Immunogenicity and Safety of Homologous and Heterologous Prime-Boost of CoronaVac® and ChAdOx1 nCoV-19 among Hemodialysis Patients: An Observational Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
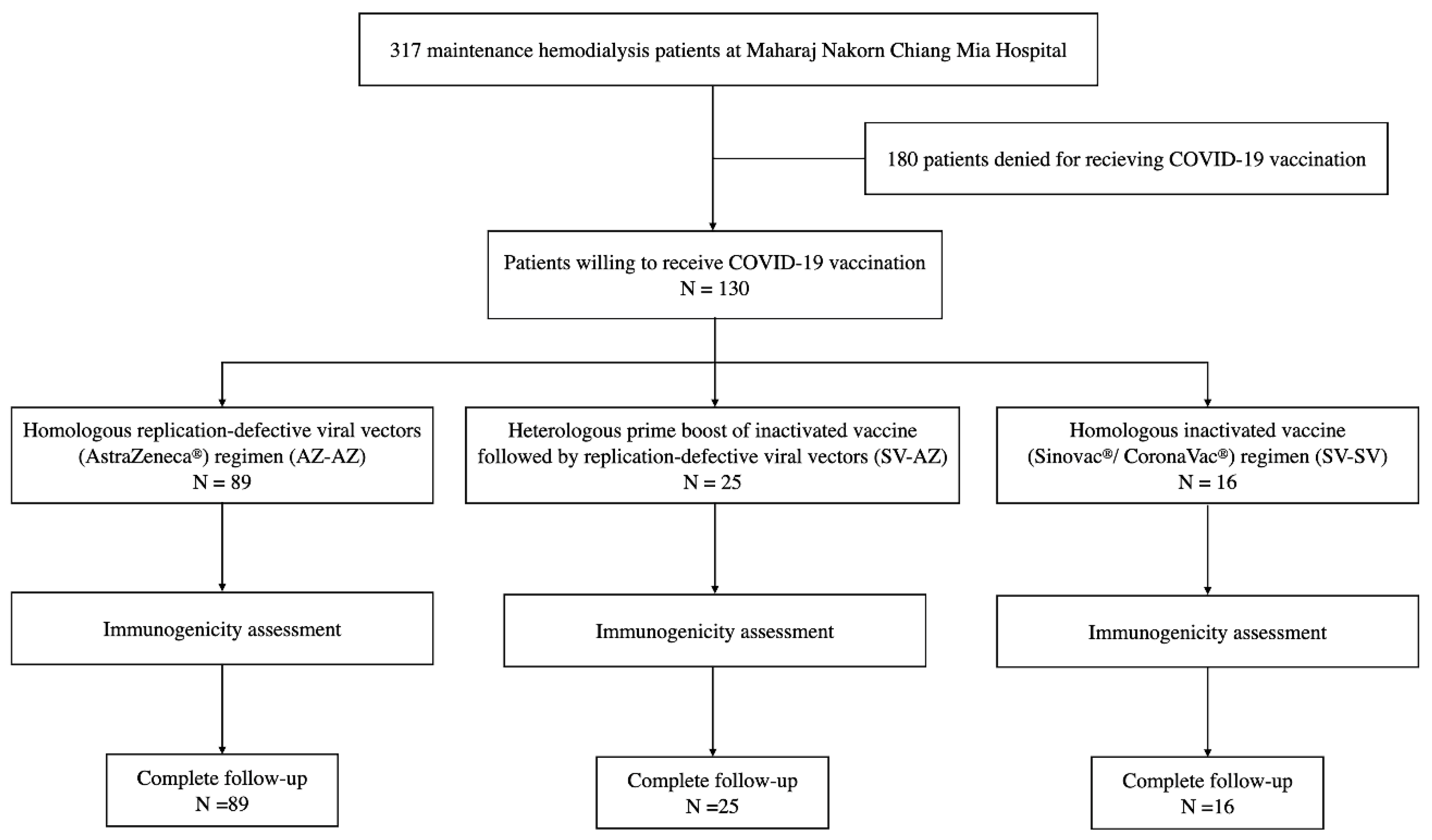
2.1. Study Design and Participants
2.2. Vaccine Regimen
2.2.1. Homologous Inactivated Vaccine against SARS-CoV-2 Regimen CoronaVac® Vaccine (Sinovac) (SV-SV)
2.2.2. Homologous Replication-Defective Viral Vectors against the SARS-CoV-2 Regimen ChAdOx1 nCoV-19 Vaccine (AZD1222) (AZ-AZ)
2.2.3. Heterologous Prime-Boost of Inactivated Vaccine Followed by the Replication-Defective Viral Vectors Vaccine (SV-AZ)
2.2.4. Data Collection, Laboratory Collection, and Immunogenicity Assessment
2.3. SARS-CoV2 Anti-RBD IgG Assay
2.4. SARS-CoV-2 sVNT
2.5. Safety Assessment and Adverse Events
2.6. Study Outcomes
2.7. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics and Laboratory Results of Participants
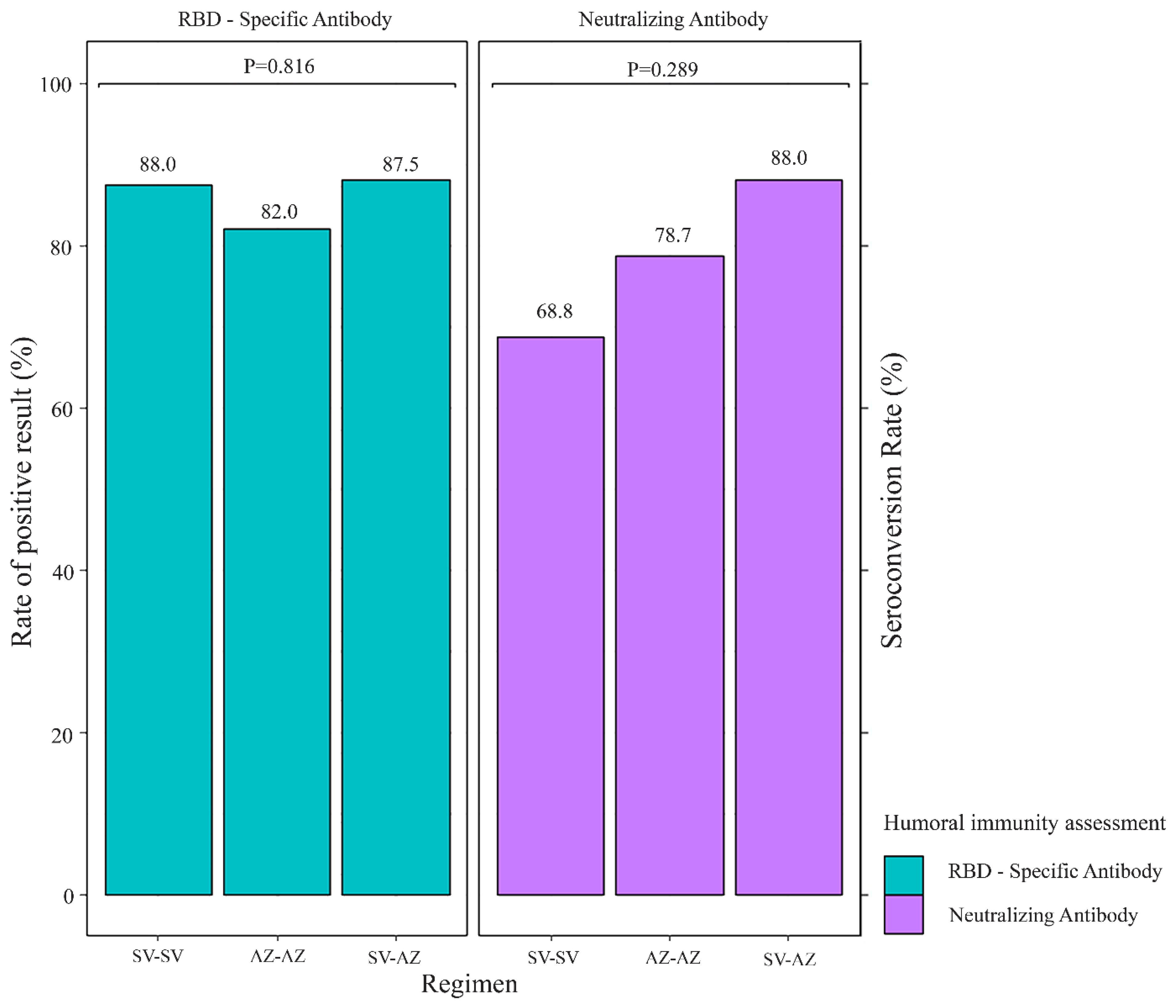
3.2. SARS-CoV-2 Vaccine Immunogenicity
3.3. Factors Associated with Seroconversion Measured by sVNT among MHD Patients
3.4. Safety and Adverse Events after Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Coronavirus Disease 2019 (COVID-19): Situation Report, 61. Available online: https://apps.who.int/iris/handle/10665/331605 (accessed on 21 March 2022).
