COVID-19 Vaccination during Pregnancy and Lactation: Attitudes and Uptakes before and after Official Recommendations in Germany
Abstract
1. Introduction
2. Methods
2.1. Materials and Procedure
2.2. Measures
2.3. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Vaccination Recommendation Awareness
3.3. Pregnant Women’s Attitudes towards COVID-19 Vaccination
3.3.1. Results from the Second Survey after the National Recommendation
3.3.2. Comparison before and after the Official Recommendation
3.4. Breastfeeding Women’s Attitudes towards COVID-19 Vaccination
3.4.1. Results from the Second Survey after the National Recommendation
3.4.2. Comparison before and after Official Recommendation
3.5. Identification of General COVID-19 Vaccination Opponents
4. Discussion
4.1. Vaccination Prevalence
4.2. Reasons for and against Vaccination
4.3. Knowledge Transfer and Dissemination of the Official Recommendation
4.4. The Role of Health Care Professionals
4.5. Vaccination Opponents
4.6. Clinical Implications
4.7. Strengths and Limitations
4.8. Research Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barchielli, B.; Cricenti, C.; Galle, F.; Sabella, E.A.; Liguori, F.; Da Molin, G.; Liguori, G.; Orsi, G.B.; Giannini, A.M.; Ferracuti, S.; et al. Climate Changes, Natural Resources Depletion, COVID-19 Pandemic, and Russian-Ukrainian War: What Is the Impact on Habits Change and Mental Health? Int. J. Environ. Res. Public Health 2022, 19, 11929. [Google Scholar] [CrossRef] [PubMed]
- Dashraath, P.; Nielsen-Saines, K.; Madhi, S.A.; Baud, D. COVID-19 vaccines and neglected pregnancy. Lancet 2020, 396, e22. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Perinatale Medizin DGPM); Deutsche Gesellschaft für Gynäkologie und Geburtshilfe (DGGG); Deutsche Gesellschaft für Pränatal- und Geburtsmedizin (DGPGM); Deutsche Gesellschaft für Reproduktionsmedizin (DGRM); Deutsche Gesellschaft für Gynäkologische Endokrinologie und Fortpflanzungsmedizin (DGGEF); AG Geburtshilfe und Pränatalmedizin in der DGGG (AGG); Arbeitsgemeinschaft für Infektionen und Infektionsimmunologie in der Gynäkologie und Geburtshilfe (AGII); AG Universitäre Reproduktionsmedizinische Zentren der DGGG (URZ); Dachverband Reproduktionsbiologie und -Medizin (DVR); Bundesarbeitsgemeinschaft Leitender Ärztinnen und Ärzte in der Frauenheilkunde und Geburtshilfe (BLFG); et al. Empfehlung der COVID-19-Impfung für Schwangere und Stillende Frauen. Available online: https://www.dggg.de/fileadmin/data/Stellungnahmen/DGGG/2021/COVID-19_Impfung_bei_schwangeren_und_stillenden_Frauen.pdf (accessed on 20 November 2022).
- Robert Koch Institut. Empfehlung der STIKO zur Impfung Gegen COVID-19 von Schwangeren und Stillenden und Die Dazugehörige Wissenschaftliche Begründung. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2021/Ausgaben/38_21.pdf?__blob=publicationFile (accessed on 2 January 2023).
- Robert Koch Institut. Coronavirus Disease 2019 (COVID-19) Daily Situation Report of the Robert Koch Institute, Germany. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Nov_2021/2021-11-26-en.pdf?__blob=publicationFile (accessed on 2 January 2023).
- Robert Koch Institut. Coronavirus Disease 2019 (COVID-19) Weekly Situation Report of the Robert Koch Institute, Germany. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Apr_2021/2021-04-01-en.pdf?__blob=publicationFile (accessed on 2 January 2023).
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef] [PubMed]
- Leiner, D.J. SoSci Survey (Version 2.1.06). 2021. Available online: https://www.sosci.survey.de (accessed on 2 April 2021).
