Clinical Outcomes of COVID-19 Infection in Pregnant and Nonpregnant Women: Results from The Philippine CORONA Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Setting
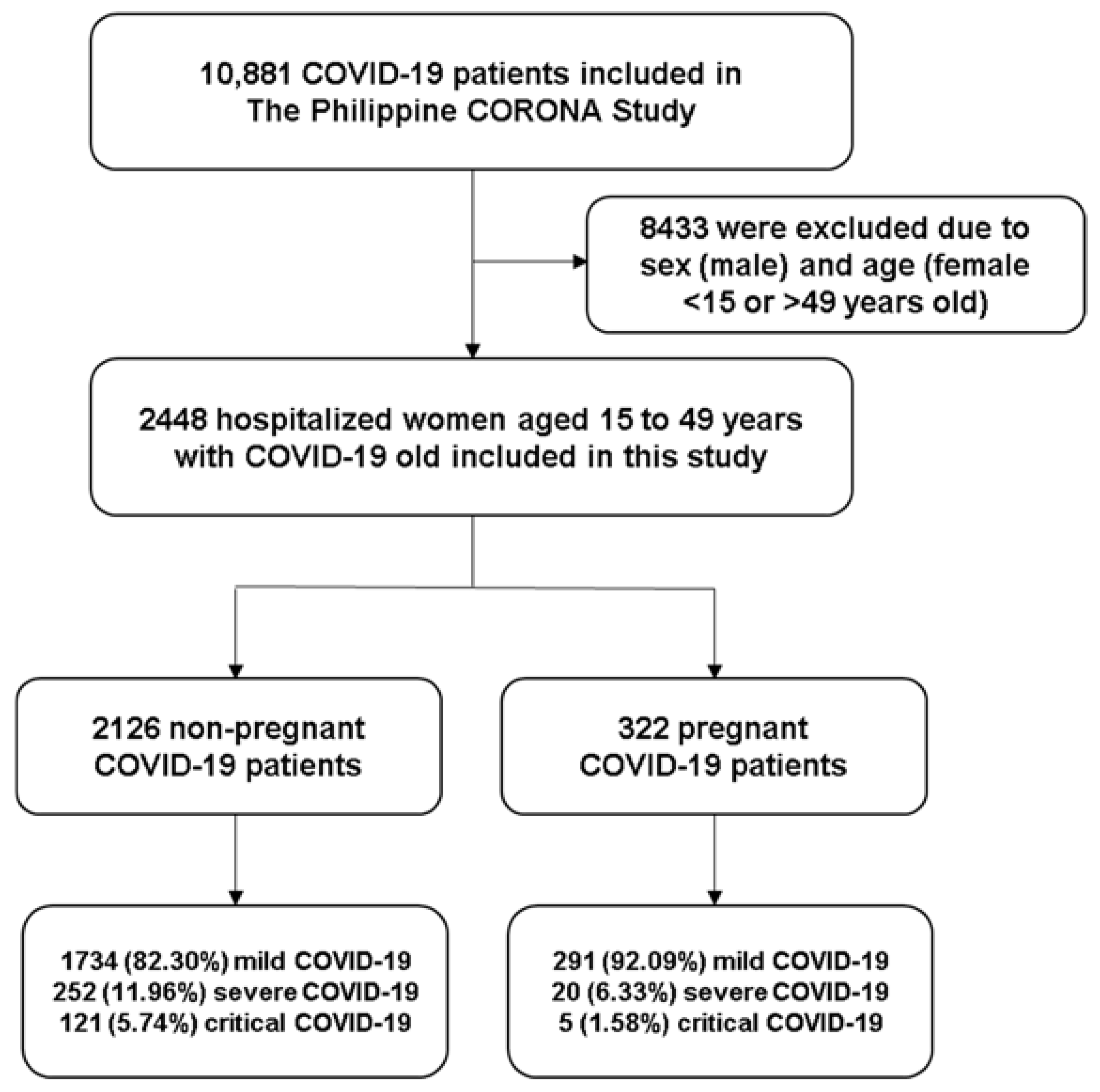
2.3. Patient Selection, Sampling, and Cohort Description
2.4. Information Sources, Data Collection, Patient Variables, and Bias
2.5. Outcome Variables
2.6. Statistical Analysis
3. Results
3.1. Demographic Data and Clinical/ Neurological Profile of Included Patients
3.2. Comparison of Clinical/ Neurological Outcomes of Included Pregnant and Nonpregnant Women
3.3. Estimated Odds Ratio of Outcomes Comparing Pregnant and Nonpregnant Women (Logistic Regression Models)
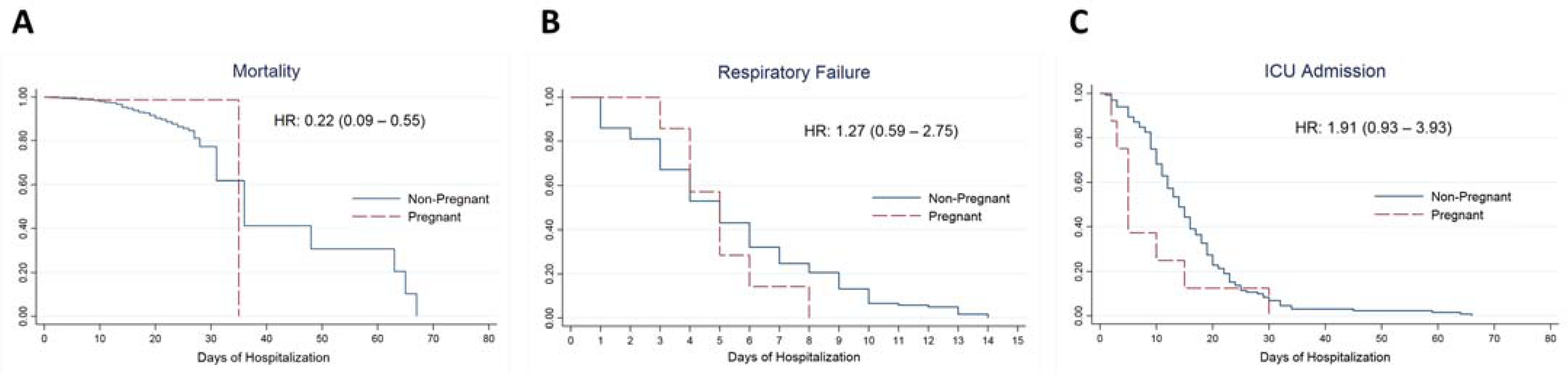
3.4. Kaplan–Meier Plots and Estimated Hazard Ratios of Outcomes Comparing Pregnant and Nonpregnant Women (Cox Regression Models)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leal, L.F.; Merckx, J.; Fell, D.B.; Kuchenbecker, R.; Miranda, A.E.; de Oliveira, W.K.; Platt, R.W.; Antunes, L.; Silveira, M.F.; Barbieri, N.B. Characteristics and Outcomes of Pregnant Women with SARS-CoV-2 Infection and Other Severe Acute Respiratory Infections (SARI) in Brazil from January to November 2020. Braz. J. Infect. Dis. 2021, 25, 101620. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.J.; Amosco, M.; Octavio, M.B.R.; Bravo, S.L.; Villanueva-Uy, E. Maternal and Neonatal Outcomes of Pregnant Women with Clinically Confirmed COVID-19 Admitted at the Philippine General Hospital. Acta Med. Philipp. 2021, 55, 183–190. [Google Scholar] [CrossRef]