- COVID-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef] [PubMed]
- Clift, A.K.; Coupland, C.A.C.; Keogh, R.H.; Diaz-Ordaz, K.; Williamson, E.; Harrison, E.M.; Hayward, A.; Hemingway, H.; Horby, P.; Mehta, N.; et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: National derivation and validation cohort study. BMJ 2020, 371, m3731. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Draft Landscape of COVID-19 Candidate Vaccines. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 20 October 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. NEJM 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 Vaccine against the B.1.351 Variant. NEJM 2021, 384, 1885–1898. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. NEJM 2020, 384, 403–416. [Google Scholar] [CrossRef]
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, Ş.; Erdinç, F.; Akalın, E.H.; Tabak Ö, F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- World Health Organization. Interim Recommendations for Use of the AZD1222 (ChAdOx1-S (Recombinant)) Vaccine against COVID-19 Developed by Oxford University and AstraZeneca. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1 (accessed on 11 February 2022).
- Lu, S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009, 21, 346–351. [Google Scholar] [CrossRef]
- He, Q.; Mao, Q.; An, C.; Zhang, J.; Gao, F.; Bian, L.; Li, C.; Liang, Z.; Xu, M.; Wang, J. Heterologous prime-boost: Breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerg. Microbes Infect. 2021, 10, 629–637. [Google Scholar] [CrossRef]
- Spencer, A.J.; McKay, P.F.; Belij-Rammerstorfer, S.; Ulaszewska, M.; Bissett, C.D.; Hu, K.; Samnuan, K.; Blakney, A.K.; Wright, D.; Sharpe, H.R.; et al. Heterologous vaccination regimens with self-amplifying RNA and adenoviral COVID vaccines induce robust immune responses in mice. Nat. Commun. 2021, 12, 2893. [Google Scholar] [CrossRef]
- Chew, K.L.; Tan, S.S.; Saw, S.; Pajarillaga, A.; Zaine, S.; Khoo, C.; Wang, W.; Tambyah, P.; Jureen, R.; Sethi, S.K. Clinical evaluation of serological IgG antibody response on the Abbott Architect for established SARS-CoV-2 infection. Clin. Microbiol. Infect. 2020, 26, 1256.e9–1256.e11. [Google Scholar] [CrossRef]
- EUROIMMUN. SARS-CoV-2 NeutraLISA. Available online: https://www.coronavirus-diagnostics.com/documents/Indications/Infections/Coronavirus/EI_2606_D_UK_F.pdf (accessed on 11 February 2022).
