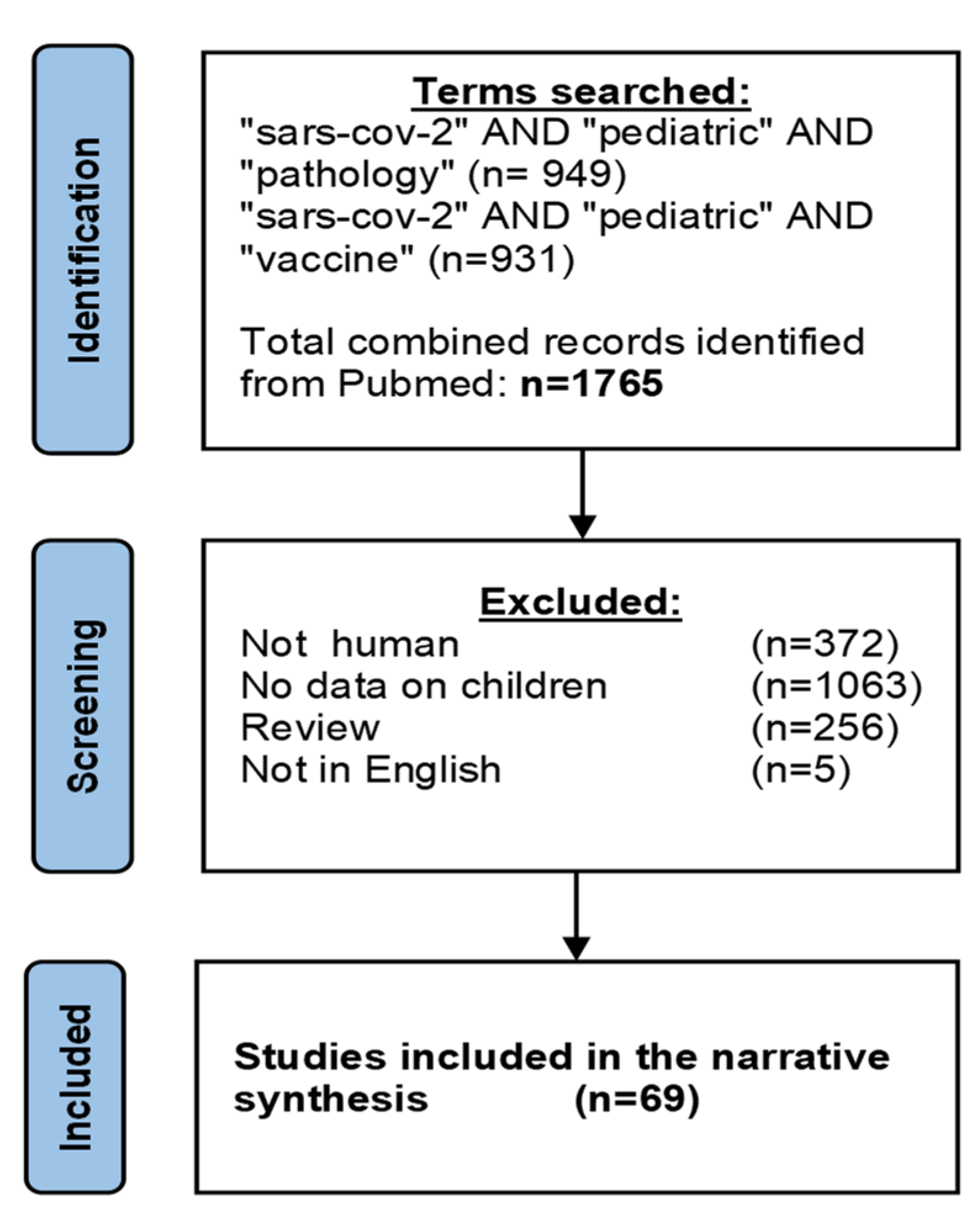
Molecular Determinants of the Early Life Immune Response to COVID-19 Infection and Immunization
Abstract
1. Introduction
2. Infection
2.1. Pathogenesis
2.2. Clinical Presentation (Children vs. Adults)
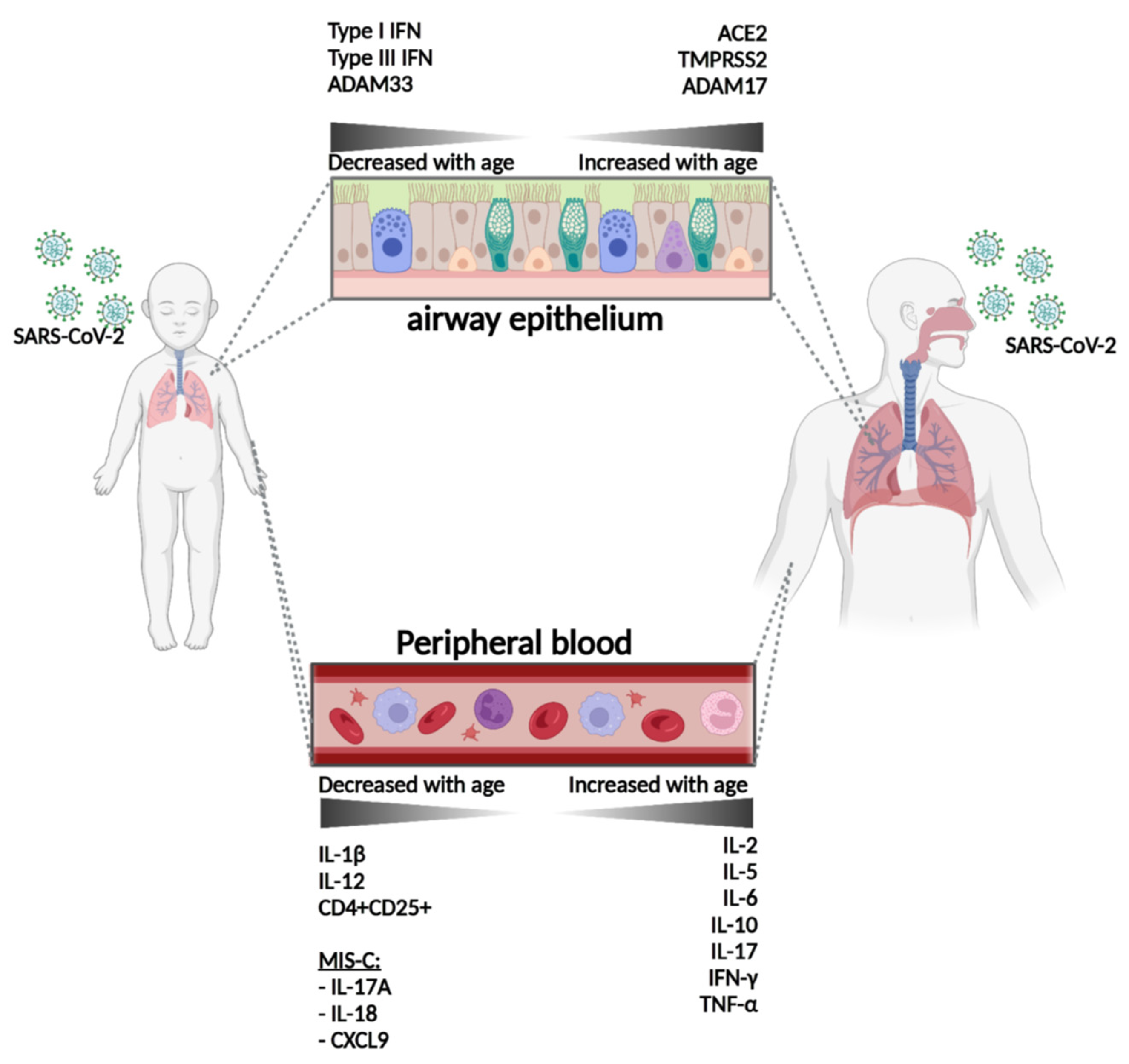
2.3. Differences in Entry Mechanisms between Children and Adults
| Molecule | Expression in Children versus Adults |
|---|---|
| ACE2 | Lower in children than in adults [66,67], Higher in children than in adults [68] higher in children than older (>50 years) adults [69] |
| TMPRSS2 | Lower in children than in adults [66,70] |
| ADAM17 (TACE) | Lower in children than in adults [71] |
2.4. Cytokine Profiles and Innate Immunity in Children versus Adults
2.5. Adaptive Immunity in Children versus Adults
| Molecule | Adults/Children with COVID-19 | Possible Impact on Outcome (Negative or Positive) |
|---|---|---|
| Il-6 | Higher in adults than in children [101,102,103,104,105] | None in children [106,107]/negative in adults [108,109,110] |
| Il-8 | Unknown | Negative in adults [110] |
| Il-10 | Higher in adults than in children [101] No differences [102] | Negative in adults [109,111] |
| Il-2 | Higher in adults than in children [102] | None in children [107] |
| Il-7 | ? | Negative in adults [111] |
| Il-5 | Higher in adults than in children [101] | |
| TNFα | Higher in children than in adults [86] No differences [102,105] | None in children [106,107] |
| TGFβ | ? | Negative in adults [112] |
| IL-17A | Higher in children than in adults [86,113] | Negative in adults [114] None in children [72] |
| IFNγ | Higher in children than in adults [86] No differences [102,105] | Negative in adults [115] None in children [107] |