- Lassi, Z.S.; Ana, A.; Das, J.K.; Salam, R.A.; Padhani, Z.A.; Irfan, O.; Bhutta, Z.A. A Systematic Review and Meta-Analysis of Data on Pregnant Women with Confirmed COVID-19: Clinical Presentation, and Pregnancy and Perinatal Outcomes Based on COVID-19 Severity. J. Glob. Health 2021, 11, 05018. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Gonzaga, Z.; Hernandez-Nazal, M. Characteristics of COVID-19 Positive Pregnant Patients Admitted in a Private Tertiary Hospital and Their Maternal and Neonatal Outcomes. Philipp. J. Obstet. Gynecol. 2022, 46, 69–79. [Google Scholar] [CrossRef]
- Reale, S.C.; Lumbreras-Marquez, M.I.; King, C.H.; Burns, S.L.; Fields, K.G.; Diouf, K.; Goldfarb, I.T.; Ciaranello, A.L.; Robinson, J.N.; Gregory, K.E.; et al. Patient Characteristics Associated with SARS-CoV-2 Infection in Parturients Admitted for Labour and Delivery in Massachusetts during the Spring 2020 Surge: A Prospective Cohort Study. Paediatr. Perinat. Epidemiol. 2021, 35, 24–33. [Google Scholar] [CrossRef]
- Jering, K.S.; Claggett, B.L.; Cunningham, J.W.; Rosenthal, N.; Vardeny, O.; Greene, M.F.; Solomon, S.D. Clinical Characteristics and Outcomes of Hospitalized Women Giving Birth With and Without COVID-19. JAMA Intern. Med. 2021, 181, 714. [Google Scholar] [CrossRef]