- Corp, S. Stata Statistical Software: Release 16; StataCorp LLC: College Station, TX, USA, 2019. [Google Scholar]
- Krueger, K.M.; Ison, M.G.; Ghossein, C. Practical Guide to Vaccination in All Stages of CKD, Including Patients Treated by Dialysis or Kidney Transplantation. Am. J. Kidney Dis. 2020, 75, 417–425. [Google Scholar] [CrossRef]
- Akyol, M.; Çevik, E.; Ucku, D.; Tanrıöver, C.; Afşar, B.; Kanbay, A.; Covic, A.; Ortiz, A.; Basile, C.; Kanbay, M. Immunogenicity of SARS-CoV-2 mRNA vaccine in dialysis and kidney transplant patients: A systematic review. Tuberk. Toraks 2021, 69, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Agur, T.; Ben-Dor, N.; Goldman, S.; Lichtenberg, S.; Herman-Edelstein, M.; Yahav, D.; Rozen-Zvi, B.; Zingerman, B. Antibody response to mRNA SARS-CoV-2 vaccine among dialysis patients—A prospectivecohort study. Nephrol. Dial. Transplant. 2021, gfab155. [Google Scholar] [CrossRef]
- Simon, B.; Rubey, H.; Treipl, A.; Gromann, M.; Hemedi, B.; Zehetmayer, S.; Kirsch, B. Hemodialysis Patients Show a Highly Diminished Antibody Response after COVID-19 mRNA Vaccination Compared to Healthy Controls. Nephrol. Dial. Transplant. 2021, 36, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Danthu, C.; Hantz, S.; Dahlem, A.; Duval, M.; Ba, B.; Guibbert, M.; El Ouafi, Z.; Ponsard, S.; Berrahal, I.; Achard, J.M.; et al. Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 32, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Frantzen, L.; Cavaille, G.; Thibeaut, S.; El-Haik, Y. Efficacy of the BNT162b2 mRNA Covid-19 Vaccine in a hemodialysis cohort. Nephrol. Dial. Transplant. 2021, gfab165. [Google Scholar] [CrossRef]
- Grupper, A.; Sharon, N.; Finn, T.; Cohen, R.; Israel, M.; Agbaria, A.; Rechavi, Y.; Schwartz, I.F.; Schwartz, D.; Lellouch, Y.; et al. Humoral Response to the Pfizer BNT162b2 Vaccine in Patients Undergoing Maintenance Hemodialysis. Clin. J. Am. Soc. Nephrol. 2021, 16, 1037–1042. [Google Scholar] [CrossRef]
- Jahn, M.; Korth, J.; Dorsch, O.; Anastasiou, O.E.; Sorge-Hädicke, B.; Tyczynski, B.; Gäckler, A.; Witzke, O.; Dittmer, U.; Dolff, S.; et al. Humoral Response to SARS-CoV-2-Vaccination with BNT162b2 (Pfizer-BioNTech) in Patients on Hemodialysis. Vaccines 2021, 9, 360. [Google Scholar] [CrossRef]
- Broseta, J.J.; Rodríguez-Espinosa, D.; Rodríguez, N.; Mosquera, M.D.M.; Marcos, M.; Egri, N.; Pascal, M.; Soruco, E.; Bedini, J.L.; Bayés, B.; et al. Humoral and Cellular Responses to mRNA-1273 and BNT162b2 SARS-CoV-2 Vaccines Administered to Hemodialysis Patients. Am. J. Kidney Dis. 2021, 78, 571–581. [Google Scholar] [CrossRef]