| NF-kb | ? | Negative in adults [116] |
| Il-12 | Higher in children than in adults [101] | |
| IL-1β | Higher in children than in adults [101] | |
| CD25+ (on CD4+ cells) | Higher expression in adults than in children [86] |
3. Immune Response to Immunization
4. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lotfi, M.; Hamblin, M.R.; Rezaei, N. COVID-19: Transmission, Prevention, and Potential Therapeutic Opportunities. Clin. Chim. Acta 2020, 508, 254–266. [Google Scholar] [CrossRef]
- Grant, M.C.; Geoghegan, L.; Arbyn, M.; Mohammed, Z.; McGuinness, L.; Clarke, E.L.; Wade, R.G. The Prevalence of Symptoms in 24,410 Adults Infected by the Novel Coronavirus (SARS-CoV-2; COVID-19): A Systematic Review and Meta-Analysis of 148 Studies from 9 Countries. PLoS ONE 2020, 15, e0234765. [Google Scholar] [CrossRef]
- Kompaniyets, L.; Agathis, N.T.; Nelson, J.M.; Preston, L.E.; Ko, J.Y.; Belay, B.; Pennington, A.F.; Danielson, M.L.; DeSisto, C.L.; Chevinsky, J.R.; et al. Underlying Medical Conditions Associated With Severe COVID-19 Illness Among Children. JAMA Netw. Open 2021, 4, e2111182. [Google Scholar] [CrossRef]
- Shi, T.; Pan, J.; Katikireddi, S.V.; McCowan, C.; Kerr, S.; Agrawal, U.; Shah, S.A.; Simpson, C.R.; Ritchie, L.D.; Robertson, C.; et al. Risk of COVID-19 Hospital Admission among Children Aged 5–17 Years with Asthma in Scotland: A National Incident Cohort Study. Lancet Respir. Med. 2021, 10, P191–P198. [Google Scholar] [CrossRef]
- CDC Estimated COVID-19 Burden. Available online: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html (accessed on 14 December 2021).