- Schaal, N.K.; Zollkau, J.; Hepp, P.; Fehm, T.; Hagenbeck, C. Pregnant and breastfeeding women’s attitudes and fears regarding the COVID-19 vaccination. Arch. Gynecol. Obstet. 2022, 306, 365–372. [Google Scholar] [CrossRef] [PubMed]
- OurWorldInData.org. Share of People Who Received at Least One Dose of COVID-19 Vaccine. Available online: https://ourworldindata.org/grapher/share-people-vaccinated-covid?country=USA~OWID_WRL~DEU~ISR~FRA~GBR~CHE (accessed on 20 November 2022).
- MacDonald, N.E.; Eskola, J.; Liang, X.; Chaudhuri, M.; Dube, E.; Gellin, B.; Goldstein, S.; Larson, H.; Manzo, M.L.; Reingold, A.; et al. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef]
- Majid, U.; Ahmad, M.; Zain, S.; Akande, A.; Ikhlaq, F. COVID-19 vaccine hesitancy and acceptance: A comprehensive scoping review of global literature. Health Promot. Int. 2022, 37, daac078. [Google Scholar] [CrossRef]
- Skjefte, M.; Ngirbabul, M.; Akeju, O.; Escudero, D.; Hernandez-Diaz, S.; Wyszynski, D.F.; Wu, J.W. COVID-19 vaccine acceptance among pregnant women and mothers of young children: Results of a survey in 16 countries. Eur. J. Epidemiol. 2021, 36, 197–211. [Google Scholar] [CrossRef]
- Janik, K.; Nietupska, K.; Iwanowicz-Palus, G.; Cybulski, M. Fear of COVID-19 and Vaccine Hesitancy among Pregnant Women in Poland: A Cross-Sectional Study. Vaccines 2022, 10, 1700. [Google Scholar] [CrossRef]
- Riad, A.; Jouzova, A.; Ustun, B.; Lagova, E.; Hruban, L.; Janku, P.; Pokorna, A.; Klugarova, J.; Koscik, M.; Klugar, M. COVID-19 Vaccine Acceptance of Pregnant and Lactating Women (PLW) in Czechia: An Analytical Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 3373. [Google Scholar] [CrossRef] [PubMed]
