Mechanisms of Immune-Related Long Non-Coding RNAs in Spleens of Mice Vaccinated with 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Treatment and Sample Collection
2.2. RNA Isolation, Library Preparation, and Sequencing
2.3. Quality Control and Mapping
2.4. Identification of lncRNAs
2.5. Analysis of DE mRNAs and lncRNAs
2.6. Target Gene Prediction and Functional Analysis of lncRNAs
2.7. GO and KEGG Enrichment Analysis
2.8. RNA-Seq Result Validation by qRT-PCR
3. Results
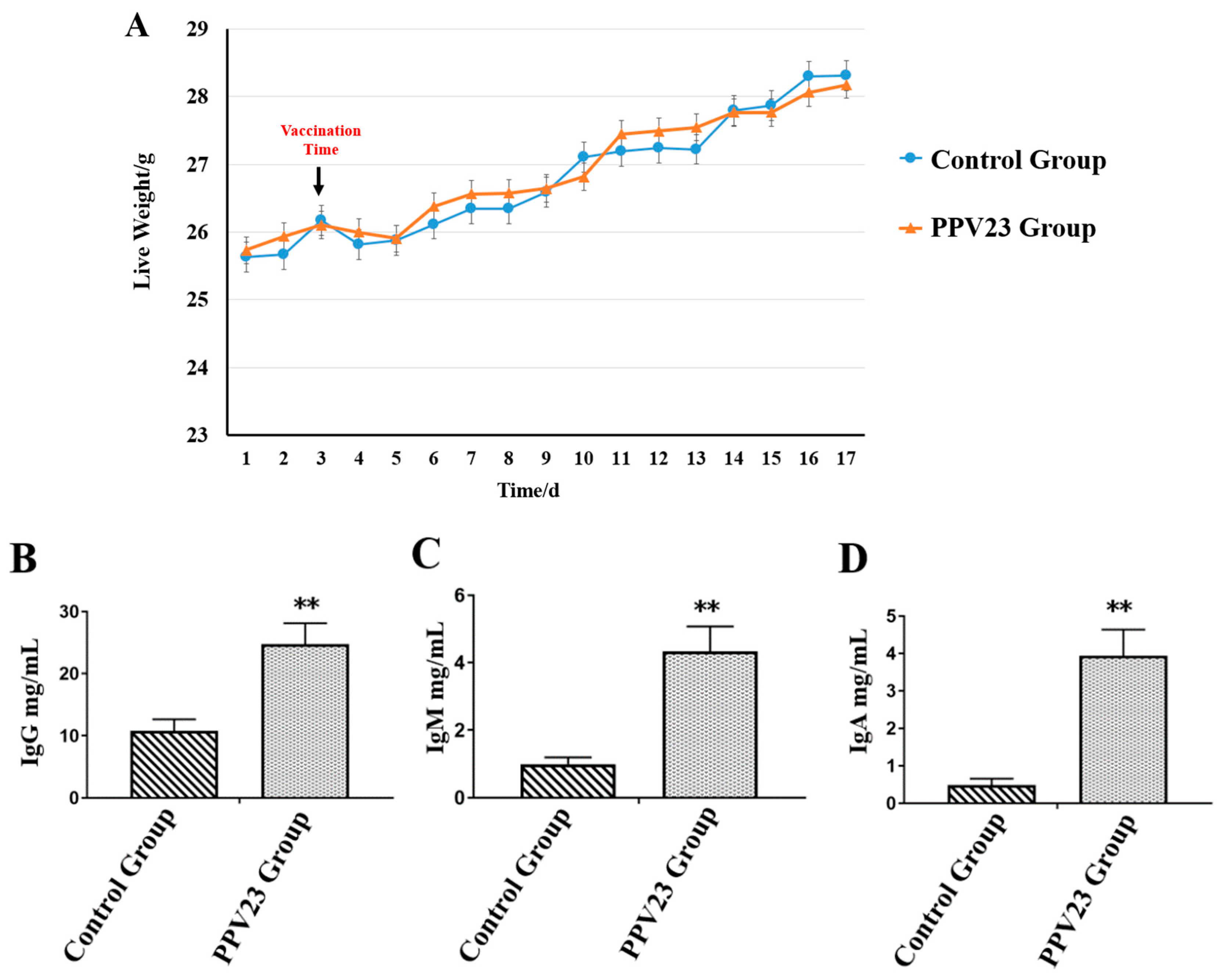
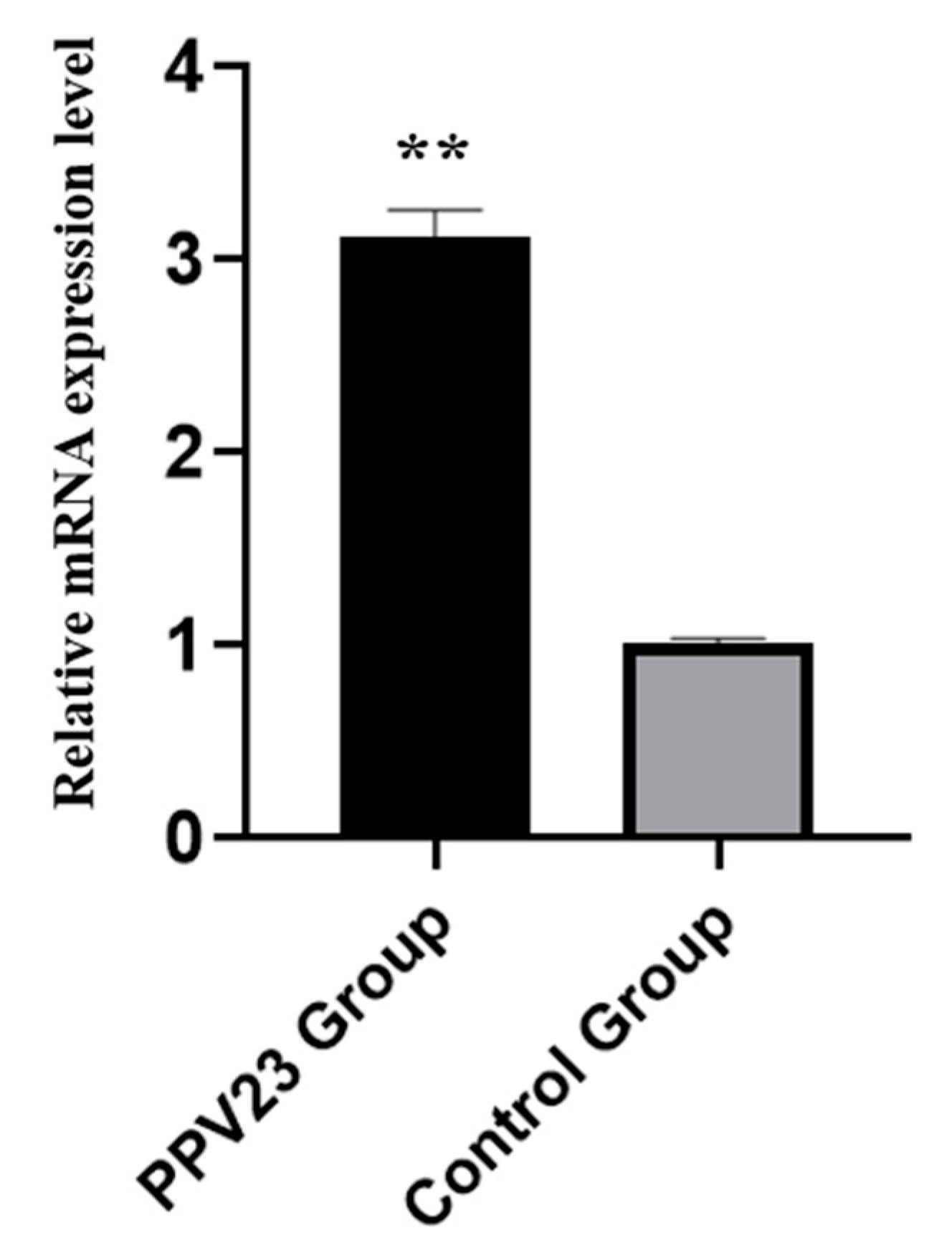
3.1. Phenotypic Data Analysis
3.2. Sequencing Data Summary
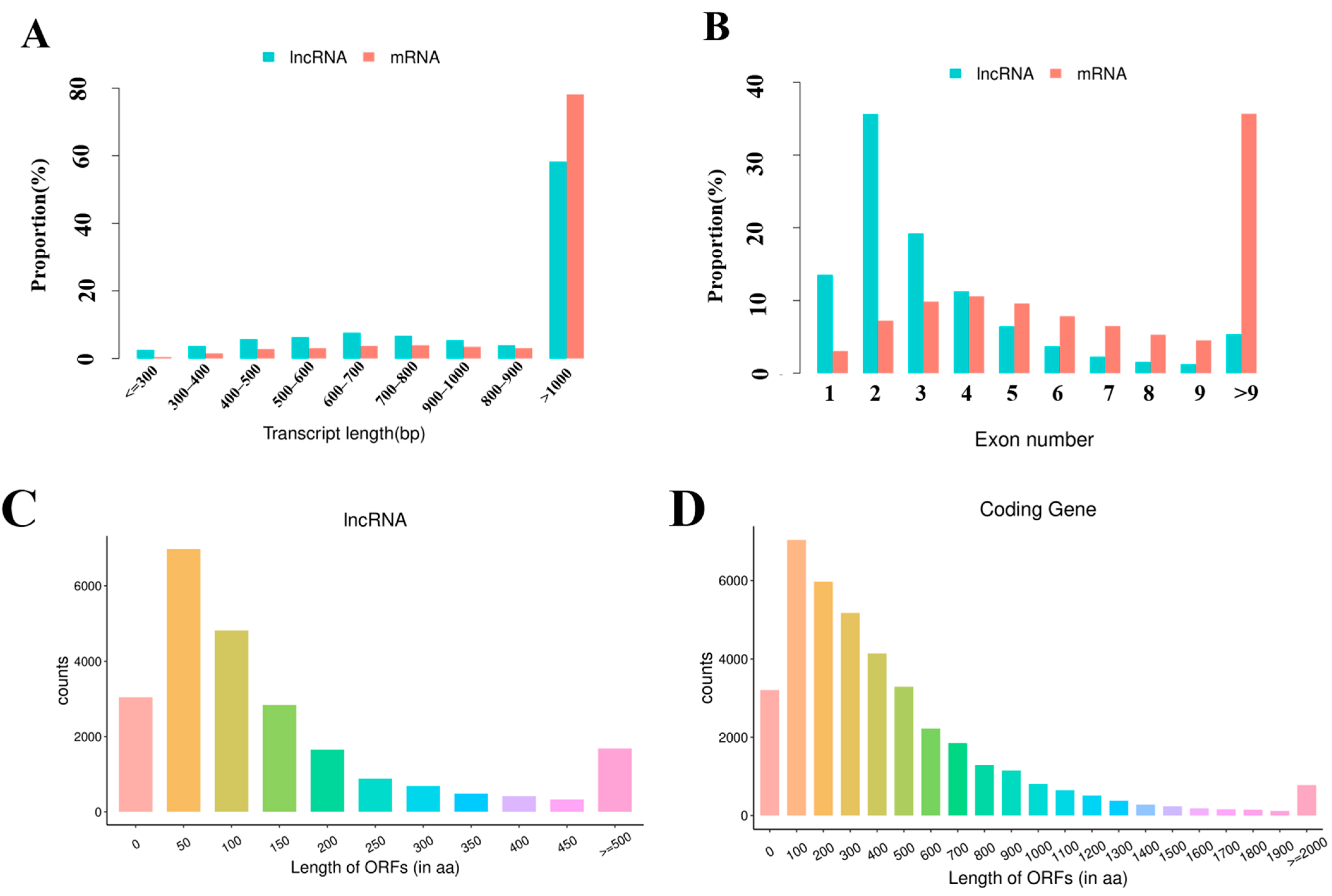
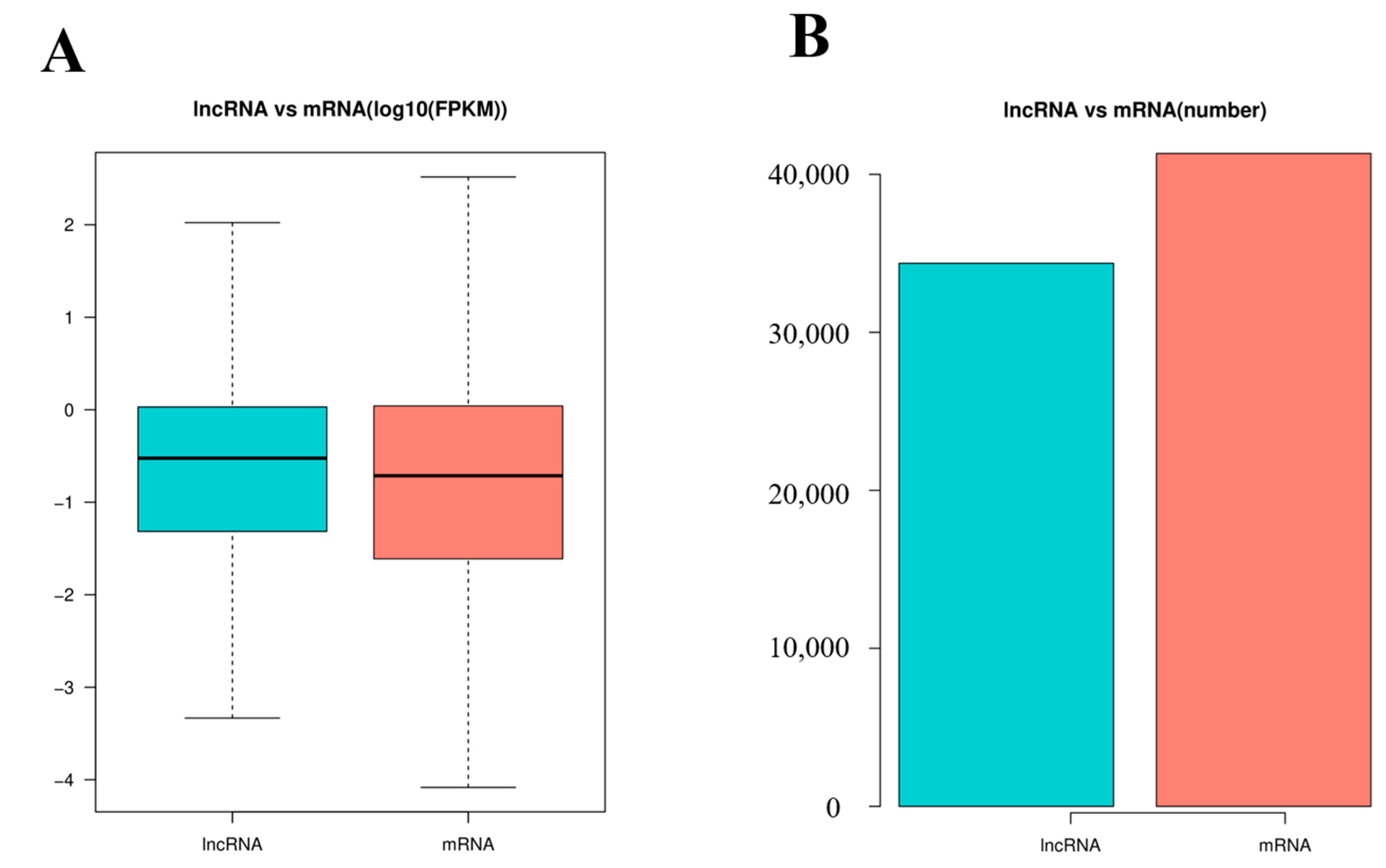
3.3. Identification of lncRNAs and mRNAs in Mouse Spleens
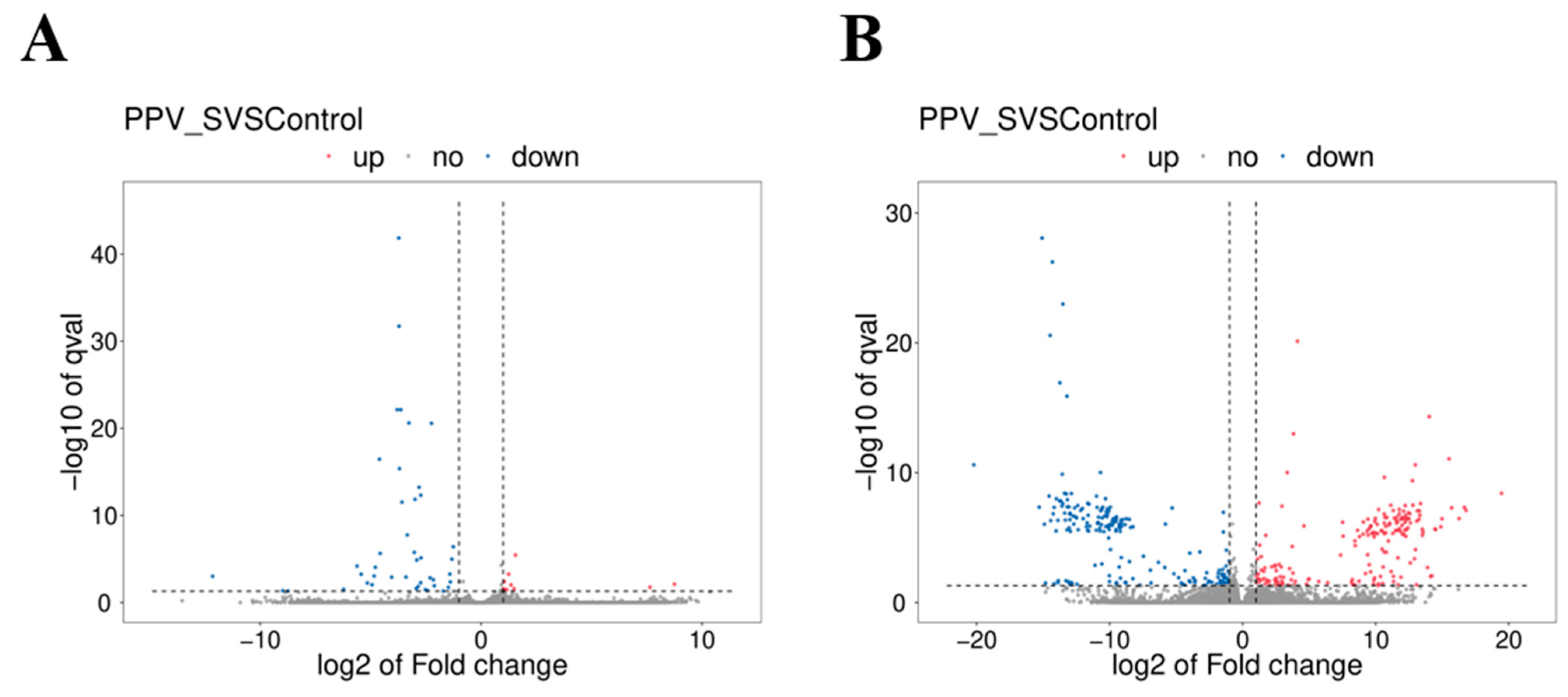
3.4. Identification of DE mRNAs and DE lncRNAs
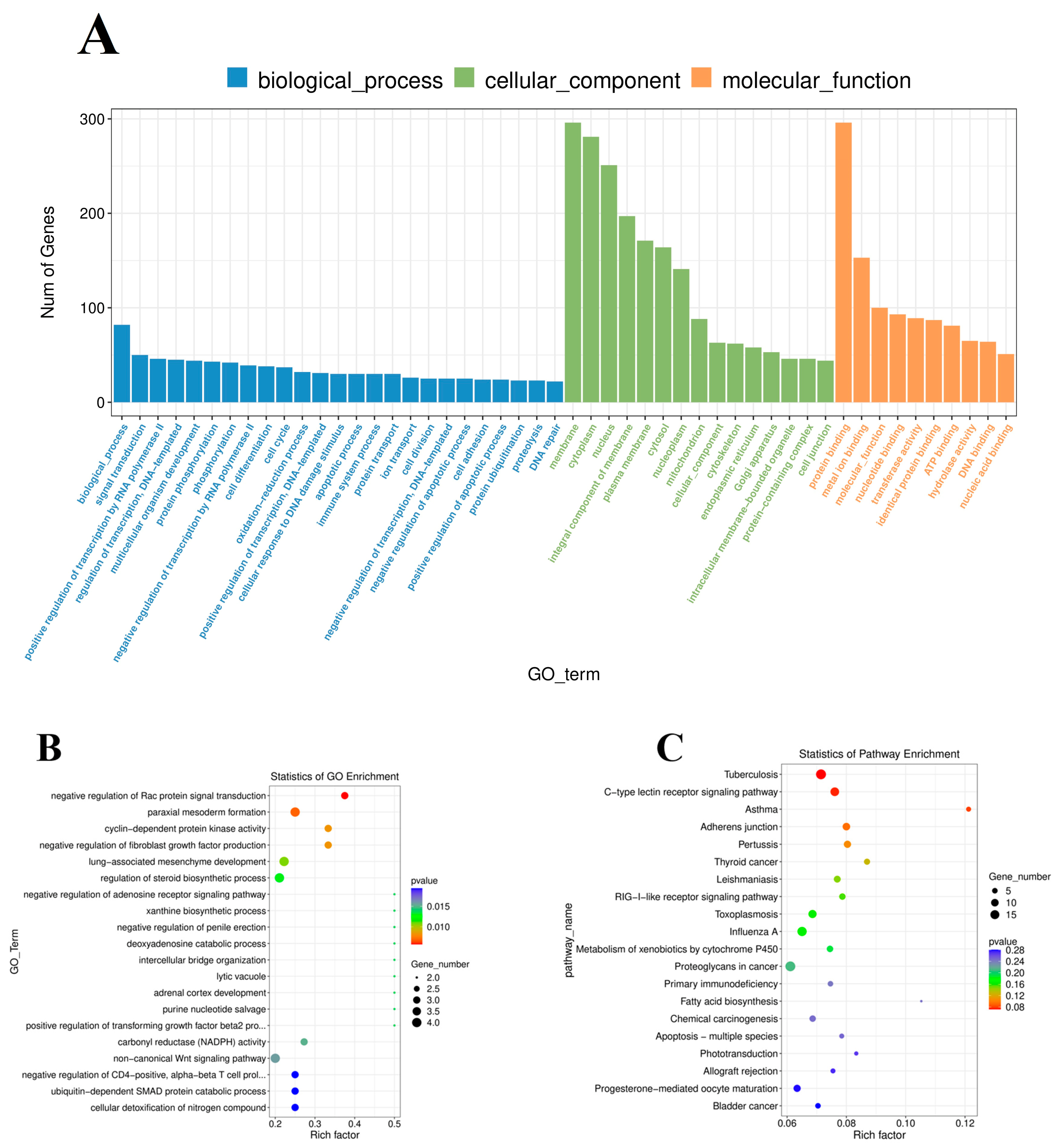
3.5. Functional Enrichment of DE mRNAs
3.6. Cis-Regulatory Roles of DE lncRNAs in Spleen Tissues of Mice
3.7. Co-Enriched GO Terms of DE lncRNAs and mRNAs
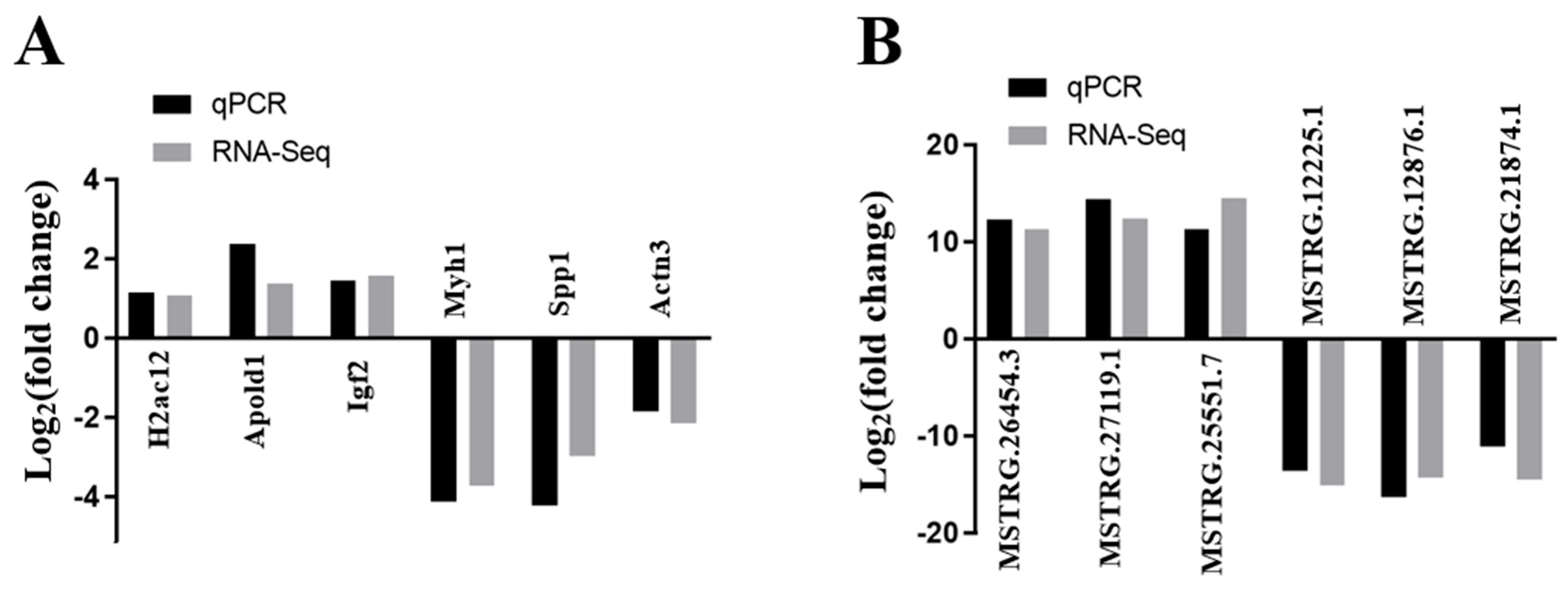
3.8. DE lncRNAs and DE mRNAs Validation by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudis, M.I.; Stone, S.C.; Goad, J.A.; Lee, V.W.; Chitchyan, A.; and Newton, K.I. Pneumococcal vaccination in the emergency department: An assessment of need. Ann. Emerg. Med. 2004, 44, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Paradisi, F.; Corti, G.; and Cinelli, R. Streptococcus pneumoniae as an agent of nosocomial infection: Treatment in the era of penicillin-resistant strains. Clin. Microbiol. Infec. 2001, 7, 34–42. