure and Classification. 2019. Available online: https://www.news-medical.net/health/Rotavirus-Structure-and-Classification.aspx (accessed on 13 January 2023).
- Taniguchi, K.; Hoshino, Y.; Nishikawa, K.; Green, K.Y.; Maloy, W.L.; Morita, Y.; Urasawa, S.; Kapikian, A.Z.; Chanock, R.M.; Gorziglia, M. Cross-reactive and serotype-specific neutralization epitopes on VP7 of human rotavirus: Nucleotide sequence analysis of antigenic mutants selected with monoclonal antibodies. J. Virol. 1988, 62, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Coulson, B.S.; Kirkwood, C. Relation of VP7 amino acid sequence to monoclonal antibody neutralization of rotavirus and rotavirus monotype. J. Virol. 1991, 65, 5968–5974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuvo, M.S.R.; Mukharjee, S.K.; Ahmed, F. In Silico screening of T-cell and B-cell Epitopes of Rotavirus VP7 and VP4 proteins for Effective Vaccine Design. Bangladesh J. Microbiol. 2018, 35, 45–55. [Google Scholar] [CrossRef]
- Manavalan, B.; Shin, T.H.; Kim, M.O.; Lee, G. AIPpred: Sequence-based prediction of anti-inflammatory peptides using random forest. Front. Pharmacol. 2018, 9, 276. [Google Scholar] [CrossRef] [Green Version]
- Burnett, E.; Parashar, U.D.; Tate, J.E. Real-world effectiveness of rotavirus vaccines, 2006–19: A literature review and meta-analysis. Lancet Glob. Health 2020, 8, e1195–e1202. [Google Scholar] [CrossRef]
- Folorunso, O.S.; Sebolai, O.M. Overview of the Development, Impacts, and Challenges of Live-Attenuated Oral Rotavirus Vaccines. Vaccines 2020, 8, 341. [Google Scholar] [CrossRef]
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018, 172, 958–965. [Google Scholar] [CrossRef] [Green Version]
- Rotavirus: Current Vaccine Intro Status. Available online: https://view-hub.org/vaccine/rota (accessed on 8 January 2023).
- Sadiq, A.; Bostan, N.; Bokhari, H.; Matthijnssens, J.; Yinda, K.C.; Raza, S.; Nawaz, T. Molecular characterization of human group A rotavirus genotypes circulating in Rawalpindi, Islamabad, Pakistan during 2015–2016. PLoS ONE 2019, 14, e0220387. [Google Scholar] [CrossRef] [Green Version]
- Sadiq, A.; Bostan, N.; Khan, J.; Aziz, A. Effect of rotavirus genetic diversity on vaccine impact. Rev. Med. Virol. 2022, 32, e2259. [Google Scholar] [CrossRef] [PubMed]
- Martella, V.; Bányai, K.; Matthijnssens, J.; Buonavoglia, C.; Ciarlet, M. Zoonotic aspects of rotaviruses. Vet. Microbiol. 2010, 140, 246–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, Y.S.; Bhat, S.; Dar, P.S.; Sircar, S.; Dhama, K.; Singh, R.K. Evolving rotaviruses, interspecies transmission and zoonoses. Open Virol. J. 2020, 14, 1–6. [Google Scholar] [CrossRef]
- Dennehy, P.H. Rotavirus vaccines: An overview. Clin. Microbiol. Rev. 2008, 21, 198–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladstone, B.P.; Ramani, S.; Mukhopadhya, I.; Muliyil, J.; Sarkar, R.; Rehman, A.M.; Jaffar, S.; Gomara, M.I.; Gray, J.J.; Brown, D.W.G.; et al. Protective effect of natural rotavirus infection in an Indian birth cohort. N. Engl. J. Med. 2011, 365, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Koch, J.; Harder, T.; von Kries, R.; Wichmann, O. Risk of Intussusception After Rotavirus Vaccination: A Systematic Literature Review and Meta-Analysis. Dtsch. Ärzteblatt Int. 2017, 114, 255–262. [Google Scholar]