- Januszek, S.M.; Faryniak-Zuzak, A.; Barnas, E.; Lozinski, T.; Gora, T.; Siwiec, N.; Szczerba, P.; Januszek, R.; Kluz, T. The Approach of Pregnant Women to Vaccination Based on a COVID-19 Systematic Review. Medicina 2021, 57, 977. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, M.K.; Mehl, R.; Costantine, M.M.; Johnson, A.; Cohen, J.; Summerfield, T.L.; Landon, M.B.; Rood, K.M.; Venkatesh, K.K. Characteristics and perceptions associated with COVID-19 vaccination hesitancy among pregnant and postpartum individuals: A cross-sectional study. Br. J. Obstet. Gynaecol. 2022, 129, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Eitze, S.; Felgendreff, L.; Korn, L.; Sprengholz, P.; Allen, J.; Jenny, M.A.; Wieler, L.H.; Thaiss, H.; De Bock, F.; Betsch, C. Public trust in institutions in the first half of the Corona pandemic: Findings from the COVID-19 Snapshot Monitoring (COSMO) project. Bundesgesundheitsblatt Gesundh. Gesundh. 2021, 64, 268–276. [Google Scholar] [CrossRef]
- Collier, A.Y.; McMahan, K.; Yu, J.; Tostanoski, L.H.; Aguayo, R.; Ansel, J.; Chandrashekar, A.; Patel, S.; Apraku Bondzie, E.; Sellers, D.; et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. J. Am. Med. Assoc. 2021, 325, 2370–2380. [Google Scholar] [CrossRef]
- Theiler, R.N.; Wick, M.; Mehta, R.; Weaver, A.L.; Virk, A.; Swift, M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am. J. Obstet. Gynecol. MFM 2021, 3, 100467. [Google Scholar] [CrossRef] [PubMed]
- Wainstock, T.; Yoles, I.; Sergienko, R.; Sheiner, E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine 2021, 39, 6037–6040. [Google Scholar] [CrossRef]
- O’Leary, S.T.; Narwaney, K.J.; Wagner, N.M.; Kraus, C.R.; Omer, S.B.; Glanz, J.M. Efficacy of a Web-Based Intervention to Increase Uptake of Maternal Vaccines: An RCT. Am. J. Prev. Med. 2019, 57, e125–e133. [Google Scholar] [CrossRef]