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; and Cherian, T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimate. Lancet 2009, 374, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Li, L.F.; Ma, J.Y.; Yu, Z.Y.; Li, M.C.; Zhang, W.C.; and Sun, H.Q. Epidemiological characteristics and antibiotic resistance mechanisms of Streptococcus pneumoniae: An updated review. Microbiol. Res. 2023, 226, 127221. [Google Scholar] [CrossRef] [PubMed]
- Watkins, E.R.; Kalizang’Oma, A.; Gori, A.; Gupta, S.; and Heyderman, R.S. Factors affecting antimicrobial resistance in Streptococcus pneumoniae following vaccination introduction. Trends. Microbiol. 2022, 30, 1135–1145. [Google Scholar] [CrossRef]
- O’Brien, K.L.; Hochman, M.; and Goldblatt, D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: Is hyporesponsiveness an issue? Lancet. Infect. Dis. 2007, 7, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Masomian, M.; Ahmad, Z.; Gew, L.T.; and Poh, C.L. Development of Next Generation Streptococcus pneumoniae Vaccines Conferring Broad Protection. Vaccines 2020, 8, 132. [Google Scholar] [CrossRef]
- Jackson, L.A.; Neuzil, K.M.; Nahm, M.H.; Whitney, C.G.; Yu, O.; Nelson, J.C.; Starkovich, P.T.; Dunstan, M.; Carste, B.; Shay, D.K.; et al. Immunogenicity of varying dosages of 7-valent pneurnococcal polysaccharide-protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2007, 25, 4029–4037. [Google Scholar] [CrossRef]
- WHO. Pneumococcal conjugate vaccines in infants and childrenunder 5 years of age: WHO position paper-February. Wkly. Epidemiol. Rec. 2019, 94, 85–104. [Google Scholar]