- Vartak, A.; Sucheck, S.J. Recent advances in subunit vaccine carriers. Vaccines 2016, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-D.; Chi, W.Y.; Su, J.H.; Ferrall, L.; Hung, C.F.; Wu, T.C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef]
- Martinelli, D.D. In silico vaccine design: A tutorial in immunoinformatics. Healthc. Anal. 2022. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [Green Version]
- Reynisson, B.; Alvarez, B.; Paul, S.; Peters, B.; Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: Improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020, 48, W449–W454. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; Ugene Team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.; Raghava, G.P.S. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins Struct. Funct. Bioinform. 2006, 65, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Doytchinova, I.A.; Flower, D.R. VaxiJen: A server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinform. 2007, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Dimitrov, I.; Bangov, I.; Flower, D.R.; Doytchinova, I. AllerTOP v. 2-a server for in silico prediction of allergens. J. Mol. Model. 2014, 20, 2278. [Google Scholar] [CrossRef]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Open Source Drug Discovery Consortium; Raghava, G.P. In silico approach for predicting toxicity of peptides and proteins. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bui, H.-H.; Sidney, J.; Dinh, K.; Southwood, S.; Newman, M.J.; Sette, A. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinform. 2006, 7, 153. [Google Scholar] [CrossRef] [Green Version]
- Devi, Y.D.; Devi, A.; Gogoi, H.; Dehingia, B.; Doley, R.; Buragohain, A.K.; Singh, C.S.; Borah, P.P.; Rao, C.D.; Ray, P.; et al. Exploring rotavirus proteome to identify potential B- and T-cell epitope using computational immunoinformatics. Heliyon 2020, 6, e05760. [Google Scholar] [CrossRef]
- Bibi, S.; Ullah, I.; Zhu, B.; Adnan, M.; Liaqat, R.; Kong, W.B.; Niu, S. In silico analysis of epitope-based vaccine candidate against tuberculosis using reverse vaccinology. Sci. Rep. 2021, 11, 1249. [Google Scholar] [CrossRef]
- Du, Z.; Su, H.; Wang, W.; Ye, L.; Wei, H.; Peng, Z.; Anishchenko, I.; Baker, D.; Yang, J. The trRosetta server for fast and accurate protein structure prediction. Nat. Protocols 2021, 16, 5634–5651. [Google Scholar] [CrossRef]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Rapin, N.; Lund, O.; Bernaschi, M.; Castiglione, F. Computational immunology meets bioinformatics: The use of prediction tools for molecular binding in the simulation of the immune system. PLoS ONE 2010, 5, e9862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, S.; Bagchi, P.; Dutta, D.; Mukherjee, A.; Kobayashi, N.; Chawlasarkar, M. Computational identification of post-translational modification sites and functional families reveal possible moonlighting role of rotaviral proteins. Bioinformation 2010, 4, 448–451. [Google Scholar] [CrossRef]
- Gupta, R.; Brunak, S. Prediction of Glycosylation across the Human Proteome and the Correlation to Protein Function. Pacific Symp. Biocomput. 2002, 310–322. Available online: https://books.google.com.pk/books?hl=en&lr=&id=LX4zvTqSgLMC&oi=fnd&pg=PA310&dq=39.%09Gupta,+R.%3B+Brunak,+S.+Prediction+of+glycosylation+across+the+human+proteome+and+the+correlation+to+protein+function.+2001%3B+pp.+310%E2%80%93322&ots=gpxsakUmoQ&sig=CeXDwIz9iIMUa8jGNLW7VghHd3c&redir_esc=y#v=onepage&q&f=false (accessed on 17 January 2023).