- Nyhan, B.; Reifler, J. When corrections fail: The persistence of political misperceptions. Political Behav. 2010, 32, 303–330. [Google Scholar]
- Wilson, R.J.; Paterson, P.; Jarrett, C.; Larson, H.J. Understanding factors influencing vaccination acceptance during pregnancy globally: A literature review. Vaccine 2015, 33, 6420–6429. [Google Scholar] [CrossRef]
- Myers, K.L. Predictors of maternal vaccination in the United States: An integrative review of the literature. Vaccine 2016, 34, 3942–3949. [Google Scholar] [CrossRef]
- Chervenak, F.A.; McCullough, L.B.; Bornstein, E.; Johnson, L.; Katz, A.; McLeod-Sordjan, R.; Nimaroff, M.; Rochelson, B.L.; Tekbali, A.; Warman, A.; et al. Professionally responsible coronavirus disease 2019 vaccination counseling of obstetrical and gynecologic patients. Am. J. Obstet. Gynecol. 2021, 224, 470–478. [Google Scholar] [CrossRef]
- Motta, M.; Callaghan, T.; Sylvester, S.; Lunz-Trujillo, K. Identifying the prevalence, correlates, and policy consequences of anti-vaccine social identity. Politics Groups Identities 2021, 9, 1–15. [Google Scholar] [CrossRef]
- Statistisches Bundesamt (Federal Statistical Office of Germany). Daten zum Durchschnittlichen Alter der Eltern bei Geburt nach der Geburtenfolge für 1. Kind, 2. Kind, 3. Kind der Mutter und Insgesamt. 2021. Available online: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/Geburten/Tabellen/geburten-eltern-biologischesalter.html (accessed on 27 January 2023).
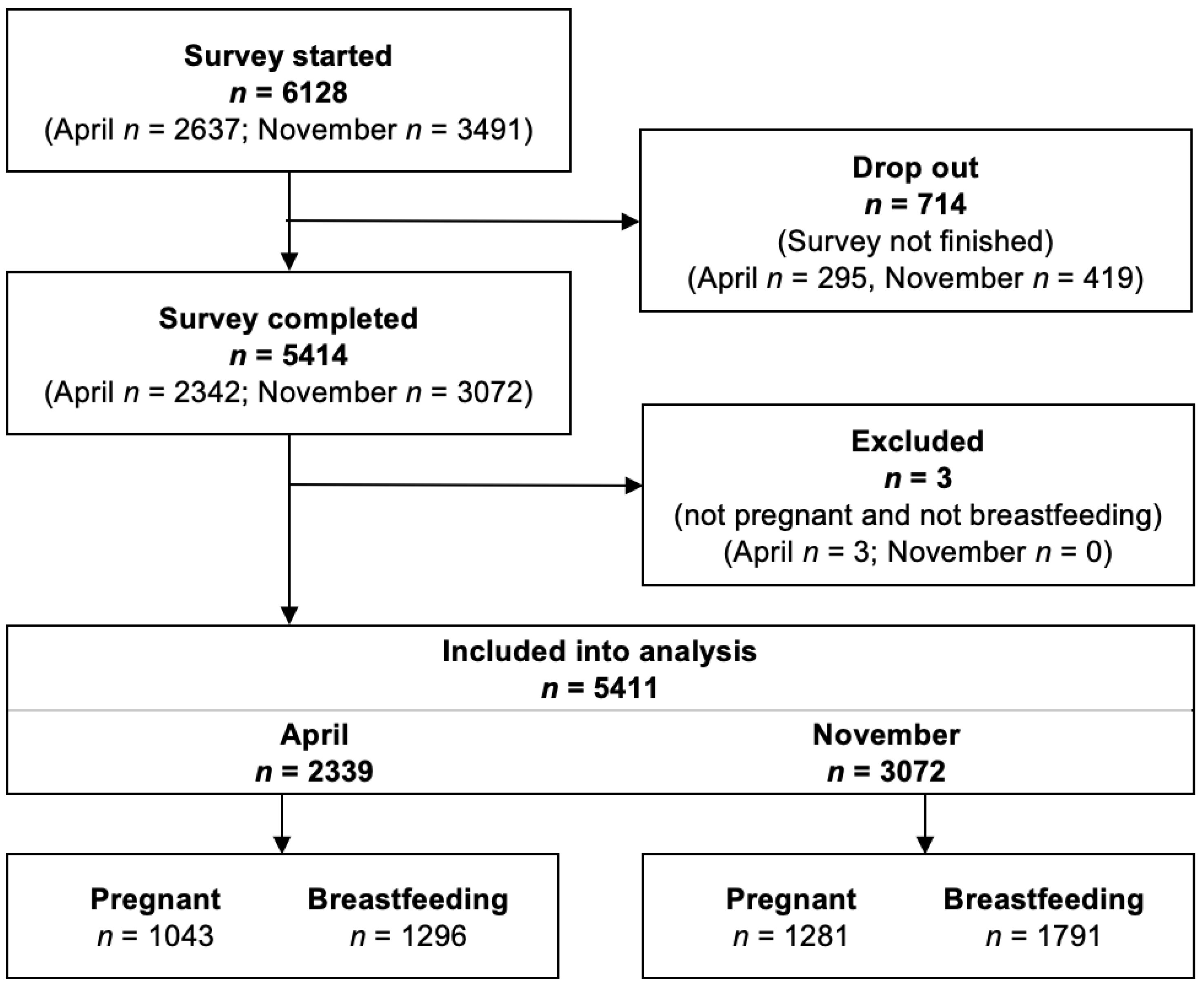




| Before Official Recommendation (n = 2339) | After Official Recommendation (n = 3072) | |||
|---|---|---|---|---|
| Pregnant Women | Breastfeeding Women | Pregnant Women | Breastfeeding Women | |
| n | 1043 | 1296 | 1281 | 1791 |