- Vizheh, M.; Muhidin, S.; Aghajani, F.; Maleki, Z.; Bagheri, F.; Hosamirudsari, H.; Aleyasin, A.; Tehranian, A. Characteristics and Outcomes of COVID-19 Pneumonia in Pregnancy Compared with Infected Nonpregnant Women. Int. J. Gynecol. Obstet. 2021, 153, 462–468. [Google Scholar] [CrossRef]
- Zha, Y.; Chen, G.; Gong, X.; Wu, Y.-Y.; Lin, X.-G.; Wu, J.-L.; Huang, Y.-F.; Li, Y.-Q.; Zhang, Y.; Deng, D.-R.; et al. Coronavirus Disease 2019 in Pregnant and Non-Pregnant Women: A Retrospective Study. Chin. Med. J. 2021, 134, 1218–1220. [Google Scholar] [CrossRef]
- Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID-19) Infection in Pregnancy. Available online: https://www.rcog.org.uk/guidance/coronavirus-covid-19-pregnancy-and-women-s-health/coronavirus-covid-19-infection-in-pregnancy/ (accessed on 1 August 2022).
- Knight, M.; Ramakrishnan, R.; Bunch, K.; Vousden, N.; Jennifer, J.; Dunn, S.; Norman, L.; Barry, A.; Harrison, E. UKOSS/ISARIC/CO-CIN: Females in Hospital with SARS-CoV-2 Infection, the Association with Pregnancy and Pregnancy Outcomes, 25 March 2021. Available online: https://www.gov.uk/government/publications/ukossisaricco-cin-females-in-hospital-with-sars-cov-2-infection-the-association-with-pregnancy-and-pregnancy-outcomes-25-march-2021 (accessed on 1 August 2022).