- Myers, V.; Saban, M.; Wilf-Miron, R. COVID-19 in Children Aged 5 to 11: Examining the Issues Surrounding Vaccination and Public Health Policy. Paediatr. Respir. Rev. 2022, 43, 85–90. [Google Scholar] [CrossRef]
- Plotkin, S.A.; Levy, O. Considering Mandatory Vaccination of Children for COVID-19. Pediatrics 2021, 147, e2021050531. [Google Scholar] [CrossRef]
- Ke, R.; Martinez, P.P.; Smith, R.L.; Gibson, L.L.; Mirza, A.; Conte, M.; Gallagher, N.; Luo, C.; Jarrett, J.; Zhou, R.; et al. Daily Longitudinal Sampling of SARS-CoV-2 Infection Reveals Substantial Heterogeneity in Infectiousness. Nat. Microbiol. 2022, 7, 640–652. [Google Scholar] [CrossRef]
- Hurst, J.H.; Heston, S.M.; Chambers, H.N.; Cunningham, H.M.; Price, M.J.; Suarez, L.; Crew, C.G.; Bose, S.; Aquino, J.N.; Carr, S.T.; et al. SARS-CoV-2 Infections Among Children in the Biospecimens from Respiratory Virus-Exposed Kids (BRAVE Kids) Study. Clin. Infect. Dis. 2020, 73, ciaa1693. [Google Scholar] [CrossRef]
- Ochoa, V.; Díaz, F.; Ramirez, E.; Fentini, M.; Carobene, M.; Geffner, J.; Arruvito, L.; Lenicov, F.; Group, I.; Adamczyk, A.; et al. Infants Younger than 6 Months Old Infected by SARS-CoV-2 Show the Highest Respiratory Viral Loads. J. Infect. Dis. 2021, 225, 392–395. [Google Scholar] [CrossRef]
- Krogh-Jensen, O.; Nikitina, I.; Donnikov, A.; Lenyushkina, A.; Degtyareva, N.; Degtyareva, A. Why Children Are Less Affected by COVID-19 Than Adults: Potential Immunological Factors and the Renin-Angiotensin System Associated Mechanisms. Preprints 2020, 2020060120. [Google Scholar] [CrossRef]
- Borrelli, M.; Corcione, A.; Castellano, F.; Nastro, F.F.; Santamaria, F. Coronavirus Disease 2019 in Children. Front. Pediatr. 2021, 9, 668484. [Google Scholar] [CrossRef]
- Warner, S.; Richter, A.; Stamataki, Z.; Kelly, D. Understanding COVID-19: Are Children the Key? BMJ Paediatr. Open 2021, 5, e001063. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Dowling, D.J.; Ahmed, S.; Choi, H.; Brightman, S.; Bergelson, I.; Berger, S.T.; Sauld, J.F.; Pettengill, M.; Kho, A.T.; et al. Adjuvant-Induced Human Monocyte Secretome Profiles Reveal Adjuvant- and Age-Specific Protein Signatures. Mol. Cell Proteom. 2016, 15, 1877–1894. [Google Scholar] [CrossRef]
- Burl, S.; Townend, J.; Njie-Jobe, J.; Cox, M.; Adetifa, U.J.; Touray, E.; Philbin, V.J.; Mancuso, C.; Kampmann, B.; Whittle, H.; et al. Age-Dependent Maturation of Toll-like Receptor-Mediated Cytokine Responses in Gambian Infants. PLoS ONE 2011, 6, e18185. [Google Scholar] [CrossRef]
- Lee, A.H.; Shannon, C.P.; Amenyogbe, N.; Bennike, T.B.; Diray-Arce, J.; Idoko, O.T.; Gill, E.E.; Ben-Othman, R.; Pomat, W.S.; van Haren, S.D.; et al. Dynamic Molecular Changes during the First Week of Human Life Follow a Robust Developmental Trajectory. Nat. Commun. 2019, 10, 1092. [Google Scholar] [CrossRef]
- England, R.; Pak, J.; Liu, M.; Rao, S.; Ozonoff, A.; Levy, O.; van Haren, S.D. Human Blood Plasma Shapes Distinct Neonatal TLR-Mediated Dendritic Cell Activation via Expression of the MicroRNA Let-7g. Immunohorizons 2021, 5, 246–256. [Google Scholar] [CrossRef] [PubMed]
- van Haren, S.D.; Ganapathi, L.; Bergelson, I.; Dowling, D.J.; Banks, M.; Samuels, R.C.; Reed, S.G.; Marshall, J.D.; Levy, O. In Vitro Cytokine Induction by TLR-Activating Vaccine Adjuvants in Human Blood Varies by Age and Adjuvant. Cytokine 2016, 83, 99–109. [Google Scholar] [CrossRef]
- Belderbos, M.E.; Levy, O.; Stalpers, F.; Kimpen, J.L.; Meyaard, L.; Bont, L. Neonatal Plasma Polarizes TLR4-Mediated Cytokine Responses towards Low IL-12p70 and High IL-10 Production via Distinct Factors. PLoS ONE 2012, 7, e33419. [Google Scholar] [CrossRef]
- Smolen, K.K.; Plotkin, A.L.; Shannon, C.P.; Idoko, O.T.; Pak, J.; Darboe, A.; van Haren, S.; Amenyogbe, N.; Tebbutt, S.J.; Kollmann, T.R.; et al. Ontogeny of Plasma Cytokine and Chemokine Concentrations across the First Week of Human Life. Cytokine 2021, 148, 155704. [Google Scholar] [CrossRef]
- Bennike, T.; Fatou, B.; Angelidou, A.; Diray-Arce, J.; Falsafi, R.; Ford, R.; Gill, E.E.; van Haren, S.D.; Idoko, O.T.; Lee, A.H.; et al. Preparing for Life: Plasma Proteome Changes and Immune System Development During the First Week of Human Life. Front. Immunol. 2020, 11, 578505. [Google Scholar] [CrossRef]
- Beijnen, E.M.; van Haren, S.D. Vaccine-Induced CD8+ T Cell Responses in Children: A Review of Age-Specific Molecular Determinants Contributing to Antigen Cross-Presentation. Front. Immunol. 2020, 11, 607977. [Google Scholar] [CrossRef] [PubMed]
- van Haren, S.D.; Dowling, D.J.; Foppen, W.; Christensen, D.; Andersen, P.; Reed, S.G.; Hershberg, R.M.; Baden, L.R.; Levy, O. Age-Specific Adjuvant Synergy: Dual TLR7/8 and Mincle Activation of Human Newborn Dendritic Cells Enables Th1 Polarization. J. Immunol. 2016, 197, 4413–4424. [Google Scholar] [CrossRef]
- Philbin, V.J.; Dowling, D.J.; Gallington, L.C.; Cortés, G.; Tan, Z.; Suter, E.E.; Chi, K.W.; Shuckett, A.; Stoler-Barak, L.; Tomai, M.; et al. Imidazoquinoline Toll-like Receptor 8 Agonists Activate Human Newborn Monocytes and Dendritic Cells through Adenosine-Refractory and Caspase-1-Dependent Pathways. J. Allergy Clin. Immun. 2012, 130, 195–204.e9. [Google Scholar] [CrossRef]
- Levy, O.; Suter, E.E.; Miller, R.L.; Wessels, M.R. Unique Efficacy of Toll-like Receptor 8 Agonists in Activating Human Neonatal Antigen-Presenting Cells. Blood 2006, 108, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Belderbos, M.E.; Levy, O.; Meyaard, L.; Bont, L. Plasma-mediated Immune Suppression: A Neonatal Perspective. Pediatr. Allergy Immunol. 2013, 24, 102–113. [Google Scholar] [CrossRef]