- Weiser, J.N.; Ferreira, D.M.; and Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 354–367. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Zhang, X.M.; Wang, X.F.; Wang, L.B.; Zhang, J.H.; Yin, Y.B. ComE, an essential response regulator, negatively regulates the expression of the capsular polysaccharide locus and attenuates the bacterial virulence in Streptococcus pneumoniae. Front. Microbiol. 2018, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, U.B.S.; Yao, K.H.; Yang, Y.H.; Tettelin, H.; Kilian, M. Capsular Polysaccharide Expression in Commensal Streptococcus Species: Genetic and Antigenic Similarities to Streptococcus pneumoniae. MBIO 2017, 7, e01844-16. [Google Scholar] [CrossRef] [PubMed]
- Aliaksandr, A.Y.; Igor, V.K. Long noncoding RNAs: A potential novel class of cancer biomarkers. Front. Genet. 2015, 6, 145–154. [Google Scholar]
- Mallory, A.C.; Shkumatava, A. LncRNAs in vertebrates: Advances and challenges. Biochimie 2015, 117, 3–14. [Google Scholar] [CrossRef]
- Shen, Y.-F.; Mao, H.-G.; Huang, M.J.; Chen, L.-X.; Chen, J.-C.; Cai, Z.-W.; Wang, X.; Xu, N. Long noncoding RNA and mRNA expression profiles in the thyroid gland of two phenotypically extreme pig breeds using Ribo-zero RNA sequencing. Genes 2016, 7, 34–48. [Google Scholar] [CrossRef]
- Nojima, T.; Proudfoot, N.J. Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat. Rev. Mol. Cell. Bio. 2022, 23, 389–406. [Google Scholar] [CrossRef]
- Wang, P.; Xu, J.; Wang, Y.; Cao, X. An interferon-independent lncRNA promotes viral replication by modulating cellular metabolism. Science 2017, 358, 1051–1055. [Google Scholar] [CrossRef]
- Engreitz, J.M.; Haines, J.E.; Perez, E.M.; Munson, G.; Chen, J.; Kane, M.; McDonel, P.E.; Guttman, M.; Lander, E.S. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 2016, 539, 452–455. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Z.M.; Chang, Y.N.; Hu, Z.M.; Qi, H.X.; Hong, W. The ways of action of long non-coding RNAs in cytoplasm and nucleus. Gene 2014, 547, 1–9. [Google Scholar] [CrossRef]