- Molteni, E.; Astley, C.M.; Ma, W.; Sudre, C.H.; Magee, L.A.; Murray, B.; Fall, T.; Gomez, M.F.; Tsereteli, N.; Franks, P.W.; et al. Symptoms and Syndromes Associated with SARS-CoV-2 Infection and Severity in Pregnant Women from Two Community Cohorts. Sci. Rep. 2021, 11, 6928. [Google Scholar] [CrossRef]
- Crossette-Thambiah, C.; Nicolson, P.; Rajakaruna, I.; Langridge, A.; Sayar, Z.; Perelta, M.R.; Essex, S.; Oakes, R.; Mounter, P.; Lewis, S.; et al. The Clinical Course of COVID-19 in Pregnant versus Non-pregnant Women Requiring Hospitalisation: Results from the Multicentre UK CA-COVID-19 Study. Br. J. Haematol. 2021, 195, 85–89. [Google Scholar] [CrossRef]
- Garcia, J.J.; Turalde, C.W.; Bagnas, M.A.; Anlacan, V.M. Intravenous Immunoglobulin in COVID-19 Associated Guillain–Barré Syndrome in Pregnancy. BMJ Case Rep. 2021, 14, e242365. [Google Scholar] [CrossRef] [PubMed]
- Gama, M.D.P.; Angelo Júnior, J.R.L.; Cunha-Correia, C.D. Stroke in COVID-19 and Pregnancy: A Case Report. Rev. Soc. Bras. Med. Trop. 2021, 54. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent Neurologic Symptoms and Cognitive Dysfunction in Non-hospitalized COVID-19 “Long Haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef]
- Pajo, A.T.; Espiritu, A.I.; Apor, A.D.A.O.; Jamora, R.D.G. Neuropathologic Findings of Patients with COVID-19: A Systematic Review. Neurol. Sci. 2021, 42, 1255–1266. [Google Scholar] [CrossRef]
- Espiritu, A.I.; Sy, M.C.C.; Anlacan, V.M.M.; Jamora, R.D.G.; Macalintal, C.M.S.A.; Robles, J.B.; Cataniag, P.L.; Flores, M.K.C.; Tangcuangco-Trinidad, N.J.C.; Juangco, D.N.A.; et al. COVID-19 Outcomes of 10,881 Patients: Retrospective Study of Neurological Symptoms and Associated Manifestations (Philippine CORONA Study). J. Neural Transm. 2021, 128, 1687–1703. [Google Scholar] [CrossRef]
- Espiritu, A.I.; Sy, M.C.C.; Anlacan, V.M.M.; Jamora, R.D.G. The Philippine COVID-19 Outcomes: A Retrospective Study of Neurological Manifestations and Associated Symptoms (The Philippine CORONA Study): A Protocol Study. BMJ Open 2020, 10, e040944. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical Manifestations, Risk Factors, and Maternal and Perinatal Outcomes of Coronavirus Disease 2019 in Pregnancy: Living Systematic Review and Meta-Analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Intensive Care National Audit Research Centre (ICNARC). ICNARC Report on COVID-19 in Critical Care: England, Wales and Northern Ireland; Intensive Care National Audit Research Centre (ICNARC): London, UK, 2021; Available online: https://www.icnarc.org/DataServices/Attachments/Download/fa086899-6dde-eb11-9132-00505601089b (accessed on 1 August 2022).
