Durability of Immune Response after Application of a Third Dose of SARS-CoV-2 Vaccination in Liver Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
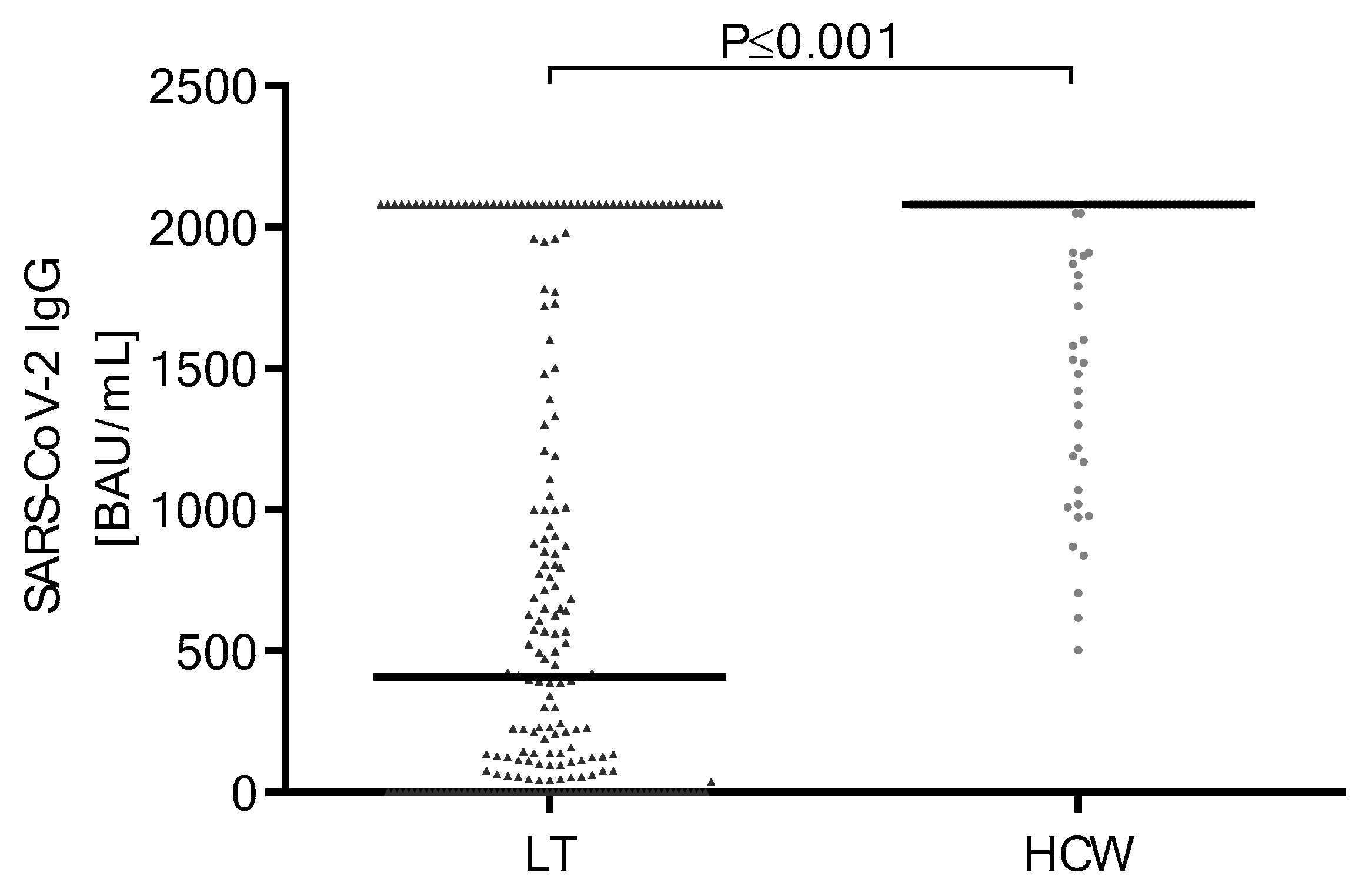
3.2. Antibody Response and Titer after Second SARS-CoV-2 Vaccine Doses
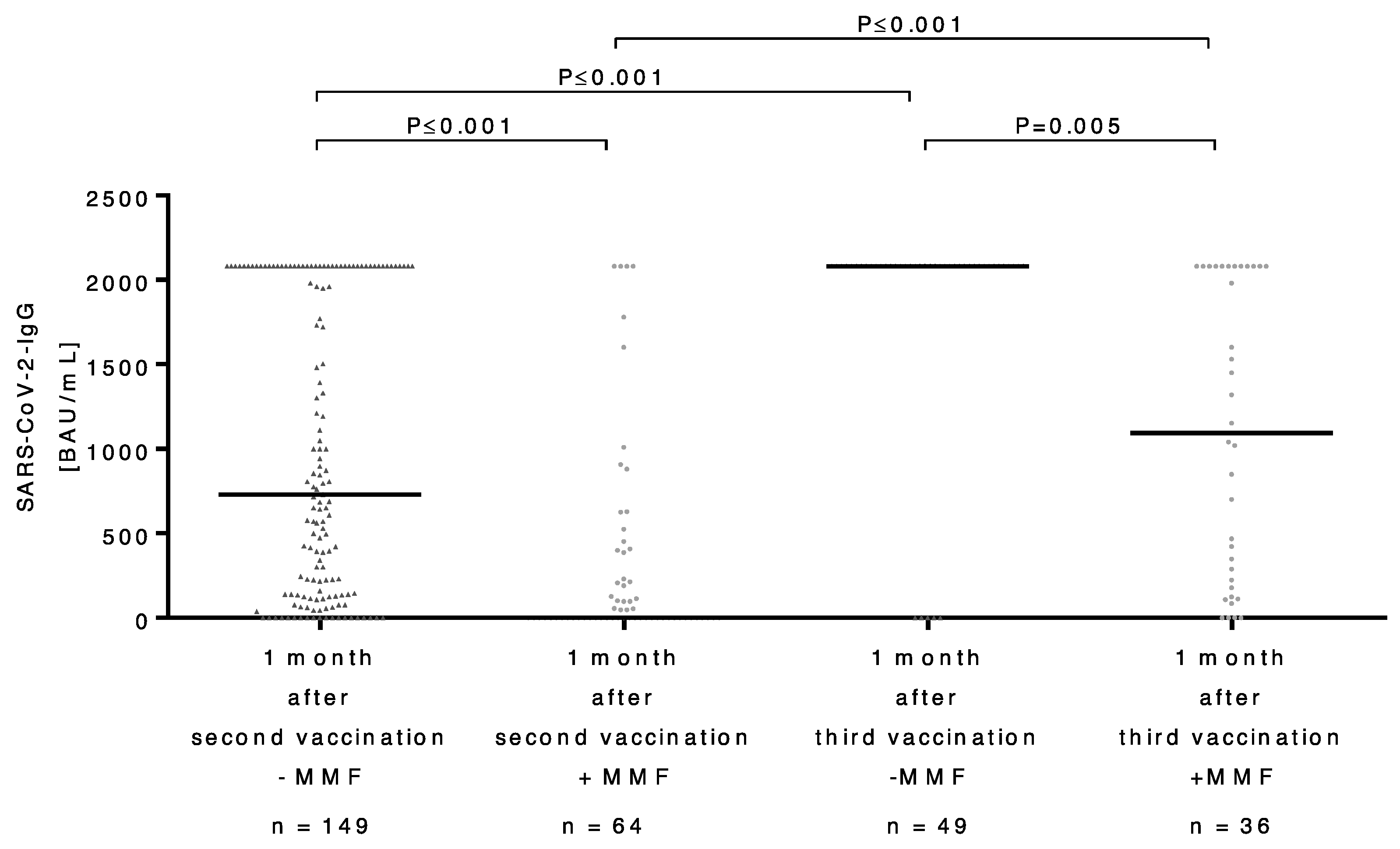
3.3. Course of Antibody Titers after Second and Third SARS-CoV-2 Vaccinations in LT Recipients
3.4. Comparison of the Antibody Response in Patients with and without MMF after Application of Third Dose of SARS-CoV-2 Vaccine
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazzola, A.; Todesco, E.; Drouin, S.; Hazan, F.; Marot, S.; Thabut, D.; Varnous, S.; Soulié, C.; Barrou, B.; Marcelin, A.G.; et al. Poor Antibody Response After Two Doses of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine in Transplant Recipients. Clin. Infect. Dis. 2022, 74, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Rabinowich, L.; Grupper, A.; Baruch, R.; Ben-Yehoyada, M.; Halperin, T.; Turner, D.; Katchman, E.; Levi, S.; Houri, I.; Lubezky, N.; et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J. Hepatol. 2021, 75, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Strauss, A.T.; Hallett, A.M.; Boyarsky, B.J.; Ou, M.T.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Hamilton, J.P.A.; Garonzik-Wang, J.M.; et al. Antibody Response to Severe Acute Respiratory Syndrome-Coronavirus-2 Messenger RNA Vaccines in Liver Transplant Recipients. Liver Transplant. 2021, 27, 1852–1856. [Google Scholar] [CrossRef]
- John, B.V.; Deng, Y.; Khakoo, N.S.; Taddei, T.H.; Kaplan, D.E.; Dahman, B. Coronavirus Disease 2019 Vaccination Is Associated With Reduced Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Death in Liver Transplant Recipients. Gastroenterology 2022, 162, 645–647.e2. [Google Scholar] [CrossRef]
- Cholankeril, G.; Al-Hillan, A.; Tarlow, B.; Abrams, D.; Jacobs, J.S.; Flores, N.P.; Rana, A.; Kanwal, F.; Goss, J.A. Clinical Factors Associated With Lack of Serological Response to SARS-CoV-2 Messenger RNA Vaccine in Liver Transplantation Recipients. Liver Transpl. 2022, 28, 123–126. [Google Scholar] [CrossRef]