- Harinarayanan, J.; Reniqua, P.H.; Vamsi, K.G.; Alan, D.J.; Philip, H.H.; Viswanathan, P. The long (lncRNA) and short (miRNA) of it: TGFβ-mediated control of RNA-binding proteins and noncoding RNAs. Mol. Cancer Res. 2018, 16, 567–579. [Google Scholar]
- Marina, R.H.; Mark, A.L.P. Long Non-Coding RNAs and the Innate Immune Response. Non-Coding Rna. 2019, 5, 34. [Google Scholar]
- Sigdel, K.R.; Cheng, A.; Wang, Y.; Duan, L.H.; Zhang, Y.L. The Emerging Functions of Long Noncoding RNA in Immune Cells: Autoimmune Diseases. J. Immunol. Res. 2015, 2015, 848790–848798. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.A.; Wapinski, O.L.; Yang, Y.W.; Bureau, J.-F.; Gopinath, S.; Monack, D.M.; Chang, H.Y.; Brahic, M.; Kirkegaard, K. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cell 2013, 152, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Bolland, D.J.; Wood, A.L.; Johnston, C.M.; Bunting, S.F.; Morgan, G.; Chakalova, L.; Fraser, P.J.; Corcoran, A.E. Antisense intergenic transcription in V(D)J recombination. Nat. Immunol. 2004, 5, 630–637. [Google Scholar] [CrossRef]
- Wu, X.-B.; Yang, L.; Wang, J.; Hao, Y.-Y.; Wang, C.-Y.; Lu, Z.-M. The Involvement of Long Non-Coding RNAs in Glioma: From Early Detection to Immunotherapy. Front. Immunol. 2022, 13, 897754. [Google Scholar] [CrossRef] [PubMed]