- Qeadan, F.; Mensah, N.A.; Tingey, B.; Stanford, J.B. The Risk of Clinical Complications and Death among Pregnant Women with COVID-19 in the Cerner COVID-19 Cohort: A Retrospective Analysis. BMC Pregnancy Childbirth 2021, 21, 305. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Smulian, J.C.; Lednicky, J.A.; Wen, T.S.; Jamieson, D.J. Coronavirus Disease 2019 (COVID-19) and Pregnancy: What Obstetricians Need to Know. Am. J. Obstet. Gynecol. 2020, 222, 415–426. [Google Scholar] [CrossRef]
- Blitz, M.J.; Grünebaum, A.; Tekbali, A.; Bornstein, E.; Rochelson, B.; Nimaroff, M.; Chervenak, F.A. Intensive Care Unit Admissions for Pregnant and Nonpregnant Women with Coronavirus Disease 2019. Am. J. Obstet. Gynecol. 2020, 223, 290–291. [Google Scholar] [CrossRef]
- Asghar, M.S.; Siddiqui, M.A.; Iqbal, S.; Avinash; Tahir, M.J.; Yasmin, F.; Chughtai, N.; Khan, F.; Kirmani, T.A.; Lareeb, I. COVID-19 Infection among Pregnant and Non-Pregnant Women: Comparison of Biochemical Markers and Outcomes during COVID-19 Pandemic, A Retrospective Cohort Study. Ann. Med. Surg. 2022, 76, 103527. [Google Scholar] [CrossRef] [PubMed]
- Pineles, B.L.; Goodman, K.E.; Pineles, L.; O’Hara, L.M.; Nadimpalli, G.; Magder, L.S.; Baghdadi, J.D.; Parchem, J.G.; Harris, A.D. In-Hospital Mortality in a Cohort of Hospitalized Pregnant and Nonpregnant Patients With COVID-19. Ann. Intern. Med. 2021, 174, 1186–1188. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.S.A.; Pirzada, A.N.; Ali, A.; Salam, R.A.; Das, J.K.; Lassi, Z.S. The Differences in Clinical Presentation, Management, and Prognosis of Laboratory-Confirmed Covid-19 between Pregnant and Non-Pregnant Women: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 5613. [Google Scholar] [CrossRef] [PubMed]
- Philippine Infectious Diseases Society for Obstetrics and Gynecology. The PIDSOG Handbook: A Guidance for Clinicians on the Obstetric Management of Patients with Coronavirus Disease 2019, 2nd ed.; Angelo-Dela Cruz, K.A., Gementiza, J.R., Taladtad, F.F., Tiuseco, C.T., Eds.; Philippine Infectious Diseases Society for Obstetrics and Gynecology: Quezon City, Philippine, 2020. [Google Scholar]
| Characteristics | Overall | Nonpregnant | Pregnant | p Value |
|---|---|---|---|---|
| Frequency | 2448 | 2126 (86.85%) | 322 (13.15%) | - |
| Age in years | 33 (13) | 33 (14) | 30 (9) | |
| 15–20 years | 64 (2.61%) | 40 (1.88%) | 24 (7.45%) | <0.01 * |
| 21–30 years | 957 (39.09%) | 803 (37.77%) | 154 (47.83%) | |
| 31–40 years | 827 (33.78%) | 697 (32.78%) | 130 (40.37%) | |
| 41–49 years | 600 (24.51%) | 586 (27.56%) | 14 (4.35%) | |
| Presence of comorbid conditions | ||||
| Hypertension | 267 (10.91%) | 249 (11.71%) | 18 (5.59%) | <0.01 * |
| Diabetes mellitus | 167 (6.82%) | 161 (7.57%) | 6 (1.86%) | <0.01 * |
| Kidney disease | 79 (3.23%) | 78 (3.67%) | 1 (0.31%) | <0.01 * |
| Bronchial asthma | 132 (5.39%) | 126 (5.93%) | 6 (1.86%) | <0.01 * |
| Coronary artery disease | 19 (0.78%) | 19 (0.89%) | - | 0.16 |