| Age (years) | 31.8 ± 4.3 | 32.4 ± 4.4 | 30.8 ± 4.0 (20–46) | 31.7 ± 4.3 |
| Age distribution (n) | ||||
| ≤24 y | 48 | 47 | 58 | 65 |
| 25–39 y | 959 | 1172 | 1189 | 1652 |
| ≥40 y | 36 | 77 | 34 | 74 |
| Medical condition with higher risk for COVID-19 | 210 (20.1%) | 262 (20.2%) | 198 (15.5%) | 207 (11.5%) |
| SARS-CoV-2 positive previously | 44 (4.2%) | 45 (3.7%) | 96 (7.5%) | 106 (5.9%) |
COVID-19 symptoms
| 6 (13.6%) 30 (68.2%) 8 (18.2%) - | 5 (11.1%) 35 (77.8%) 5 (11.1%) - | 8 (8.3%) 61 (63.5%) 25 (26%) 2 (2.1%) | 4 (3.8%) 80 (75.5%) 20 (18.9%) 2 (1.9%) |
| Fear of COVID-19 symptoms (VAS *; mm) | 57.12 ± 27.09 | 55.14 ± 26.60 | 54.1 ± 27.0 | 54.2 ± 26.1 |
| Fear of SARS-CoV-2 infection (VAS *; mm) | 51.18 ± 25.55 | 48.06 ± 24.53 | 52.1 ± 25.0 | 50.6 ± 24.5 |
| Gestational age (weeks) | 24.7 ± 9.1 | - | 26.2 ± 9.5 (4–42) | - |
| 1. Trimester | 12% (116/966) | 8.4% (108/1279) | ||
| 2. Trimester | 34% (330/966) | 34.5% (442/1279) | ||
| 3. Trimester | 53.8% (520/966) | 57.0% (729/1279) | ||
| First pregnancy | 494 (47.4%) | - | 804 (63%) | - |
| High-risk pregnancy | 418 (40.1%) | - | 401 (31.1%) | - |
| COVID-19 vaccination | ||||
| 1018 (97.6%) 25 (2.4%) 1 dose 13 (52%) 2 doses 12 (48%) | 1108 (85.5%) 188 (14.5%) During pregnancy 2 (1.1%) Post-partum 186 (98.9%) | 529 (41.3%) 752 (58.7%) 1 dose 86 (11.4%) 2 doses 660 (87.8%) 3 doses 6 (0.8%) | 452 (25.3%) 1339 (74.8%) During pregnancy 453 (33.8%) Post-partum 1116 (83.3%) |
| Before Official Recommendation n = 2339 | After Official Recommendation n = 3072 | p-Values | Effect Size ϕ | |
|---|---|---|---|---|
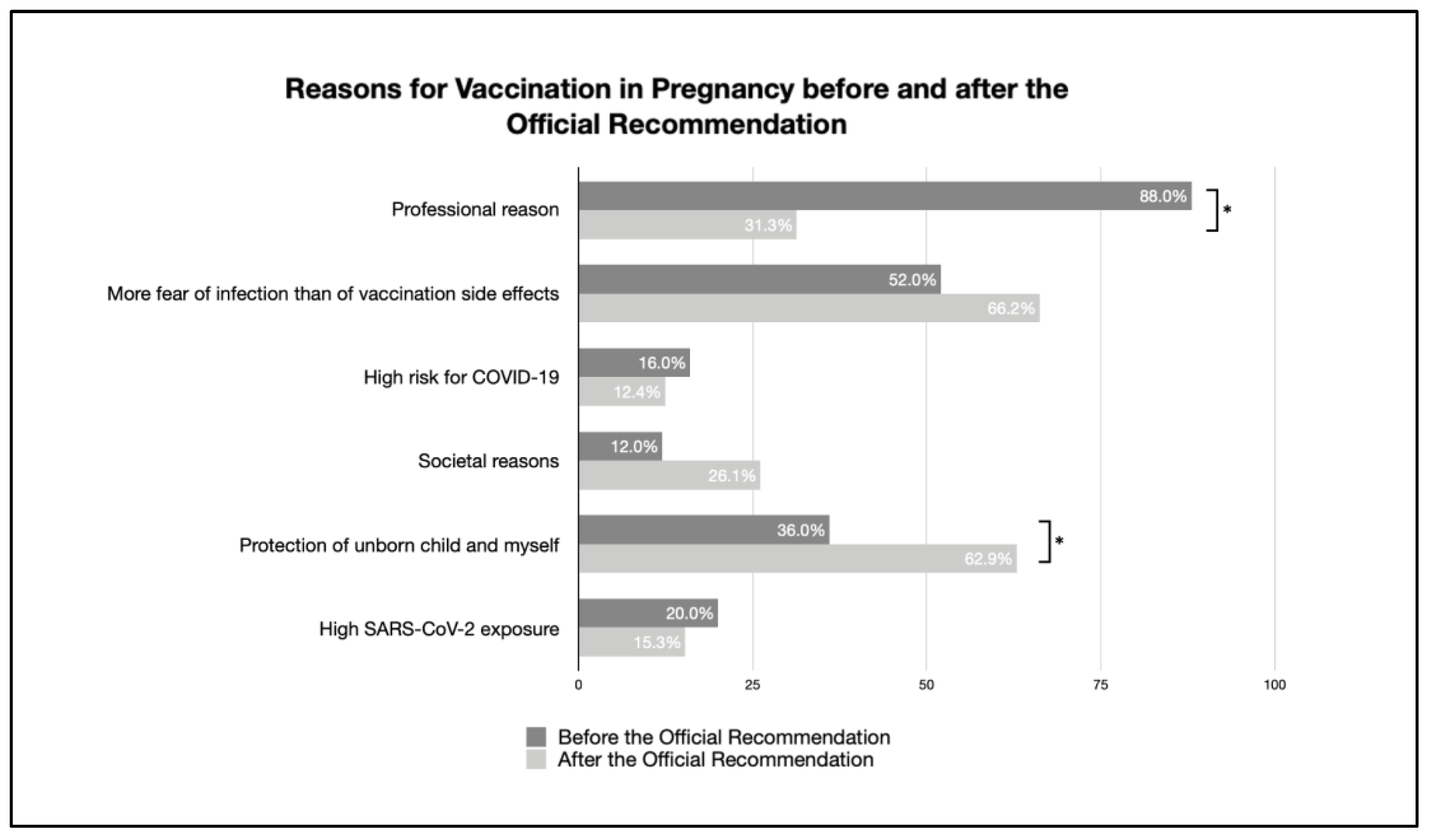
| Pregnant women’s reason for vaccination | ||||
| Professional reason | 88.0% (22/25) | 31.3% (235/752) | <0.001 | 0.213 |
| More fear of infection than of vaccination side effects | 52.0% (13/25) | 66.2% (498/752) | 0.052 | . |