- Oh, S.; Purja, S.; Shin, H.; Kim, M.S.; Park, S.; Kronbichler, A.; Smith, L.; Eisenhut, M.; Shin, J.I.; Kim, E. Efficacy, Immunogenicity, and Safety of COVID-19 Vaccines in Randomized Control Trials in the Pre-Delta Era: A Systematic Review and Network Meta-Analysis. Vaccines 2022, 10, 1572. [Google Scholar] [CrossRef]
- Borobia, A.M.; Carcas, A.J.; Pérez-Olmeda, M.; Castaño, L.; Bertran, M.J.; García-Pérez, J.; Campins, M.; Portolés, A.; González-Pérez, M.; García Morales, M.T.; et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Tenbusch, M.; Schumacher, S.; Vogel, E.; Priller, A.; Held, J.; Steininger, P.; Beileke, S.; Irrgang, P.; Brockhoff, R.; Salmanton-García, J.; et al. Heterologous prime–boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect. Dis. 2021, 21, 1212–1213. [Google Scholar] [CrossRef]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir. Med. 2021, 9, 1255–1265. [Google Scholar] [CrossRef]
- Zhang, J.; He, Q.; An, C.; Mao, Q.; Gao, F.; Bian, L.; Wu, X.; Wang, Q.; Liu, P.; Song, L.; et al. Boosting with heterologous vaccines effectively improves protective immune responses of the inactivated SARS-CoV-2 vaccine. Emerg. Microbes Infect. 2021, 10, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Quach, T.H.T.; Tran, T.M.; Phuoc, H.N.; Nguyen, H.T.; Vo, T.K.; Vo, G.V. Reactogenicity and immunogenicity of heterologous prime-boost immunization with COVID-19 vaccine. Biomed. Pharmacother. 2022, 147, 112650. [Google Scholar] [CrossRef]


| Characteristic | Total (N = 130) | SV-SV (N = 16) | AZ-AZ (N = 89) | SV-AZ (N = 25) | p Value |
|---|---|---|---|---|---|
| Age—year | 64.2 ± 14.3 | 49.8 ± 10.2 | 69.5 ± 10.2 | 54.5 ± 17.7 | <0.001 |
| Female | 59 (45.3) | 5 (31.3) | 41 (46.1) | 13 (52.0) | 0.417 |
| Body mass index—kg/m2 | 24.1 ± 5.4 | 27.9 ± 8.8 | 23.9 ± 4.4 | 22.7 ± 5.1 | 0.007 |
| Comorbid disease | |||||
| Hypertension | 124 (95.4) | 16 (100.0) | 84 (94.4) | 24 (96.0) | 1.000 |
| Dyslipidemia | 53 (40.8) | 6 (37.5) | 36 (40.5) | 11 (44.0) | 0.913 |
| Diabetes mellitus | 57 (43.9) | 3 (18.8) | 48 (53.9) | 6 (24.0) | 0.003 |
| Cardiovascular disease (MI, HF) | 25 (19.2) | 3 (18.8) | 17 (19.1) | 5 (20.0) | 1.000 |
| Cerebrovascular disease | 4 (3.1) | 1 (6.3) | 3 (3.4) | 0 (0) | 0.535 |
| Chronic obstructive pulmonary disease | 2 (1.5) | 0 (0) | 1 (1.1) | 1 (4.0) | 0.533 |