- Necsulea, A.; Necsulea, A.; Kaessmann, H.; Kaessmann, H. Evolutionary dynamics of coding and non-coding transcriptomes. Nat. Rev. Genet. 2014, 15, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat Methods. 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Frazee, A.C.; Pertea, G.; Jaffe, A.E.; Langmead, B.; Salzberg, S.L.; Leek, J.T. Ballgown bridges the gap between transcriptome assembly and expression analysis. Nat. Biotechnol. 2015, 33, 243–246. [Google Scholar] [CrossRef]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic. Acids. Res. 2013, 41, e166. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, Y.; Ye, Z.Q.; Liu, X.Q.; Zhao, S.Q.; Wei, L.; Gao, G. CPC: Assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic. Acids. Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Duan, L.H.; Cyster, J.G. Chemo- and mechanosensing by dendritic cells facilitate antigen surveillance in the spleen. Immunol. Rev. 2022, 306, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Yeo, S.G.; Kim, Y.I.; Lee, J.W.; Kim, S.H.; Park, D.C. Comparison of innate immunity mediators in peritoneal fluid and spleen between young and aged rats. Aging. Clin. Exp. Res. 2016, 28, 775–779. [Google Scholar] [CrossRef]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. Cell. 2009, 136, 629–641. [Google Scholar] [CrossRef]
- Du, Y.; Chu, C.M.; Zhuo, D.; Ning, J.Z. The inhibition of TRIM35-mediated TIGAR ubiquitination enhances mitochondrial fusion and alleviates renal ischemia-reperfusion injury. Int. J. Biol. Macromol. 2022, 209, 725–736. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Y.; Chen, X.; Zhang, Y.; Hu, J.; Ren, C.; Ding, J.; Jiang, D.; Li, L. Porcine TRIM35 positively regulate TRAF3-mediated IFN-beta production and inhibit Japanese encephalitis virus replication. Dev. Comp. Immunol. 2021, 127, 104290. [Google Scholar] [CrossRef]