- Blom, N.; Sicheritz-Pontén, T.; Gupta, R.; Gammeltoft, S.; Brunak, S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 2004, 4, 1633–1649. [Google Scholar] [CrossRef]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.B.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, S.; et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef] [Green Version]
- Graham, K.L.; Halasz, P.; Tan, Y.; Hewish, M.J.; Takada, Y.; Mackow, E.R.; Robinson, M.K.; Coulson, B.S. Integrin-using rotaviruses bind α2β1 integrin α2 I domain via VP4 DGE sequence and recognize αXβ2 and αVβ3 by using VP7 during cell entry. J. Virol. 2003, 77, 9969–9978. [Google Scholar] [CrossRef] [Green Version]
- Abdelhakim, A.H.; Salgado, E.N.; Fu, X.; Pasham, M.; Nicastro, D.; Kirchhausen, T.; Harrison, S.C. Structural correlates of rotavirus cell entry. PLoS Pathog. 2014, 10, e1004355. [Google Scholar] [CrossRef]
- Villena, J.; Vizoso-Pinto, M.; Kitazawa, H. Intestinal innate antiviral immunity and immunobiotics: Beneficial effects against rotavirus infection. Front. Immunol. 2016, 7, 563. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein–protein docking. Nat. Protocols 2017, 12, 255–278. [Google Scholar] [CrossRef]
- Abraham, M.J.; Van Der Spoel, D.; Lindahl, E.; Hess, B.; The GROMACS development team. GROMACS Reference Manual Version 2019. Available online: https://manual.gromacs.org/documentation/2019/manual-2019.pdf (accessed on 2 March 2023).
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef]
- Sadiq, A.; Bostan, N. Comparative Analysis of G1P[8] Rotaviruses Identified Prior to Vaccine Implementation in Pakistan With Rotarix™ and RotaTeq™ Vaccine Strains. Front. Immunol. 2020, 11, 562282. [Google Scholar] [CrossRef]
- Steele, A.D.; Neuzil, K.M.; Cunliffe, N.A.; Madhi, S.A.; Bos, P.; Ngwira, B.; Witte, D.; Todd, S.; Louw, C.; Kirsten, M.; et al. Human rotavirus vaccine Rotarix™ provides protection against diverse circulating rotavirus strains in African infants: A randomized controlled trial. BMC Infect. Dis. 2012, 12, 213. [Google Scholar] [CrossRef] [Green Version]
- Bowen, M.D.; Mijatovic-Rustempasic, S.; Esona, M.D.; Teel, E.N.; Gautam, R.; Sturgeon, M.; Azimi, P.H.; Baker, C.J.; Bernstein, D.I.; Boom, J.A.; et al. Rotavirus strain trends during the postlicensure vaccine era: United States, 2008–2013. J. Infect. Dis. 2016, 214, 732–738. [Google Scholar] [CrossRef] [Green Version]
- Cates, J.E.; Amin, A.B.; Tate, J.E.; Lopman, B.; Parashar, U. Do rotavirus strains affect vaccine effectiveness? A systematic review and meta-analysis. Pediatr. Infect. Dis. J. 2021, 40, 1135–1143. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Zeller, M.; Heylen, E.; De Coster, S.; Vercauteren, J.; Braeckman, T.; Herk, K.V.; Meyer, N.; PirCon, J.Y.; Soriano-Gabbaro, M.; et al. Higher proportion of G2P[4] rotaviruses in vaccinated hospitalized cases compared with unvaccinated hospitalized cases, despite high vaccine effectiveness against heterotypic G2P[4] rotaviruses. Clin. Microbiol. Infect. 2014, 20, O702–O710. [Google Scholar] [CrossRef] [Green Version]