| Malignancy | 41 (1.67%) | 41 (1.93%) | - | 0.01 * |
| COPD | 1 (0.04%) | - | 1 (0.31%) | 0.13 |
| Heart failure | 6 (0.25%) | 5 (0.24%) | 1 (0.31%) | 0.80 |
| Liver disease | 6 (0.25%) | 5 (0.24%) | 1 (0.31%) | 0.80 |
| Immunocompromised conditions | 3 (0.12%) | 3 (0.14%) | - | 0.50 |
| Obesity | 22 (0.90%) | 21 (0.99%) | 1 (0.31%) | 0.23 |
| Other conditions | 309 (12.62%) | 289 (13.59%) | 20 (6.21%) | <0.01 * |
| Relevant history | ||||
| Smoker | 55 (2.25%) | 55 (2.59%) | - | <0.01 * |
| Healthcare worker | 393 (16.05%) | 387 (18.20%) | 6 (1.86%) | <0.01 * |
| COVID-19 disease severity | ||||
| Mild | 2025 (83.57%) | 1734 (82.30%) | 291 (92.09%) | <0.01 * |
| Severe | 272 (11.23%) | 252 (11.96%) | 20 (6.33%) | |
| Critical | 126 (5.20%) | 121 (5.74%) | 5 (1.58%) | |
| Respiratory and constitutional symptoms | ||||
| Cough | 712 (29.08%) | 669 (31.47%) | 43 (13.35%) | <0.01 * |
| Fever | 619 (25.29%) | 598 (28.13%) | 21 (6.52%) | <0.01 * |
| Dyspnea | 352 (14.38%) | 337 (15.85%) | 15 (4.66%) | <0.01 * |
| Sore throat | 198 (8.09%) | 191 (8.98%) | 7 (2.17%) | <0.01 * |
| Fatigue | 112 (4.58%) | 108 (5.08%) | 4 (1.24%) | <0.01 * |
| Sputum production | 94 (3.84%) | 88 (4.14%) | 6 (1.86%) | 0.05 * |
| Rhinorrhea | 182 (7.43%) | 169 (7.95%) | 13 (4.04%) | 0.01 * |
| Diarrhea | 136 (5.56%) | 132 (6.21%) | 4 (1.24%) | <0.01 * |
| Arthralgia | 34 (1.39%) | 32 (1.51%) | 2 (0.62%) | 0.21 |
| Hemoptysis | 1 (0.04%) | 1 (0.05%) | - | 0.70 |
| Treatment Received | ||||
| Systematic glucocorticoids | 314 (12.83%) | 300 (14.11%) | 14 (4.35%) | <0.01 * |
| Remdesivir | 132 (5.39%) | 129 (6.07%) | 3 (0.93%) | <0.01 * |
| Tocilizumab | 65 (2.66%) | 63 (2.96%) | 2 (0.62%) | <0.01 * |
| Lopinavir-Ritonavir | 64 (2.61%) | 64 (3.01%) | - | <0.01 * |
| Hydroxychloroquine | 112 (4.58%) | 110 (5.17%) | 2 (0.62%) | <0.01 * |
| Chloroquine | 38 (1.55%) | 37 (1.74%) | 1 (0.31%) | 0.06 |
| Convalescent plasma | 21 (0.86%) | 21 (0.99%) | - | 0.10 |
| Antibiotic therapy | 831 (33.95%) | 641 (30.15%) | 190 (59.01%) | <0.01 * |
| Other regimens | 600 (24.51%) | 527 (24.79%) | 73 (22.67%) | 0.41 |
| Outcomes | Overall | Nonpregnant | Pregnant | p-Value |
|---|---|---|---|---|
| Frequency | 2448 | 2126 (86.85%) | 322 (13.15%) | - |
| Mortality and associated causes | ||||
| Mortality | 123 (5.02%) | 118 (5.55%) | 5 (1.55%) | <0.01 * |
| Acute respiratory distress syndrome | 55 (44.72%) | 52 (44.07%) | 3 (60%) | 0.48 |
| Septic shock | 35 (28.46%) | 32 (27.12%) | 3 (60%) | 0.11 |
| Multi-organ dysfunction syndrome | 14 (11.38%) | 14 (11.86%) | - | 0.41 |
| Acute coronary syndrome | 7 (5.69%) | 7 (5.93%) | - | 0.58 |
| Cardiac arrhythmia | 5 (4.07%) | 5 (4.24%) | - | 0.64 |
| Brain herniation | 7 (5.69%) | 7 (5.93%) | - | 0.58 |
| Decompensated heart failure | 1 (0.81%) | 1 (0.85%) | - | 0.84 |