- Sun, N.; Jiang, L.; Ye, M.; Wang, Y.; Wang, G.; Wan, X.; Zhao, Y.; Wen, X.; Liang, L.; Ma, S.; et al. TRIM35 mediates protection against influenza infection by activating TRAF3 and degrading viral PB2. Protein. Cell. 2020, 11, 894–914. [Google Scholar] [CrossRef]
- Wang, Y.M.; Yan, S.S.; Yang, B.; Wang, Y.; Zhou, H.Y.; Lian, Q.S.; Sun, B. TRIM35 negatively regulates TLR7-and TLR9-mediated type I interferon production by targeting IRF7. Febs. Lett. 2015, 589, 1322–1330. [Google Scholar] [CrossRef]
- Liu, G.; Cooley, M.A.; Jarnicki, A.G.; Hsu, A.C.Y.; Nair, P.M.; Haw, T.J.; Fricker, M.; Gellatly, S.L.; Kim, R.Y.; Inman, M.D.; et al. Fibulin-1 regulates the pathogenesis of tissue remodeling in respiratory diseases. JCI Insight. 2016, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Shah, S.D.; Panettieri, R.A.; Deshpande, D.A. Bnip3 regulates airway smooth muscle cell focal adhesion and proliferation. Am. J. Physiol-Lung C 2019, 317, L758–L767. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Wei, T.; Cox, C.W.; Jiang, Y.; Roche, W.R.; Walls, A.F. Mast cell chymase impairs bronchial epithelium integrity by degrading cell junction molecules of epithelial cells. Allergy 2019, 76, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Erturk-Hasdemir, D.; Kasper, D.L. Finding a needle in a haystack: Bacteroides fragilis polysaccharide A as the archetypical symbiosis factor. Ann. N. Y. Acad. Sci. 2018, 1417, 116–129. [Google Scholar] [CrossRef]
- Hsieh, S.A.; Allen, P.M. Immunomodulatory roles of polysaccharide capsules in the intestine. Front. Immunol. 2020, 11, 690. [Google Scholar] [CrossRef]
- Avci, F.Y.; Kasper, D.L. How bacterial carbohydrates influence the adaptive immune system. Annu. Rev. Immunol. 2010, 28, 107–130. [Google Scholar] [CrossRef]