- Muhsen, K.; Anis, E.; Rubinstein, U.; Kassem, E.; Goren, S.; Shulman, L.M.; Ephros, M.; Cohen, D. Effectiveness of rotavirus pentavalent vaccine under a universal immunization programme in Israel, 2011–2015: A case–control study. Clin. Microbiol. Infect. 2018, 24, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthijnssens, J.; Nakagomi, O.; Kirkwood, C.D.; Ciarlet, M.; Desselberger, U.; Ranst, M.V. Group A rotavirus universal mass vaccination: How and to what extent will selective pressure influence prevalence of rotavirus genotypes? Expert Rev. Vaccines 2012, 11, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K. Multi valent DNA vaccine against group A human rotavirus: An in-silico investigation. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Jafarpour, S.; Ayat, H.; Ahadi, A.M. Design and antigenic epitopes prediction of a new trial recombinant multiepitopic rotaviral vaccine: In silico analyses. Viral Immunol. 2015, 28, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Nirwati, H.; Donato, C.M.; Ikram, A.; Aman, A.T.; Wibawa, T.; Kirkwood, C.D.; Soenarto, Y.; Pan, Q.; Hakim, M.S. Phylogenetic and immunoinformatic analysis of VP4, VP7, and NSP4 genes of rotavirus strains circulating in children with acute gastroenteritis in Indonesia. J. Med. Virol. 2019, 91, 1776–1787. [Google Scholar] [CrossRef]
- Arguelles, M.; Villegas, G.A.; Castello, A.; Abrami, A.; Ghiringhelli, P.D.; Semorile, L.; Glikmann, G. VP7 and VP4 genotyping of human group A rotavirus in Buenos Aires, Argentina. J. Clin. Microbiol. 2000, 38, 252–259. [Google Scholar] [CrossRef]
- McCowin, S.E.; Moreau, G.B.; Haque, R.; Noble, J.A.; McDevitt, S.L.; Donowitz, J.R.; Alam, M.M.; Kirkpatrick, B.D.; Petri, W.A.; Marie, C. HLA class I and II associations with common enteric pathogens in the first year of life. EBioMedicine 2021, 67, 103346. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, T.; Yu, Q.; Zhang, H.; Du, J.; Zhang, Y.; Xia, S.; Yang, H.; Li, Q. Association of human leukocyte antigen alleles and supertypes with immunogenicity of oral rotavirus vaccine given to infants in China. Medicine 2018, 97, e12706. [Google Scholar] [CrossRef]
- Wei, J.; Li, J.; Zhang, X.; Tang, Y.; Wang, J.; Wu, Y. A naturally processed epitope on rotavirus VP7 glycoprotein recognized by HLA-A2. 1-restricted cytotoxic CD8+ T cells. Viral Immunol. 2009, 22, 189–194. [Google Scholar] [CrossRef]
- Herrmann, J.E.; Chen, S.C.; Jones, D.H.; Tinsley-Bown, A.; Fynan, E.F.; Greenberg, H.B.; Farrar, G.H. Immune Responses and Protection Obtained by Oral Immunization with Rotavirus VP4 and VP7 DNA Vaccines Encapsulated in Microparticles. Virology 1999, 259, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Desselberger, U.; Huppertz, H.-I. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J. Infect. Dis. 2011, 203, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sana, M.; Javed, A.; Jamal, S.B.; Junaid, M.; Faheem, M. Development of multivalent vaccine targeting M segment of Crimean Congo Hemorrhagic Fever Virus (CCHFV) using immunoinformatic approaches. Saudi J. Biol. Sci. 2022, 29, 2372–2388. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Rungta, T.; Rawat, M.; Goyal, K.; Gupta, Y.; Singh, M.P. Excavating SARS-coronavirus 2 genome for epitope-based subunit vaccine synthesis using immunoinformatics approach. J. Cell. Physiol. 2021, 236, 1131–1147. [Google Scholar] [CrossRef]