| Other causes | 31 (35.20%) | 29 (24.58%) | 2 (40%) | 0.44 |
| Respiratory failure and causes | ||||
| Respiratory failure | 128 (5.23%) | 121 (5.69%) | 7 (2.17%) | 0.01 * |
| Pneumonia | 71 (55.47%) | 67 (55.37%) | 4 (57.14%) | 0.93 |
| Acute respiratory distress syndrome | 58 (45.31%) | 56 (46.28%) | 2 (28.57%) | 0.36 |
| Shock | 9 (7.03%) | 8 (6.61%) | 1 (14.29%) | 0.44 |
| Central neurological cause | 4 (3.13%) | 4 (3.31%) | - | 0.63 |
| Pulmonary edema | 2 (1.56%) | 2 (1.65%) | - | 0.73 |
| Pulmonary embolism | 5 (3.91%) | 5 (4.13%) | - | 0.58 |
| Duration of ventilator dependence | 11 (10) | 12 (9) | 5 (11) | 0.20 |
| 1–5 days | 75 (58.89%) | 70 (57.85%) | 5 (71.43%) | 0.48 |
| 6 or more days | 53 (41.41%) | 51 (42.15%) | 2 (28.57%) | |
| ICU Admission and reasons | ||||
| Needed intensive care | 139 (5.68%) | 131 (6.16%) | 8 (2.48%) | 0.01 * |
| Respiratory failure | 84 (60.43%) | 78 (59.54%) | 6 (75%) | 0.39 |
| Acute respiratory distress syndrome | 63 (45.32%) | 61 (46.56%) | 2 (25%) | 0.23 |
| Shock | 13 (9.35%) | 11 (8.40%) | 2 (25%) | 0.12 |
| Impaired level of consciousness | 12 (8.63%) | 10 (7.63%) | 2 (25%) | 0.09 |
| Acute myocardial infarction | 4 (2.88%) | 4 (3.05%) | - | 0.62 |
| Acute kidney injury | 10 (7.19%) | 9 (6.87%) | 1 (12.50%) | 0.55 |
| Treatment-related indication | 13 (9.35%) | 11 (8.40%) | 2 (25%) | 0.12 |
| Acute stroke | 5 (3.60%) | 4 (3.05%) | 1 (12.50%) | 0.16 |
| Cardiac arrhythmia | 2 (1.44%) | 2 (1.53%) | - | 0.73 |
| Post-cardiac arrest | 2 (1.44%) | 2 (1.53%) | - | 0.73 |
| Cerebral edema | 2 (1.44%) | 1 (0.76%) | 1 (12.50%) | 0.01 * |
| Venous thromboembolism | 1 (0.72%) | 1 (0.76%) | - | 0.80 |
| Time to ICU Admission | 5 (4) | 5 (4) | 5 (4) | 0.65 |
| Duration of ICU Stay | 14 (11) | 14 (11) | 5 (8) | 0.02 * |
| 1–7 days | 80 (57.55%) | 73 (55.73%) | 7 (87.50%) | 0.08 |
| 8 or more days | 59 (42.45%) | 58 (44.27%) | 1 (12.50%) | |
| Duration of Hospital Stay | 13 (8) | 13 (7) | 14 (10) | <0.01 * |
| 1–14 days | 1353 (55.27%) | 1213 (57.06%) | 140 (43.48%) | <0.01 * |
| 15 or more days | 1095 (44.73%) | 913 (42.94%) | 182 (56.52%) | |
| Neurologic Outcomes | ||||
| Full recovery | 1973 (80.60%) | 1762 (82.88%) | 211 (65.53%) | <0.01 * |
| Stable with improvement | 333 (13.60%) | 227 (10.68%) | 106 (32.92%) | |
| Stable without improvement | 40 (1.63%) | 40 (1.88%) | - | |
| Not applicable | 102 (4.17%) | 97 (4.56%) | 5 (1.55%) | |
| Final Disposition | ||||
| Discharged | 2308 (94.28%) | 1991 (93.65%) | 317 (98.45%) | <0.01 * |
| HAMA | 17 (0.69%) | 17 (0.80%) | - | |
| Died | 123 (5.02%) | 118 (5.55%) | 5 (1.55%) | |
| Outcomes | OR (95% CI) | p-Value |
|---|---|---|
| Mortality | ||
| Crude estimates for pregnancy | 0.26 (0.11–0.66) | <0.01 * |
| Adjusted for age, disease severity, and NNS | 0.51 (0.16–1.68) | 0.27 |
| Respiratory Failure | ||