- Blandford, L.E.; Johnston, E.L.; Sanderson, J.D.; Wade, W.G.; Lax, A.G. Promoter orientation of the immunomodulatory Bacteroides fragilis capsular polysaccharide A (PSA) is off in individuals with inflammatory bowel disease (IBD). Gut. Microbes. 2019, 10, 569–577. [Google Scholar] [CrossRef]
- Troy, E.B.; Kasper, D.L. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front. Biosci. 2010, 15, 25–34. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef]
- Ochoa-Reparaz, J.; Mielcarz, D.W.; Wang, Y.; Begum-Haque, S.; Dasgupta, S.; Kasper, D.L.; Kasper, L.H. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal. Immunol. 2010, 3, 487–495. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Cantin, E.M. IFN gamma inhibits G-CSF induced neutrophil expansion and invasion of the CNS to prevent viral encephalitis. PLoS. Pathog. 2018, 14, e1006822. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, C.; Kujawski, M.; Chug, H.; Li, L.; Mazmanian, S.K.; Cantin, E.M. Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat. Commun. 2019, 10, 2153. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Jones, M.B.; Cobb, B.A. Polysaccharide-experienced effector T cells induce IL-10 in FoxP3+ regulatory T cells to prevent pulmonary inflammation. Glycobiology. 2018, 28, 50–58. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | lncRNA Transcript Name | Cislocation (bp) | Pearson Correlation Coefficient |
|---|---|---|---|
| St6galnac1 | MSTRG.5738.1 | 100 K | 1 |
| Tmem106a | MSTRG.5460.2 | 100 K | 1 |
| St6galnac1 | ENSMUST00000156293 | 100 K | 1 |
| St6galnac1 | ENSMUST00000147508 | 100 K | 1 |
| Cdo1 | MSTRG.13054.1 | 100 K | −0.90 |
| Vamp8 | MSTRG.21907.5 | 1 K | −0.77 |
| Lin7b | ENSMUST00000211098 | 100 K | −0.74 |