- Rashidi-Alavijeh, J.; Frey, A.; Passenberg, M.; Korth, J.; Zmudzinski, J.; Anastasiou, O.; Saner, F.; Jahn, M.; Lange, C.; Willuweit, K. Humoral Response to SARS-CoV-2 Vaccination in Liver Transplant Recipients—A Single-Center Experience. Vaccines 2021, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Davidov, Y.; Indenbaum, V.; Tsaraf, K.; Cohen-Ezra, O.; Likhter, M.; Yakov, G.B.; Halperin, R.; Levy, I.; Mor, O.; Agmon-Levin, N.; et al. A third dose of the BNT162b2 mRNA vaccine significantly improves immune responses among liver transplant recipients. J. Hepatol. 2022, 77, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Del Bello, A.; Abravanel, F.; Marion, O.; Couat, C.; Esposito, L.; Lavayssière, L.; Izopet, J.; Kamar, N. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am. J. Transplant. 2022, 22, 322–323. [Google Scholar] [CrossRef]
- Hall, V.G.; Ferreira, V.H.; Ku, T.; Ierullo, M.; Majchrzak-Kita, B.; Chaparro, C.; Selzner, N.; Schiff, J.; McDonald, M.; Tomlinson, G.; et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N. Engl. J. Med. 2021, 385, 1244–1246. [Google Scholar] [CrossRef]
- Harberts, A.; Schaub, G.M.; Ruether, D.F.; Duengelhoef, P.M.; Brehm, T.T.; Karsten, H.; Fathi, A.; Jahnke-Triankowski, J.; Fischer, L.; Addo, M.M.; et al. Humoral and Cellular Immune Response After Third and Fourth SARS-CoV-2 mRNA Vaccination in Liver Transplant Recipients. Clin. Gastroenterol. Hepatol. 2022, 20, 2558–2566.e5. [Google Scholar] [CrossRef]
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; Del Bello, A. Three Doses of an mRNA COVID-19 Vaccine in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Toniutto, P.; Cussigh, A.; Cmet, S.; Bitetto, D.; Fornasiere, E.; Fumolo, E.; Fabris, M.; D’Aurizio, F.; Fabris, C.; Grillone, L.; et al. Immunogenicity and safety of a third dose of anti-SARS-CoV-2 BNT16b2 vaccine in liver transplant recipients. Liver Int. 2023, 43, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef] [PubMed]
- McDade, T.W.; Demonbreun, A.R.; Sancilio, A.; Mustanski, B.; D’Aquila, R.T.; McNally, E.M. Durability of antibody response to vaccination and surrogate neutralization of emerging variants based on SARS-CoV-2 exposure history. Sci. Rep. 2021, 11, 17325. [Google Scholar] [CrossRef]
- Chang, A.; Strauss, A.T.; Alejo, J.L.; Chiang, T.P.Y.; Hernandez, N.F.; Zeiser, L.B.; Boyarsky, B.J.; Avery, R.K.; Tobian, A.A.; Levan, M.L.; et al. Letter to the editor: Six-month antibody kinetics and durability in liver transplant recipients after two doses of SARS-CoV-2 mRNA vaccination. Hepatol. Commun. 2022, 6, 2990–2992. [Google Scholar] [CrossRef]