- Lemoine, S.; Jaron, B.; Tabka, S.; Ettreiki, C.; Deriaud, E.; Zhivaki, D.; Ray, C.; Launay, O.; Majlessi, L.; Tissieres, P.; et al. Dectin-1 Activation Unlocks IL12A Expression and Reveals the TH1 Potency of Neonatal Dendritic Cells. J. Allergy Clin. Immunol. 2015, 136, 1355–1368.e15. [Google Scholar] [CrossRef]
- Kraft, J.D.; Horzempa, J.; Davis, C.; Jung, J.; Peña, M.M.O.; Robinson, C.M. Neonatal Macrophages Express Elevated Levels of Interleukin-27 That Oppose Immune Responses. Immunology 2013, 139, 484–493. [Google Scholar] [CrossRef]
- Prescott, S.L.; Taylor, A.; King, B.; Dunstan, J.; Upham, J.W.; Thornton, C.A.; Holt, P.G. Neonatal Interleukin-12 Capacity Is Associated with Variations in Allergen-specific Immune Responses in the Neonatal and Postnatal Periods. Clin. Exp. Allergy 2003, 33, 566–572. [Google Scholar] [CrossRef]
- Upham, J.W.; Rate, A.; Rowe, J.; Kusel, M.; Sly, P.D.; Holt, P.G. Dendritic Cell Immaturity during Infancy Restricts the Capacity To Express Vaccine-Specific T-Cell Memory. Infect. Immun. 2006, 74, 1106–1112. [Google Scholar] [CrossRef]
- Rudd, B.D. Neonatal T Cells: A Reinterpretation. Annu. Rev. Immunol. 2020, 38, 229–247. [Google Scholar] [CrossRef]
- Doganci, A.; Birkholz, J.; Gehring, S.; Puhl, A.G.; Zepp, F.; Meyer, C.U. In the Presence of IL-21 Human Cord Blood T Cells Differentiate to IL-10-Producing Th1 but Not Th17 or Th2 Cells. Int. Immunol. 2013, 25, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; You, D.; Saravia, J.; Siefker, D.T.; Jaligama, S.; Lee, G.I.; Sallam, A.A.; Harding, J.N.; Cormier, S.A. IL-4Rα on Dendritic Cells in Neonates and Th2 Immunopathology in Respiratory Syncytial Virus Infection. J. Leukoc. Biol. 2017, 102, 153–161. [Google Scholar] [CrossRef]
- Chirkova, T.; Ha, B.; Rimawi, B.H.; Oomens, A.G.; Hartert, T.V.; Anderson, L.J. In Vitro Model for the Assessment of Human Immune Responses to Subunit RSV Vaccines. PLoS ONE 2020, 15, e0229660. [Google Scholar] [CrossRef]
- Jia, R.; Lu, L.; Liang, X.; Sun, Z.; Tan, L.; Xu, M.; Su, L.; Xu, J. Poly(U) and CpG Ameliorate the Unbalanced T Cell Immunity and Pneumonia of Mice with RSV Vaccine-Enhanced Disease. Biosci. Trends 2017, 11, 450–459. [Google Scholar] [CrossRef]
- Russell, C.D.; Unger, S.A.; Walton, M.; Schwarze, J. The Human Immune Response to Respiratory Syncytial Virus Infection. Clin. Microbiol. Rev. 2017, 30, 481–502. [Google Scholar] [CrossRef]
- van Haren, S.D.; Pedersen, G.K.; Kumar, A.; Ruckwardt, T.J.; Moin, S.; Moore, I.N.; Minai, M.; Liu, M.; Pak, J.; Borriello, F.; et al. CAF08 Adjuvant Enables Single Dose Protection against Respiratory Syncytial Virus Infection in Murine Newborns. Nat. Commun. 2022, 13, 4234. [Google Scholar] [CrossRef] [PubMed]
- Odumade, O.A.; van Haren, S.D.; Angelidou, A. Implications of the SARS-CoV-2 Pandemic on the Epidemiology of Pediatric Respiratory Syncytial Virus (RSV) Infection. Clin. Infect. Dis. 2022, 75, S130–S135. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of Spike Glycoprotein of SARS-CoV-2 on Virus Entry and Its Immune Cross-Reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef]
- Hikmet, F.; Méar, L.; Edvinsson, Å.; Micke, P.; Uhlén, M.; Lindskog, C. The Protein Expression Profile of ACE2 in Human Tissues. Mol. Syst. Biol. 2020, 16, e9610. [Google Scholar] [CrossRef]
- Murgolo, N.; Therien, A.G.; Howell, B.; Klein, D.; Koeplinger, K.; Lieberman, L.A.; Adam, G.C.; Flynn, J.; McKenna, P.; Swaminathan, G.; et al. SARS-CoV-2 Tropism, Entry, Replication, and Propagation: Considerations for Drug Discovery and Development. PLoS Pathog. 2021, 17, e1009225. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Li, Z.; Buck, M. Neuropilin-1 Assists SARS-CoV-2 Infection by Stimulating the Separation of Spike Protein S1 and S2. Biophys. J. 2021, 120, 2828–2837. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.-J.; Tortorici, A.M.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef] [PubMed]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; Logan, N.; Lorenzo, G.D.; Furnon, W.; Scott, S.; Manali, M.; Szemiel, A.; et al. The Hyper-Transmissible SARS-CoV-2 Omicron Variant Exhibits Significant Antigenic Change, Vaccine Escape and a Switch in Cell Entry Mechanism. Medrxiv 2022. [Google Scholar] [CrossRef]
- Fantini, J.; Yahi, N.; Colson, P.; Chahinian, H.; Scola, B.L.; Raoult, D. The Puzzling Mutational Landscape of the SARS-2-variant Omicron. J. Med. Virol. 2022, 94, 2019–2025. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, L.; Peng, Z.; Chen, L.-L.; Meng, X.; Zhang, C.; Ip, J.D.; Chan, W.-M.; Chu, A.W.-H.; Chan, K.-H.; et al. SARS-CoV-2 Omicron Variant Shows Less Efficient Replication and Fusion Activity When Compared with Delta Variant in TMPRSS2-Expressed Cells. Emerg. Microbes Infect. 2022, 11, 277–283. [Google Scholar] [CrossRef]
- Hu, Z.; Huang, X.; Zhang, J.; Fu, S.; Ding, D.; Tao, Z. Differences in Clinical Characteristics Between Delta Variant and Wild-Type SARS-CoV-2 Infected Patients. Front. Med. 2022, 8, 792135. [Google Scholar] [CrossRef]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk Factors and Disease Profile of Post-Vaccination SARS-CoV-2 Infection in UK Users of the COVID Symptom Study App: A Prospective, Community-Based, Nested, Case-Control Study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Shiehzadegan, S.; Alaghemand, N.; Fox, M.; Venketaraman, V. Analysis of the Delta Variant B.1.617.2 COVID-19. Clin. Pract. 2021, 11, 778–784. [Google Scholar] [CrossRef] [PubMed]
- WHO. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern (accessed on 16 February 2022).