- Guerrero, C.A.; Méndez, E.; Zárate, S.; Isa, P.; López, S.; Arias, C.F. Integrin alpha(v)beta(3) mediates rotavirus cell entry. Proc. Natl. Acad. Sci. USA 2000, 97, 14644–14649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, A.; Onozuka, A.; Matin, K.; Imai, S.; Hanada, N.; Nisizawa, T. RGD motif enhances immunogenicity and adjuvanicity of peptide antigens following intranasal immunization. Vaccine 2003, 22, 237–243. [Google Scholar] [CrossRef]
- Sheikh, A.; Alhakamy, N.A.; Md, S.; Kesharwani, P. Recent Progress of RGD Modified Liposomes as Multistage Rocket Against Cancer. Front. Pharmacol. 2022, 12, 4024. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.-C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef] [Green Version]
- Ayyagari, V.S.; TC, V.; Srirama, K. Design of a multi-epitope-based vaccine targeting M-protein of SARS-CoV2: An immunoinformatics approach. J. Biomol. Struct. Dyn. 2022, 40, 2963–2977. [Google Scholar] [CrossRef]
- Ali, M.; Pandey, R.K.; Khatoon, N.; Narula, A.; Mishra, A.; Prajapati, V.K. Exploring dengue genome to construct a multi-epitope based subunit vaccine by utilizing immunoinformatics approach to battle against dengue infection. Sci. Rep. 2017, 7, 9232. [Google Scholar] [CrossRef] [Green Version]
- Saha, R.; Prasad, B.V. In silico approach for designing of a multi-epitope based vaccine against novel Coronavirus (SARS-COV-2). BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Goel, H.; Hazel, A.; Ustach, V.D.; Jo, S.; Yu, W.; MacKerell, A.D. Rapid and accurate estimation of protein–ligand relative binding affinities using site-identification by ligand competitive saturation. Chem. Sci. 2021, 12, 8844–8858. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Zou, X. An iterative knowledge-based scoring function for protein–protein recognition. Proteins Struct. Funct. Bioinform. 2008, 72, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, E.A.; Zhang, Y. Protein–Ligand Docking in the Machine-Learning Era. Molecules 2022, 27, 4568. [Google Scholar] [CrossRef]
- Ikram, A.; Zaheer, T.; Awan, F.M.; Obaid, A.; Naz, A.; Hanif, R.; Paracha, R.Z.; Ali, A.; Naveed, A.K.; Janjua, H.A. Exploring NS3/4A, NS5A and NS5B proteins to design conserved subunit multi-epitope vaccine against HCV utilizing immunoinformatics approaches. Sci. Rep. 2018, 8, 16107. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Protein | Epitope Type | Peptide | Vaxijen Score; ≥0.5 | Allergenicity | IFNγ Induction | Toxicity | Anti- Inflammatory Response | Total Amino Acids | MW | pI |
|---|---|---|---|---|---|---|---|---|---|---|
| VP4 | B cell | VTLSTQFTDFVSLNSLRFRF | 1.1633 | Non-allergen | + | Non-toxic | AIP | 20 | 2378.71 | 9.57 |
| DSPVISAIIDFKTLKNLNDN | 0.47 | Non-allergen | + | Non-toxic | AIP | 20 | 2217.5 | 4.43 | ||
| MSFDDISAAVLKTKIDMSTQ | 0.8802 | Non-allergen | + | Non-toxic | AIP | 20 | 2201.53 | 4.43 | ||
| MHC-I | NMVYVRSLA | 0.4292 | Non-allergen | − | Non-toxic | AIP | 9 | 1052.26 | 8.75 | |
| YINNGLPPI | 0.9097 | Non-allergen | − | Non-toxic | AIP | 9 | 1000.16 | 5.52 | ||