| Connective tissue disease | 8 (6.2) | 1 (6.3) | 3 (3.4) | 4 (16.0) | 0.048 |
| Liver disease | 6 (4.6) | 0 (0) | 5 (5.6) | 1 (4.0) | 1.000 |
| Cause of end-stage kidney disease | 0.010 | ||||
| Diabetic kidney disease | 54 (41.5) | 2 (12.5) | 46 (51.7) | 6 (24.0) | |
| Hypertensive nephropathy | 18 (13.9) | 2 (12.5) | 14 (15.7) | 2 (8.0) | |
| Obstructive uropathy | 9 (6.9) | 2 (12.5) | 4 (4.5) | 3 (12.0) | |
| Glomerular disease | 28 (21.5) | 7 (43.8) | 13 (14.6) | 8 (32.0) | |
| Others causes | 6 (4.6) | 1 (6.3) | 3 (3.4) | 2 (8.0) | |
| Unknown cause | 15 (11.5) | 2 (12.5) | 9 (10.1) | 4 (16.0) | |
| Dialysis vintage—months (min, max) | 44 (26, 70) | 32.5 (23, 69.5) | 45 (30, 69) | 32 (17, 75) | 0.812 |
| Dialysis schedule | 0.802 | ||||
| 1–2 time/week | 25 (19.2) | 4 (25.0) | 16 (18.0) | 5 (20.0) | |
| 3–4 time/week | 105 (80.8) | 12 (75.0) | 73 (82.0) | 20 (80.0) | |
| Mode of hemodialysis | 0.582 | ||||
| Conventional hemodialysis | 119 (91.5) | 14 (87.5) | 83 (93.3) | 22 (88.0) | |
| Online hemodiafiltration | 11 (8.5) | 2 (12.5) | 6 (6.7) | 3 (12.0) | |
| Presence of urine output | 83 (63.9) | 10 (62.5) | 56 (62.9) | 17 (68.0) | 0.890 |
| <200 mL | 28 (33.7) | 3 (30.0) | 19 (33.9) | 6 (35.3) | 1.000 |
| ≥200 mL | 55 (66.3) | 7 (70.0) | 37 (66.1) | 11 (64.7) | |
| Iron supplement | 82 (63.1) | 12 (75.0) | 57 (64.0) | 13 (52.0) | 0.346 |
| Intravenously | 68 (82.9) | 10 (83.3) | 46 (80.7) | 12 (92.3) | 0.743 |
| Oral | 14 (17.1) | 2 (16.67) | 11 (19.3) | 1 (7.7) | |
| Laboratory result | |||||
| Hemoglobin—g/dL | 10.3 ± 1.4 | 10.3 ± 1.0 | 10.3 ± 1.34 | 10.4 ± 1.7 | 0.965 |
| White blood cells—cells/mm3 | 6738.5 ± 2160.7 | 6970.6 ± 1852.9 | 6520.7 ± 2054.9 | 7365.6 ± 2615.7 | 0.203 |
| Polymorphonuclear cell—% | 63.8 ± 9.5 | 64.1 ± 8.5 | 63.5 ± 9.0 | 64.6 ± 11.6 | 0.889 |
| Lymphocyte—% | 22.1 ± 6.4 | 21.6 ± 3.9 | 22.1 ± 6.3 | 22.0 ± 7.9 | 0.959 |
| ≤16 | 21 (16.2) | 1 (6.3) | 14 (15.7) | 6 (24.0) | 0.322 |
| >16 | 109 (83.9) | 15 (93.8) | 75 (84.3) | 19 (76.0) | |
| Platelet—×103 cells/mm3 | 219 (175, 261) | 215 (187, 246) | 216 (179, 259) | 239 (156, 278) | 0.789 |
| Transferrin saturation—% (min, max) | 31 (24, 42) | 31 (19.5, 52.5) | 30 (24, 39) | 35 (27, 47) | 0.079 |
| Ferritin—µg/L | 568.9 ± 414.4 | 478.56 ± 271.01 | 564.1 ± 436.4 | 643.8 ± 409.2 | 0.455 |
| C-reactive protein—mg/L | 3.1 (3.12, 6.2) | 3.12 (3.12, 6) | 3.1 (3.1, 6.5) | 3.1 (3.1, 4.6) | 0.785 |
| Albumin—g/dL | 4 (3.8, 4.2) | 4.1 (3.85, 4.4) | 4 (3.8, 4.2) | 4.1 (3.9, 4.2) | 0.122 |