- Wolter, N.; Jassat, W.; Walaza, S.; Welch, R.; Moultrie, H.; Groome, M.; Amoako, D.G.; Everatt, J.; Bhiman, J.N.; Scheepers, C.; et al. Early Assessment of the Clinical Severity of the SARS-CoV-2 Omicron Variant in South Africa: A Data Linkage Study. Lancet 2022, 399, 437–446. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Algarni, A.S.; Alamri, N.M.; Khayat, N.Z.; Alabdali, R.A.; Alsubhi, R.S.; Alghamdi, S.H. Clinical Practice Guidelines in Multisystem Inflammatory Syndrome (MIS-C) Related to COVID-19: A Critical Review and Recommendations. World J. Pediatr. 2022, 18, 83–90. [Google Scholar] [CrossRef]
- Wu, E.Y.; Campbell, M.J. Cardiac Manifestations of Multisystem Inflammatory Syndrome in Children (MIS-C) Following COVID-19. Curr. Cardiol. Rep. 2021, 23, 168. [Google Scholar] [CrossRef]
- Consiglio, C.R.; Cotugno, N.; Sardh, F.; Pou, C.; Amodio, D.; Rodriguez, L.; Tan, Z.; Zicari, S.; Ruggiero, A.; Pascucci, G.R.; et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020, 183, 968–981.e7. [Google Scholar] [CrossRef] [PubMed]
- Gurlevik, S.L.; Ozsurekci, Y.; Sağ, E.; Oygar, P.D.; Kesici, S.; Akca, Ü.K.; Cuceoglu, M.K.; Basaran, O.; Göncü, S.; Karakaya, J.; et al. The Difference of the Inflammatory Milieu in MIS-C and Severe COVID-19. Pediatr. Res. 2022, 92, 1805–1814. [Google Scholar] [CrossRef]
- Henderson, L.A.; Yeung, R.S.M. MIS-C: Early Lessons from Immune Profiling. Nat. Rev. Rheumatol. 2021, 17, 75–76. [Google Scholar] [CrossRef]
- Schuler, B.A.; Habermann, A.C.; Plosa, E.J.; Taylor, C.J.; Jetter, C.; Negretti, N.M.; Kapp, M.E.; Benjamin, J.T.; Gulleman, P.; Nichols, D.S.; et al. Age-Determined Expression of Priming Protease TMPRSS2 and Localization of SARS-CoV-2 in Lung Epithelium. J. Clin. Investig. 2020, 131. [Google Scholar] [CrossRef] [PubMed]
- Plaas, M.; Seppa, K.; Gaur, N.; Kasenõmm, P.; Plaas, M. Age- and Airway Disease Related Gene Expression Patterns of Key SARS-CoV-2 Entry Factors in Human Nasal Epithelia. Virology 2021, 561, 65–68. [Google Scholar] [CrossRef]
- Yeung, M.L.; Teng, J.L.L.; Jia, L.; Zhang, C.; Huang, C.; Cai, J.-P.; Zhou, R.; Chan, K.-H.; Zhao, H.; Zhu, L.; et al. Soluble ACE2-Mediated Cell Entry of SARS-CoV-2 via Interaction with Proteins Related to the Renin-Angiotensin System. Cell 2021, 184, 2212–2228.e12. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; An, Y. ACE2 Shedding and the Role in COVID-19. Front. Cell. Infect. Microbiol. 2022, 11, 789180. [Google Scholar] [CrossRef]
- del Maza, M.; Úbeda, M.; Delgado, P.; Horndler, L.; Llamas, M.A.; van Santen, H.M.; Alarcón, B.; Abia, D.; García-Bermejo, L.; Serrano-Villar, S.; et al. ACE2 Serum Levels as Predictor of Infectability and Outcome in COVID-19. Front. Immunol. 2022, 13, 836516. [Google Scholar] [CrossRef]
- Ciaglia, E.; Vecchione, C.; Puca, A. COVID-19 Infection and Circulating ACE2 Levels: Protective Role in Women and Children. Front. Pediatr. 2020, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Sharif-Askari, N.S.; Sharif-Askari, F.S.; Alabed, M.; Temsah, M.-H.; Heialy, S.A.; Hamid, Q.; Halwani, R. Airways Expression of SARS-CoV-2 Receptor, ACE2, and TMPRSS2 Is Lower in Children Than Adults and Increases with Smoking and COPD. Mol. Ther. Methods Clin. Dev. 2020, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA 2020, 323, 2427–2429. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.E.; Thurman, A.; Pezzulo, A.A.; Leidinger, M.R.; Klesney-Tait, J.A.; Karp, P.H.; Tan, P.; Wohlford-Lenane, C.; McCray, P.B.; Meyerholz, D.K. Heterogeneous Expression of the SARS-Coronavirus-2 Receptor ACE2 in the Human Respiratory Tract. Ebiomedicine 2020, 60, 102976. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Huang, L.; Zhang, C.; Luo, R.; Zeng, L.; Liang, H.; Li, Q.; Lu, X.; Wang, X.; et al. Distinct Disease Severity between Children and Older Adults with COVID-19: Impacts of ACE2 Expression, Distribution, and Lung Progenitor Cells. Clin. Infect. Dis. 2021, 73, e4154–e4165. [Google Scholar] [CrossRef]
- Chou, J.; Thomas, P.G.; Randolph, A.G. Immunology of SARS-CoV-2 Infection in Children. Nat. Immunol. 2022, 23, 177–185. [Google Scholar] [CrossRef]
- Bastolla, U.; Chambers, P.; Abia, D.; Garcia-Bermejo, M.-L.; Fresno, M. Is COVID-19 Severity Associated With ACE2 Degradation? Front. Drug Discov. 2022, 1, 789710. [Google Scholar] [CrossRef]