| MHC-II | VISAIIDFK | 1.2685 | Non-allergen | + | Non-toxic | AIP | 9 | 1005.22 | 5.81 | |
| ISAAVLKTK | 1.2881 | Non-allergen | + | Non-toxic | AIP | 9 | 930.16 | 10 | ||
| VP7 | B cell | MDITLYYYQQTDEANKWISM | 0.4852 | Non-allergen | + | Non-toxic | AIP | 20 | 2513.82 | 4.03 |
| MHC-I | YYYQQTDEA | 0.5149 | Non-allergen | + | Non-toxic | AIP | 9 | 1180.19 | 3.67 | |
| MHC-II | YYQQTDEAN | 0.5459 | Non-allergen | + | Non-toxic | AIP | 9 | 1131.12 | 3.67 |
| MHC Class | Region | Coverage | Average Hit | PC90 |
|---|---|---|---|---|
| Combined | South Asia | 99% | 2.33 | 1.81 |
| Worldwide | 98.47% | 2.82 | 1.72 | |
| West Africa | 87.35% | 1.5 | 0.79 | |
| East Africa | 78.3% | 1.27 | 0.46 | |
| North Africa | 74.03% | 1.15 | 0.39 | |
| Central Africa | 59.05% | 0.8 | 0.24 |
| Toxicity | Total Amino Acids | MW (g/mol) | pI | Formula | Total Atoms | Approx ½ Life | Instability Index | Gravy Score | Stability Status | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mammalian Cell (In Vitro) | Yeast (In Vivo) | E. coli (In Vivo) | ||||||||||
| With adjuvant | Non-toxic | 172 | 18878.3 | 8.6 | C845H1311N217O263S5 | 2641 | 1 h | 2 min | 2 min | 35.94 | −0.291 | Stable |
| Without adjuvant | Non-toxic | 169 | 18550 | 8.61 | C833H1291N211O258S5 | 2598 | 1 h | 30 min | >10 h | 36.91 | −0.246 | Stable |
| Integrin | ||||
|---|---|---|---|---|
| Cluster | Members | Representative | Weighted Score | |
| Balanced | 0 | 120 | Center | −836.7 |
| 0 | 120 | Lowest Energy | −964.2 | |
| Electrostatic favored | 0 | 45 | Center | −919.9 |
| 0 | 45 | Lowest Energy | −998.7 | |
| Hydrophobic Favored | 0 | 100 | Center | −905.2 |
| 0 | 100 | Lowest Energy | −1049 | |
| Van der Waals | 0 | 56 | Center | −280.9 |
| 0 | 56 | Lowest Energy | −295.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, M.; Ayub, A.; Habib, S.; Rana, M.S.; Rehman, Z.; Zohaib, A.; Jamal, S.B.; Jaiswal, A.K.; Andrade, B.S.; de Carvalho Azevedo, V.; et al. Vaccinomics Approach for Multi-Epitope Vaccine Design against Group A Rotavirus Using VP4 and VP7 Proteins. Vaccines 2023, 11, 726. https://doi.org/10.3390/vaccines11040726
Usman M, Ayub A, Habib S, Rana MS, Rehman Z, Zohaib A, Jamal SB, Jaiswal AK, Andrade BS, de Carvalho Azevedo V, et al. Vaccinomics Approach for Multi-Epitope Vaccine Design against Group A Rotavirus Using VP4 and VP7 Proteins. Vaccines. 2023; 11(4):726. https://doi.org/10.3390/vaccines11040726
Chicago/Turabian StyleUsman, Muhammad, Aaima Ayub, Sabahat Habib, Muhammad Suleman Rana, Zaira Rehman, Ali Zohaib, Syed Babar Jamal, Arun Kumar Jaiswal, Bruno Silva Andrade, Vasco de Carvalho Azevedo, and et al. 2023. "Vaccinomics Approach for Multi-Epitope Vaccine Design against Group A Rotavirus Using VP4 and VP7 Proteins" Vaccines 11, no. 4: 726. https://doi.org/10.3390/vaccines11040726
APA StyleUsman, M., Ayub, A., Habib, S., Rana, M. S., Rehman, Z., Zohaib, A., Jamal, S. B., Jaiswal, A. K., Andrade, B. S., de Carvalho Azevedo, V., Faheem, M., & Javed, A. (2023). Vaccinomics Approach for Multi-Epitope Vaccine Design against Group A Rotavirus Using VP4 and VP7 Proteins. Vaccines, 11(4), 726. https://doi.org/10.3390/vaccines11040726