| High risk for COVID-19 | 16.0% (4/25) | 12.4% (93/752) | 0.589 | |
| Societal reasons | 12.0% (3/25) | 26.1% (196/752) | 0.113 | |
| Protection of the unborn child and myself | 36.0% (9/25) | 62.9% (473/752) | 0.003 | 0.098 |
| High SARS-CoV-2 exposure | 20.0% (5/25) | 15.3% (115/752) | 0.522 | |
| Official STIKO recommendation | n/a | 15.7% (118/752) | - | |
| Pregnant women’s reason against vaccination | ||||
| Limited information about vaccination during pregnancy | 53.5% (229/430) | 24.4% (54/221) | 0.001 | 0.275 |
| Not enough scientific data for vaccination during pregnancy | 88.1% (379/430) | 82.8% (183/221) | 0.062 | |
| No contact person to ask about the vaccination | 8.8% (38/430) | 1.8% (4/221) | 0.006 | 0.135 |
| Fear of harm to the unborn child | 87.9% (378/430) | 88.7% (196/221) | 0.770 | |
| Fear of pregnancy complications | 80.2% (345/430) | 76.5% (169/221) | 0.265 | |
| Fear of side effects | 31.2% (134/430) | 41.6% (92/221) | 0.032 | 0.104 |
| Before Official Recommendation | After Official Recommendation | p-Values | Effect Size ϕ | |
|---|---|---|---|---|
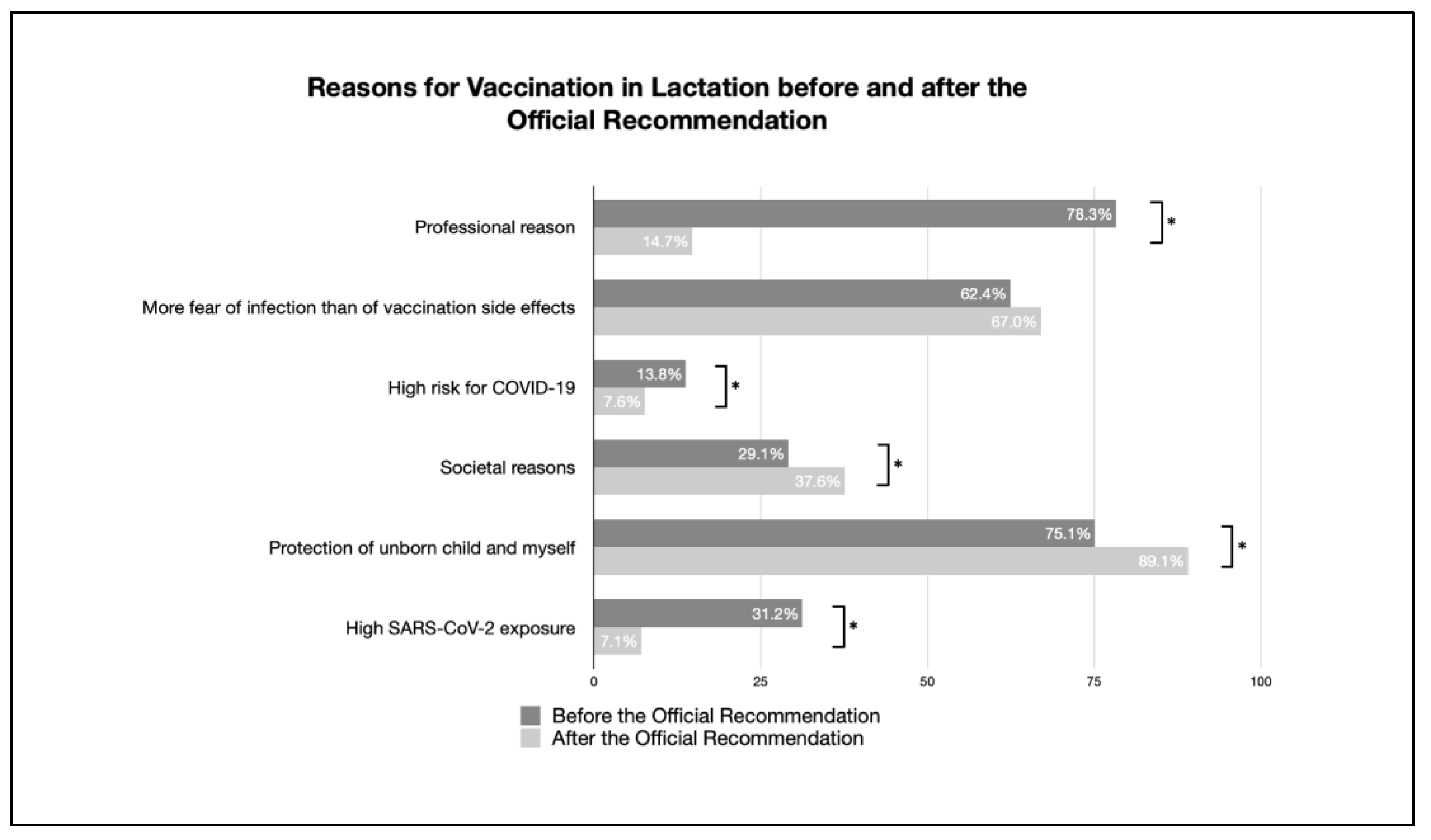
| Breastfeeding women’s reason for vaccination | ||||
| Professional reason | 78.3% (148/189) | 14.7% (197/1339) | <0.001 | 0.501 |
| More fear of infection than of vaccination side effects | 62.4% (118/189) | 67.0% (897/1339) | 0.241 | |
| High risk for COVID-19 | 13.8% (26/189) | 7.6% (102/1339) | 0.012 | 0.073 |
| Societal reasons | 29.1% (55/189) | 37.6% (502/1339) | 0.048 | 0.058 |
| Protection of my child and myself | 75.1% (142/189) | 89.1% (1193/1339) | <0.001 | 0.138 |
| High SARS-CoV-2 exposure | 31.2% (59/189) | 7.1% (95/1339) | <0.001 | 0.264 |
| Official STIKO recommendation | n/a | 46.3% (619/1338) | - | |