- Mostafa, G.A.; Ibrahim, H.M.; Shehab, A.A.S.; Magdy, S.M.; Soliman, N.A.; El-Sherif, D.F. Up-Regulated Serum Levels of Interleukin (IL)-17A and IL-22 in Egyptian Pediatric Patients with COVID-19 and MIS-C: Relation to the Disease Outcome. Cytokine 2022, 154, 155870. [Google Scholar] [CrossRef]
- Shaw, A.C.; Goldstein, D.R.; Montgomery, R.R. Age-Dependent Dysregulation of Innate Immunity. Nat. Rev. Immunol. 2013, 13, 875–887. [Google Scholar] [CrossRef]
- Bi, J. NK Cell Dysfunction in Patients with COVID-19. Cell. Mol. Immunol. 2022, 19, 127–129. [Google Scholar] [CrossRef]
- Sariol, A.; Perlman, S. SARS-CoV-2 Takes Its Toll. Nat. Immunol. 2021, 22, 801–802. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, D.; Gentili, V.; Rizzo, S.; Schiuma, G.; Beltrami, S.; Strazzabosco, G.; Fernandez, M.; Caccuri, F.; Caruso, A.; Rizzo, R. TLR3 and TLR7 RNA Sensor Activation during SARS-CoV-2 Infection. Microorganisms 2021, 9, 1820. [Google Scholar] [CrossRef]
- Panda, A.; Qian, F.; Mohanty, S.; van Duin, D.; Newman, F.K.; Zhang, L.; Chen, S.; Towle, V.; Belshe, R.B.; Fikrig, E.; et al. Age-Associated Decrease in TLR Function in Primary Human Dendritic Cells Predicts Influenza Vaccine Response. J. Immunol. 2010, 184, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Worlock, K.B.; Huang, N.; Lindeboom, R.G.; Butler, C.R.; Kumasaka, N.; Conde, C.; Mamanova, L.; Bolt, L.; Richardson, L.; et al. Local and Systemic Responses to SARS-CoV-2 Infection in Children and Adults. Nature 2022, 602, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.A.; Sy, S.; Galen, B.; Goldstein, D.Y.; Orner, E.P.; Keller, M.J.; Herold, K.C.; Herold, B.C. Natural Mucosal Barriers and COVID-19 in Children. JCI Insight 2021, 6, e148694. [Google Scholar] [CrossRef]
- Lee, J.; Shin, E.-C. The Type I Interferon Response in COVID-19: Implications for Treatment. Nat. Rev. Immunol. 2020, 20, 585–586. [Google Scholar] [CrossRef]
- Dai, J.; Wang, Y.; Wang, H.; Gao, Z.; Wang, Y.; Fang, M.; Shi, S.; Zhang, P.; Wang, H.; Su, Y.; et al. Toll-Like Receptor Signaling in Severe Acute Respiratory Syndrome Coronavirus 2-Induced Innate Immune Responses and the Potential Application Value of Toll-Like Receptor Immunomodulators in Patients With Coronavirus Disease 2019. Front. Microbiol. 2022, 13, 948770. [Google Scholar] [CrossRef]
- Zeng, C.; Evans, J.P.; King, T.; Zheng, Y.-M.; Oltz, E.M.; Whelan, S.P.J.; Saif, L.J.; Peeples, M.E.; Liu, S.-L. SARS-CoV-2 Spreads through Cell-to-Cell Transmission. Proc. Natl. Acad. Sci. USA 2022, 119, e2111400119. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Dowell, A.C.; Butler, M.S.; Jinks, E.; Tut, G.; Lancaster, T.; Sylla, P.; Begum, J.; Bruton, R.; Pearce, H.; Verma, K.; et al. Children Develop Robust and Sustained Cross-Reactive Spike-Specific Immune Responses to SARS-CoV-2 Infection. Nat. Immunol. 2022, 23, 40–49. [Google Scholar] [CrossRef]
- Cohen, C.A.; Li, A.P.Y.; Hachim, A.; Hui, D.S.C.; Kwan, M.Y.W.; Tsang, O.T.Y.; Chiu, S.S.; Chan, W.H.; Yau, Y.S.; Kavian, N.; et al. SARS-CoV-2 Specific T Cell Responses Are Lower in Children and Increase with Age and Time after Infection. Nat. Commun. 2021, 12, 4678. [Google Scholar] [CrossRef]
- Pierce, C.A.; Preston-Hurlburt, P.; Dai, Y.; Aschner, C.; Cheshenko, N.; Galen, B.; Garforth, S.J.; Herrera, N.G.; Jangra, R.K.; Morano, N.C.; et al. Immune Responses to SARS-CoV-2 Infection in Hospitalized Pediatric and Adult Patients. Sci. Transl. Med. 2020, 12, eabd5487. [Google Scholar] [CrossRef] [PubMed]
- Kalfaoglu, B.; Almeida-Santos, J.; Tye, C.; Satou, Y.; Ono, M. T-Cell Dysregulation in COVID-19. Biochem. Biophys. Res. Commun. 2020, 538, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Kaaijk, P.; Pimentel, V.O.; Emmelot, M.E.; Poelen, M.C.M.; Cevirgel, A.; Schepp, R.M.; den Hartog, G.; Reukers, D.F.M.; Beckers, L.; van Beek, J.; et al. Children and Adults With Mild COVID-19: Dynamics of the Memory T Cell Response up to 10 Months. Front. Immunol. 2022, 13, 817876. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Picard, M.; Cezar, R.; Roux-Dalvai, F.; Alleaume-Butaux, A.; Soundaramourty, C.; Cruz, A.; Mendes-Frias, A.; Gotti, C.; Leclercq, M.; et al. T Cell Apoptosis Characterizes Severe COVID-19 Disease. Cell Death Differ. 2022, 29, 1486–1499. [Google Scholar] [CrossRef]