| Pcf11 | MSTRG.23880.5 | 10 K | −0.63 |
| GO Term | GO Function | p-Value |
|---|---|---|
| CD86 biosynthetic process | biological_process | 0.03 |
| CCR chemokine receptor binding | molecular_function | 0.03 |
| positive regulation of type IIa hypersensitivity | biological_process | 0.05 |
| mRNA cleavage factor complex | cellular_component | 0.02 |
| natural killer-cell-mediated cytotoxicity biological | biological_process | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, N.; Zhang, F.; Zhou, H.; Ma, W.; Mao, H.; Wang, M.; Ke, Z.; Wang, J.; Qi, L. Mechanisms of Immune-Related Long Non-Coding RNAs in Spleens of Mice Vaccinated with 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23). Vaccines 2023, 11, 529. https://doi.org/10.3390/vaccines11030529
Zhu N, Zhang F, Zhou H, Ma W, Mao H, Wang M, Ke Z, Wang J, Qi L. Mechanisms of Immune-Related Long Non-Coding RNAs in Spleens of Mice Vaccinated with 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23). Vaccines. 2023; 11(3):529. https://doi.org/10.3390/vaccines11030529
Chicago/Turabian StyleZhu, Nan, Fan Zhang, Huan Zhou, Wei Ma, Haiguang Mao, Mengting Wang, Zhijian Ke, Jinbo Wang, and Lili Qi. 2023. "Mechanisms of Immune-Related Long Non-Coding RNAs in Spleens of Mice Vaccinated with 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23)" Vaccines 11, no. 3: 529. https://doi.org/10.3390/vaccines11030529
APA StyleZhu, N., Zhang, F., Zhou, H., Ma, W., Mao, H., Wang, M., Ke, Z., Wang, J., & Qi, L. (2023). Mechanisms of Immune-Related Long Non-Coding RNAs in Spleens of Mice Vaccinated with 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23). Vaccines, 11(3), 529. https://doi.org/10.3390/vaccines11030529