- Gilboa, M.; Regev-Yochay, G.; Mandelboim, M.; Indenbaum, V.; Asraf, K.; Fluss, R.; Amit, S.; Mendelson, E.; Doolman, R.; Afek, A.; et al. Durability of Immune Response After COVID-19 Booster Vaccination and Association With COVID-19 Omicron Infection. JAMA Netw. Open 2022, 5, e2231778. [Google Scholar] [CrossRef]
- Kamar, N.; Abravanel, F.; Marion, O.; Esposito, L.; Hebral, A.L.; Médrano, C.; Guitard, J.; Lavayssière, L.; Cointault, O.; Nogier, M.B.; et al. Anti-SARS-CoV-2 spike protein and neutralizing antibodies at 1 and 3 months after three doses of SARS-CoV-2 vaccine in a large cohort of solid organ transplant patients. Am. J. Transplant. 2022, 22, 1467–1474. [Google Scholar] [CrossRef]
- Bakasis, A.D.; Bitzogli, K.; Mouziouras, D.; Pouliakis, A.; Roumpoutsou, M.; Goules, A.V.; Androutsakos, T. Antibody Responses after SARS-CoV-2 Vaccination in Patients with Liver Diseases. Viruses 2022, 14, 207. [Google Scholar] [CrossRef]
- Ruether, D.F.; Schaub, G.M.; Duengelhoef, P.M.; Haag, F.; Brehm, T.T.; Fathi, A.; Wehmeyer, M.; Jahnke-Triankowski, J.; Mayer, L.; Hoffmann, A.; et al. SARS-CoV2-specific Humoral and T-cell Immune Response After Second Vaccination in Liver Cirrhosis and Transplant Patients. Clin. Gastroenterol. Hepatol. 2022, 20, 162–172.e9. [Google Scholar] [CrossRef]
- Willuweit, K.; Frey, A.; Passenberg, M.; Korth, J.; Saka, N.; Anastasiou, O.E.; Möhlendick, B.; Schütte, A.; Schmidt, H.; Rashidi-Alavijeh, J. Patients with Liver Cirrhosis Show High Immunogenicity upon COVID-19 Vaccination but Develop Premature Deterioration of Antibody Titers. Vaccines 2022, 10, 377. [Google Scholar] [CrossRef]
- Meunier, L.; Malezieux, E.; Bedoya, J.U.; Faure, S.; Echenne, M.; Debourdeau, A.; Meszaros, M.; Pageaux, G.P. Mycophenolate mofetil discontinuation increases severe acute respiratory syndrome coronavirus 2 vaccine response in nonresponder liver transplantation recipients: A proof of concept. Liver Transplant. 2022, 29, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. COVID-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef]
- Dimeglio, C.; Migueres, M.; Mansuy, J.M.; Saivin, S.; Miedougé, M.; Chapuy-Regaud, S.; Izopet, J. Antibody titers and breakthrough infections with Omicron SARS-CoV-2. J. Infect. 2022, 84, e13. [Google Scholar] [CrossRef]