- Yang, Y.; Kuang, L.; Li, L.; Wu, Y.; Zhong, B.; Huang, X. Distinct Mitochondria-Mediated T-Cell Apoptosis Responses in Children and Adults With Coronavirus Disease 2019. J. Infect. Dis. 2021, 224, 1333–1344. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and Immunologic Features in Severe and Moderate Coronavirus Disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Brodin, P. SARS-CoV-2 Infections in Children: Understanding Diverse Outcomes. Immunity 2022, 55, 201–209. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Connors, T.J.; Zhu, Y.; Baldwin, M.R.; Lin, W.-H.; Wontakal, S.; Szabo, P.A.; Wells, S.B.; Dogra, P.; Gray, J.; et al. Distinct Antibody Responses to SARS-CoV-2 in Children and Adults across the COVID-19 Clinical Spectrum. Nat. Immunol. 2021, 22, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Costa, V.; Racine-Brzostek, S.E.; Acker, K.P.; Yee, J.; Chen, Z.; Karbaschi, M.; Zuk, R.; Rand, S.; Sukhu, A.; et al. Association of Age With SARS-CoV-2 Antibody Response. JAMA Netw. Open 2021, 4, e214302. [Google Scholar] [CrossRef] [PubMed]
- Toh, Z.; Anderson, J.; Mazarakis, N.; Neeland, M.; Higgins, R.A.; Rautenbacher, K.; Dohle, K.; Nguyen, J.; Overmars, I.; Donato, C.; et al. Comparison of Seroconversion in Children and Adults With Mild COVID-19. JAMA Netw. Open 2022, 5, e221313. [Google Scholar] [CrossRef]
- Chiara, C.D.; Cantarutti, A.; Costenaro, P.; Donà, D.; Bonfante, F.; Cosma, C.; Ferrarese, M.; Cozzani, S.; Petrara, M.R.; Carmona, F.; et al. Long-Term Immune Response to SARS-CoV-2 Infection Among Children and Adults After Mild Infection. JAMA Netw. Open 2022, 5, e2221616. [Google Scholar] [CrossRef] [PubMed]
- Renk, H.; Dulovic, A.; Seidel, A.; Becker, M.; Fabricius, D.; Zernickel, M.; Junker, D.; Groß, R.; Müller, J.; Hilger, A.; et al. Robust and Durable Serological Response Following Pediatric SARS-CoV-2 Infection. Nat. Commun. 2022, 13, 128. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Faulkner, N.; Cornish, G.H.; Rosa, A.; Harvey, R.; Hussain, S.; Ulferts, R.; Earl, C.; Wrobel, A.G.; Benton, D.J.; et al. Preexisting and de Novo Humoral Immunity to SARS-CoV-2 in Humans. Science 2020, 370, 1339–1343. [Google Scholar] [CrossRef]
- Selva, K.J.; van de Sandt, C.E.; Lemke, M.M.; Lee, C.Y.; Shoffner, S.K.; Chua, B.Y.; Davis, S.K.; Nguyen, T.H.O.; Rowntree, L.C.; Hensen, L.; et al. Systems Serology Detects Functionally Distinct Coronavirus Antibody Features in Children and Elderly. Nat. Commun. 2021, 12, 2037. [Google Scholar] [CrossRef]
- Tian, X.; Bai, Z.; Cao, Y.; Liu, H.; Liu, D.; Liu, W.; Li, J. Evaluation of Clinical and Immune Responses in Recovered Children with Mild COVID-19. Viruses 2022, 14, 85. [Google Scholar] [CrossRef]
- Moratto, D.; Giacomelli, M.; Chiarini, M.; Savarè, L.; Saccani, B.; Motta, M.; Timpano, S.; Poli, P.; Paghera, S.; Imberti, L.; et al. Immune Response in Children with COVID-19 Is Characterized by Lower Levels of T-cell Activation than Infected Adults. Eur. J. Immunol. 2020, 50, 1412–1414. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Q.; Sun, D.; Wu, Z.; Peng, H.; Liu, X.; Liu, Y. Differences in Immune Responses between Children and Adults with COVID-19. Curr. Med. Sci. 2021, 41, 58–61. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Zhu, B.; Liang, H.; Fang, C.; Gong, Y.; Guo, Q.; Sun, X.; Zhao, D.; Shen, J.; et al. Characteristics of Pediatric SARS-CoV-2 Infection and Potential Evidence for Persistent Fecal Viral Shedding. Nat. Med. 2020, 26, 502–505. [Google Scholar] [CrossRef]
- Du, W.; Yu, J.; Wang, H.; Zhang, X.; Zhang, S.; Li, Q.; Zhang, Z. Clinical Characteristics of COVID-19 in Children Compared with Adults in Shandong Province, China. Infection 2020, 48, 445–452. [Google Scholar] [CrossRef]
- Li, H.; Chen, K.; Liu, M.; Xu, H.; Xu, Q. The Profile of Peripheral Blood Lymphocyte Subsets and Serum Cytokines in Children with 2019 Novel Coronavirus Pneumonia. J. Infect. 2020, 81, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Curatola, A.; Chiaretti, A.; Ferretti, S.; Bersani, G.; Lucchetti, D.; Capossela, L.; Sgambato, A.; Gatto, A. Cytokine Response to SARS-CoV-2 Infection in Children. Viruses 2021, 13, 1868. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, Y.; Xu, Y.; Hu, W.; Hall, I.P.; Yue, J.; Lu, H.; Ruan, L.; Ye, M.; Mei, J. Reduced Inflammatory Responses to SARS-CoV-2 Infection in Children Presenting to Hospital with COVID-19 in China. Eclinicalmedicine 2021, 34, 100831. [Google Scholar] [CrossRef] [PubMed]