| Crude estimates for pregnancy | 0.37 (0.17–0.80) | 0.01 * |
| Adjusted for age, disease severity, and NNS | 0.89 (0.32–2.49) | 0.82 |
| Ventilator dependence (>5 days) | ||
| Crude estimates for pregnancy | 0.55 (0.10–2.94) | 0.48 |
| Adjusted for age, disease severity, and NNS | 0.88 (0.14–5.56) | 0.90 |
| Need for Intensive Care | ||
| Crude estimates for pregnancy | 0.39 (0.19–0.80) | 0.01 * |
| Adjusted for age, disease severity, and NNS | 0.90 (0.39–2.11) | 0.81 |
| ICU Length of Stay (>7 days) | ||
| Crude estimates for pregnancy | 0.18 (0.02–1.50) | 0.11 |
| Adjusted for age, disease severity, and NNS | 0.15 (0.02–1.39) | 0.09 |
| Hospital Length of Stay (>14 days) | ||
| Crude estimates for pregnancy | 1.73 (1.36–2.19) | <0.01 * |
| Adjusted for age, disease severity, and NNS | 1.99 (1.56–2.54) | <0.01 * |
| Outcomes | HR (95% CI) | p-Value |
|---|---|---|
| Time to Mortality | ||
| Crude estimates for pregnancy | 0.22 (0.09–0.55) | <0.01 * |
| Adjusted for age, disease severity, NNS, hypertension, diabetes | 0.65 (0.26–1.64) | 0.35 |
| Time to Respiratory Failure | ||
| Crude estimates for pregnancy | 1.27 (0.59–2.75) | 0.54 |
| Adjusted for age, disease severity, NNS, hypertension, diabetes | 1.54 (0.68–3.49) | 0.30 |
| Time to ICU Admission | ||
| Crude estimates for pregnancy | 1.91 (0.93–3.93) | 0.08 |
| Adjusted for age, disease severity, NNS, hypertension, diabetes | 2.01 (0.94–4.29) | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espiritu, A.I.; Bravo, S.L.R.; Sombilla, H.A.A.; Tantengco, O.A.G.; Sy, M.C.C.; Sy, A.D.R.; Anlacan, V.M.M.; Jamora, R.D.G. Clinical Outcomes of COVID-19 Infection in Pregnant and Nonpregnant Women: Results from The Philippine CORONA Study. Vaccines 2023, 11, 226. https://doi.org/10.3390/vaccines11020226
Espiritu AI, Bravo SLR, Sombilla HAA, Tantengco OAG, Sy MCC, Sy ADR, Anlacan VMM, Jamora RDG. Clinical Outcomes of COVID-19 Infection in Pregnant and Nonpregnant Women: Results from The Philippine CORONA Study. Vaccines. 2023; 11(2):226. https://doi.org/10.3390/vaccines11020226
Chicago/Turabian StyleEspiritu, Adrian I., Sybil Lizanne R. Bravo, Hannah Andrea A. Sombilla, Ourlad Alzeus G. Tantengco, Marie Charmaine C. Sy, Alvin Duke R. Sy, Veeda Michelle M. Anlacan, and Roland Dominic G. Jamora. 2023. "Clinical Outcomes of COVID-19 Infection in Pregnant and Nonpregnant Women: Results from The Philippine CORONA Study" Vaccines 11, no. 2: 226. https://doi.org/10.3390/vaccines11020226
APA StyleEspiritu, A. I., Bravo, S. L. R., Sombilla, H. A. A., Tantengco, O. A. G., Sy, M. C. C., Sy, A. D. R., Anlacan, V. M. M., & Jamora, R. D. G. (2023). Clinical Outcomes of COVID-19 Infection in Pregnant and Nonpregnant Women: Results from The Philippine CORONA Study. Vaccines, 11(2), 226. https://doi.org/10.3390/vaccines11020226