- Dimeglio, C.; Migueres, M.; Bouzid, N.; Chapuy-Regaud, S.; Gernigon, C.; Da-Silva, I.; Porcheron, M.; Martin-Blondel, G.; Herin, F.; Izopet, J. Antibody Titers and Protection against Omicron (BA. 1 and BA. 2) SARS-CoV-2 Infection. Vaccines 2022, 10, 1548. [Google Scholar] [CrossRef]
- Egger, A.E.; Sahanic, S.; Gleiss, A.; Ratzinger, F.; Holzer, B.; Irsara, C.; Binder, N.; Winkler, C.; Binder, C.J.; Posch, W.; et al. One-Year Follow-Up of COVID-19 Patients Indicates Substantial Assay-Dependent Differences in the Kinetics of SARS-CoV-2 Antibodies. Microbiol. Spectr. 2022, 10, e00597-22. [Google Scholar] [CrossRef]
- Celikgil, A.; Massimi, A.B.; Nakouzi, A.; Herrera, N.G.; Morano, N.C.; Lee, J.H.; Yoon, H.A.; Garforth, S.J.; Almo, S.C. SARS-CoV-2 multi-antigen protein microarray for detailed characterization of antibody responses in COVID-19 patients. PLoS ONE 2023, 18, e0276829. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | LT Recipients n/[%] | HCWs n/[%] | p-Value |
|---|---|---|---|
| Total patient number, n [%] | 300 | 122 | - |
| Sex (male/female), n [%] | 167 [55.7]/133 [44.3] | 70 [57.4]/52 [42.6] | 0.829 |
| Age at basic immunization (years), median [IQR] | 56.5 [48–64] | 57 [51–61] | 0.505 |
| Age of recipient at LT, median [IQR] | 47 [37–55] | - | - |
| Time between first and second vaccinations, median [IQR] | 42 [35–42] | 46 [44–48] | <0.001 |
| Time between second and booster vaccinations (days), median [IQR] | 187 [174–198] | 189 [185–195] | 0.020 |
| Indication for LT | n [%] |
|---|---|
| Alcohol-induced liver cirrhosis | 45 [15] |
| Hepatocellular carcinoma | 42 [14] |
| Autoimmune hepatitis | 40 [13] |
| Primary sclerosing cholangitis | 19 [6] |
| Acute liver failure | 18 [6] |
| Hepatitis C virus | 17 [5.7] |
| NASH | 16 [5.3] |
| Cryptogenic liver cirrhosis | 15 [5] |
| Budd–Chiari syndrome | 11 [3.7] |
| Hepatitis B/D virus | 8 [2.7] |
| Bile duct atresia | 8 [2.7] |
| Cystic liver | 8 [2.7] |
| Hepatitis B virus | 6 [2] |
| Wilson’s disease | 6 [2] |
| Liver cirrhosis | 5 [1.7] |
| Primary biliary cholangitis | 4 [1] |
| Others 1 | 32 [10.7] |
| Patient Characteristics | LT Recipients SARS-CoV-2 IgG Positive | LT Recipients SARS-CoV-2 IgG Negative | p-Value |