| Kt/v | 1.6 ± 0.5 | 1.49 ± 0.32 | 1.6 ± 0.5 | 1.68 ± 0.52 | 0.329 |
| Variable | Positive | Univariate Analysis | ||
|---|---|---|---|---|
| OR | 95%CI | p Value | ||
| No. | 103/130 (79.2) | |||
| Age—(every 5-year increment in age) | 63.2 ± 14.4 | 0.81 | 0.66–0.99 | 0.040 |
| Female | 48/59 (81.4) | 1.27 | 0.54–3.00 | 0.587 |
| Vaccine regimen | ||||
| SV-AZ vs. SV-SV | 3.33 | 1.49–7.43 | 0.003 | |
| AZ-AZ vs. SV-SV | 1.67 | 0.93–3.01 | 0.085 | |
| SV-AZ vs. AZ-AZ | 1.99 | 1.03–3.83 | 0.039 | |
| Body mass index—kg/m2 | 23.8 ± 4.4 | 0.95 | 0.88–1.02 | 0.149 |
| Comorbid disease | ||||
| Diabetes mellitus | 42/57 (73.7) | 0.55 | 0.23–1.29 | 0.172 |
| Connective tissue disease | 6/8 (75.0) | 0.77 | 0.15–4.06 | 0.761 |
| Dialysis vintage—months | 44 (27, 70) | 1.00 | 0.99–1.01 | 0.696 |
| Laboratory result | ||||
| Hemoglobin—g/dL | 10.38 ± 1.44 | 1.12 | 0.83–1.51 | 0.461 |
| White blood cell—cells/mm3 | 6654.0 ± 2077.6 | 1.00 | 1.00–1.00 | 0.385 |
| Lymphocyte—% | 22.7 ± 6.1 | 1.08 | 1.01–1.17 | 0.031 |
| ≤16 | 11 (10.7) | Ref. | ||
| >16 | 92 (89.3) | 4.92 | 1.81–13.38 | 0.002 |
| Total Lymphocyte | 1462.8 ± 480.1 | 1.00 | 1.00–1.00 | 0.210 |
| Ferritin—µg/L | 460 (302, 724) | 1.00 | 1.00–1.00 | 0.796 |
| C-reactive protein—mg/L | 3.12 (3.12, 7.7) | 1.07 | 0.95–1.17 | 0.290 |
| Albumin—g/dL | 4 (3.9, 4.2) | 1.91 | 0.57–6.37 | 0.292 |
| Kt/v | 1.6 ± 0.5 | 1.94 | 0.73–5.15 | 0.182 |
| Variable | Positive | Multivariate Analysis | ||
|---|---|---|---|---|
| Adjusted OR | 95%CI | p Value | ||
| No. | 103/130 (79.2) | |||
| Age—(every 5-year increment in age) | 63.2 ± 14.4 | 0.80 | 0.64–0.99 | 0.047 |
| Vaccine regimen (Regimen vs. Ref.) | ||||
| SV-AZ vs. SV-SV | 10.12 | 1.45–70.57 | 0.020 | |
| AZ-AZ vs. SV-SV | 5.58 | 1.16–26.75 | 0.031 | |
| SV-AZ vs. AZ-AZ | 1.81 | 0.40–8.13 | 0.437 | |
| Lymphocyte—% | 22.7 ± 6.1 | |||
| ≤16 | 11 (10.7) | Ref. | ||
| >16 | 92 (89.3) | 6.47 | 2.15–19.46 | 0.001 |
| Side Effects and AE | Regimen | |||||
|---|---|---|---|---|---|---|
| SV-SV (N = 16) | SV-AZ (N = 25) | AZ-AZ (N = 89) | ||||
| After Dose 1 | After Dose 2 | After Dose 1 | After Dose 2 | After Dose 1 | After Dose 2 | |
| Event | 4/16 (25.0) | 7/16 (43.8) | 16/25 (64.0) | 20/25 (80.0) | 46/89 (51.7) | 25/89 (28.1) |
| Local reaction | 3 (18.8) | 5 (31.3) | 10 (40.0) | 18 (72.0) | 25 (28.1) | 10 (11.2) |
| Swelling | 0 | 0 | 0 | 1 (4.0) | 0 | 1 (1.1) |
| Redness | 0 | 0 | 0 | 1 (4.0) | 0 | 1 (1.1) |