| Breastfeeding women’s reason against vaccination | ||||
| Limited information about vaccination during lactation | 53.4% (141/264) | 17.8% (26/146) | <0.001 | 0.347 |
| Not enough scientific data for vaccination during lactation | 83.7% (221/264) | 52.1% (76/146) | <0.001 | 0.339 |
| No contact person to ask about the vaccination | 11.7% (31/264) | 4.1% (6/146) | 0.03 | 0.128 |
| Fear of harm to the child | 67.4% (178/264) | 53.4% (78/146) | 0.02 | 0.138 |
| Fear of breastfeeding complications | 24.2% (64/264) | 28.8% (42/146) | 0.316 | |
| Fear of side effects | 37.5% (99/264) | 37.7% (55/146) | 0.973 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagenbeck, C.; Zöllkau, J.; Helbig, M.; Fehm, T.; Schaal, N.K. COVID-19 Vaccination during Pregnancy and Lactation: Attitudes and Uptakes before and after Official Recommendations in Germany. Vaccines 2023, 11, 627. https://doi.org/10.3390/vaccines11030627
Hagenbeck C, Zöllkau J, Helbig M, Fehm T, Schaal NK. COVID-19 Vaccination during Pregnancy and Lactation: Attitudes and Uptakes before and after Official Recommendations in Germany. Vaccines. 2023; 11(3):627. https://doi.org/10.3390/vaccines11030627
Chicago/Turabian StyleHagenbeck, Carsten, Janine Zöllkau, Martina Helbig, Tanja Fehm, and Nora K. Schaal. 2023. "COVID-19 Vaccination during Pregnancy and Lactation: Attitudes and Uptakes before and after Official Recommendations in Germany" Vaccines 11, no. 3: 627. https://doi.org/10.3390/vaccines11030627
APA StyleHagenbeck, C., Zöllkau, J., Helbig, M., Fehm, T., & Schaal, N. K. (2023). COVID-19 Vaccination during Pregnancy and Lactation: Attitudes and Uptakes before and after Official Recommendations in Germany. Vaccines, 11(3), 627. https://doi.org/10.3390/vaccines11030627