|---|---|---|---|
| Total patient number, n [%] | 158/213 [74] | 55/213 [26] | - |
| Sex (male/female), n [%] | 91 [57.6]/67 [42.4] | 26 [47.3]/29 [52.7] | 0.210 |
| Age at basic immunization (years), median [IQR] | 55.5 [41–63] | 58 [51–66] | 0.015 |
| Time between first and second vaccinations, median [IQR] | 42 [35–42] | 42 [37–42] | 0.363 |
| Immunosuppressive Therapy | Median [IQR] | Median [IQR] | p-Value |
| Prednisolone (n = 16) No prednisolone (n = 197) | 9 [6] 149 [94] | 7 [13] 48 [87] | 0.133 |
| MMF (n = 64) No MMF (n = 149) | 29 [18] 129 [82] | 35 [64] 20 [36] | 0.001 |
| Tacrolimus-based immunosuppression (n = 191) No tacrolimus (n = 22) | 141 [89] 17 [11] | 50 [91] 5 [9] | 1.000 |
| Everolimus (n = 9) No everolimus (n = 204) | 6 [4] 152 [96] | 3 [6] 52 [94] | 0.698 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passenberg, M.; Authorsen-Grudmann, R.; Frey, A.; Korth, J.; Zmudzinski, J.; Anastasiou, O.E.; Möhlendick, B.; Schmidt, H.; Rashidi-Alavijeh, J.; Willuweit, K. Durability of Immune Response after Application of a Third Dose of SARS-CoV-2 Vaccination in Liver Transplant Recipients. Vaccines 2023, 11, 572. https://doi.org/10.3390/vaccines11030572
Passenberg M, Authorsen-Grudmann R, Frey A, Korth J, Zmudzinski J, Anastasiou OE, Möhlendick B, Schmidt H, Rashidi-Alavijeh J, Willuweit K. Durability of Immune Response after Application of a Third Dose of SARS-CoV-2 Vaccination in Liver Transplant Recipients. Vaccines. 2023; 11(3):572. https://doi.org/10.3390/vaccines11030572
Chicago/Turabian StylePassenberg, Moritz, Roxane Authorsen-Grudmann, Alexandra Frey, Johannes Korth, Jaqueline Zmudzinski, Olympia E. Anastasiou, Birte Möhlendick, Hartmut Schmidt, Jassin Rashidi-Alavijeh, and Katharina Willuweit. 2023. "Durability of Immune Response after Application of a Third Dose of SARS-CoV-2 Vaccination in Liver Transplant Recipients" Vaccines 11, no. 3: 572. https://doi.org/10.3390/vaccines11030572
APA StylePassenberg, M., Authorsen-Grudmann, R., Frey, A., Korth, J., Zmudzinski, J., Anastasiou, O. E., Möhlendick, B., Schmidt, H., Rashidi-Alavijeh, J., & Willuweit, K. (2023). Durability of Immune Response after Application of a Third Dose of SARS-CoV-2 Vaccination in Liver Transplant Recipients. Vaccines, 11(3), 572. https://doi.org/10.3390/vaccines11030572