| Tenderness | 3 (100.0) | 5 (100.0) | 10 (100.0) | 18 (100.0) | 25 (100.0) | 10 (100.0) |
| Grade 1 | 3 (100.0) | 3 (60.0) | 8 (80.0) | 5 (27.8) | 16 (64.0) | 8 (80.0) |
| Grade 2 | 0 | 2 (40.0) | 2 (20.0) | 11 (61.1) | 9 (36.0) | 1 (10.0) |
| Grade 3 | 0 | 0 | 0 | 2 (11.1) | 0 | 1 (10.0) |
| Fever | 3 (12.0) | 6 (24.0) | 11 (12.4) | 1 (1.1) | 0 | 1 (6.3) |
| Headache | 4 (16.0) | 5 (20.0) | 9 (10.1) | 7 (7.9) | 1 (6.3) | 0 |
| Fatigue | 5 (20.0) | 6 (24.0) | 12 (13.5) | 3 (3.4) | 0 | 0 |
| Rash | 1 (6.3) | 1 (6.3) | 0 | 1 (4.0) | 0 | 0 |
| Abdominal pain | 0 | 0 | 0 | 1 (4.0) | 1 (1.1) | 0 |
| Others | 1 (6.3) | 1 (6.3) | 5 (20.0) | 3 (12.0) | 14 (15.7) | 9 (10.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narongkiatikhun, P.; Noppakun, K.; Chaiwarith, R.; Winichakoon, P.; Vongsanim, S.; Suteeka, Y.; Pongsuwan, K.; Kusirisin, P.; Wongsarikan, N.; Fanhchaksai, K.; et al. Immunogenicity and Safety of Homologous and Heterologous Prime-Boost of CoronaVac® and ChAdOx1 nCoV-19 among Hemodialysis Patients: An Observational Prospective Cohort Study. Vaccines 2023, 11, 715. https://doi.org/10.3390/vaccines11040715
Narongkiatikhun P, Noppakun K, Chaiwarith R, Winichakoon P, Vongsanim S, Suteeka Y, Pongsuwan K, Kusirisin P, Wongsarikan N, Fanhchaksai K, et al. Immunogenicity and Safety of Homologous and Heterologous Prime-Boost of CoronaVac® and ChAdOx1 nCoV-19 among Hemodialysis Patients: An Observational Prospective Cohort Study. Vaccines. 2023; 11(4):715. https://doi.org/10.3390/vaccines11040715
Chicago/Turabian StyleNarongkiatikhun, Phoom, Kajohnsak Noppakun, Romanee Chaiwarith, Poramed Winichakoon, Surachet Vongsanim, Yuttitham Suteeka, Karn Pongsuwan, Prit Kusirisin, Nuttanun Wongsarikan, Kanda Fanhchaksai, and et al. 2023. "Immunogenicity and Safety of Homologous and Heterologous Prime-Boost of CoronaVac® and ChAdOx1 nCoV-19 among Hemodialysis Patients: An Observational Prospective Cohort Study" Vaccines 11, no. 4: 715. https://doi.org/10.3390/vaccines11040715
APA StyleNarongkiatikhun, P., Noppakun, K., Chaiwarith, R., Winichakoon, P., Vongsanim, S., Suteeka, Y., Pongsuwan, K., Kusirisin, P., Wongsarikan, N., Fanhchaksai, K., Khamwan, C., Dankai, D., & Ophascharoensuk, V. (2023). Immunogenicity and Safety of Homologous and Heterologous Prime-Boost of CoronaVac® and ChAdOx1 nCoV-19 among Hemodialysis Patients: An Observational Prospective Cohort Study. Vaccines, 11(4), 715. https://doi.org/10.3390/vaccines11040715