- Fara, A.; Mitrev, Z.; Rosalia, R.A.; Assas, B.M. Cytokine Storm and COVID-19: A Chronicle of pro-Inflammatory Cytokines. Open Biol. 2020, 10, 200160. [Google Scholar] [CrossRef]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling Serum Cytokines in COVID-19 Patients Reveals IL-6 and IL-10 Are Disease Severity Predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, Y.; Ling, Y.; Lu, G.; Liu, F.; Yi, Z.; Jia, X.; Wu, M.; Shi, B.; Xu, S.; et al. Viral and Host Factors Related to the Clinical Outcome of COVID-19. Nature 2020, 583, 437–440. [Google Scholar] [CrossRef]
- Ozger, H.S.; Karakus, R.; Kuscu, E.N.; Bagriacik, U.E.; Oruklu, N.; Yaman, M.; Turkoglu, M.; Erbas, G.; Atak, A.Y.; Senol, E. Serial Measurement of Cytokines Strongly Predict COVID-19 Outcome. PLoS ONE 2021, 16, e0260623. [Google Scholar] [CrossRef]
- Ghazavi, A.; Ganji, A.; Keshavarzian, N.; Rabiemajd, S.; Mosayebi, G. Cytokine Profile and Disease Severity in Patients with COVID-19. Cytokine 2021, 137, 155323. [Google Scholar] [CrossRef]
- Ozsurekci, Y.; Aykac, K.; Er, A.G.; Halacli, B.; Arasli, M.; Oygar, P.D.; Gürlevik, S.; Yayla, B.C.C.; Karakaya, J.; Alp, A.; et al. Predictive Value of Cytokine/Chemokine Responses for the Disease Severity and Management in Children and Adult Cases with COVID-19. J. Med. Virol. 2020, 93, 2828–2837. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal Analyses Reveal Immunological Misfiring in Severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Gadotti, A.C.; de Castro Deus, M.; Telles, J.P.; Wind, R.; Goes, M.; Ossoski, R.G.C.; de Padua, A.M.; de Noronha, L.; Moreno-Amaral, A.; Baena, C.P.; et al. IFN-γ Is an Independent Risk Factor Associated with Mortality in Patients with Moderate and Severe COVID-19 Infection. Virus Res. 2020, 289, 198171. [Google Scholar] [CrossRef] [PubMed]
- Do, L.A.H.; Anderson, J.; Mulholland, E.K.; Licciardi, P.V. Can Data from Paediatric Cohorts Solve the COVID-19 Puzzle? PLoS Pathog. 2020, 16, e1008798. [Google Scholar] [CrossRef]
- Blavatnik School of Government; University of Oxford. COVID-19 Government Response Tracker. Available online: https://www.bsg.ox.ac.uk/research/covid-19-government-response-tracker (accessed on 8 February 2023).
- Frenck, R.W.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. New. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Hause, A.M.; Baggs, J.; Marquez, P.; Myers, T.R.; Gee, J.; Su, J.R.; Zhang, B.; Thompson, D.; Shimabukuro, T.T.; Shay, D.K. COVID-19 Vaccine Safety in Children Aged 5–11 Years—United States, November 3–December 19, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1755–1760. [Google Scholar] [CrossRef]
- Oliveira, C.R.; Niccolai, L.M.; Sheikha, H.; Elmansy, L.; Kalinich, C.C.; Grubaugh, N.D.; Shapiro, E.D.; Initiative, Y.; Billig, K.; Breban, M.I.; et al. Assessment of Clinical Effectiveness of BNT162b2 COVID-19 Vaccine in US Adolescents. JAMA Netw. Open 2022, 5, e220935. [Google Scholar] [CrossRef]
- Creech, C.B.; Anderson, E.; Berthaud, V.; Yildirim, I.; Atz, A.M.; Baez, I.M.; Finkelstein, D.; Pickrell, P.; Kirstein, J.; Yut, C.; et al. Evaluation of MRNA-1273 COVID-19 Vaccine in Children 6 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 2011–2023. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; Chua, G.T.; Lu, L.; Chan, B.; Wong, J.; Chow, C.; Yu, T.-C.; Leung, A.; Lam, S.-Y.; Wong, T.-W.; et al. Omicron Variant Susceptibility to Neutralizing Antibodies Induced in Children by Natural SARS-CoV-2 Infection or COVID-19 Vaccine. Emerg. Microbes Infect. 2022, 11, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Sacco, C.; Manso, M.; Mateo-Urdiales, A.; Rota, M.; Petrone, D.; Riccardo, F.; Bella, A.; Siddu, A.; Battilomo, S.; Proietti, V.; et al. Effectiveness of BNT162b2 Vaccine against SARS-CoV-2 Infection and Severe COVID-19 in Children Aged 5–11 Years in Italy: A Retrospective Analysis of January–April, 2022. Lancet Lond. Engl. 2022, 400, 97–103. [Google Scholar] [CrossRef]
- Dorabawila, V.; Hoefer, D.; Bauer, U.E.; Bassett, M.T.; Lutterloh, E.; Rosenberg, E.S. Risk of Infection and Hospitalization Among Vaccinated and Unvaccinated Children and Adolescents in New York After the Emergence of the Omicron Variant. JAMA 2022, 327, 2242–2244. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, L.; Mo, M.; Liu, T.; Wu, C.; Gong, C.; Lu, K.; Gong, L.; Zhu, W.; Xu, Z. SARS-CoV-2 Omicron RBD Shows Weaker Binding Affinity than the Currently Dominant Delta Variant to Human ACE2. Signal Transduct. Target. Ther. 2022, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Fowlkes, A.L.; Yoon, S.K.; Lutrick, K.; Gwynn, L.; Burns, J.; Grant, L.; Phillips, A.L.; Ellingson, K.; Ferraris, M.V.; LeClair, L.B.; et al. Effectiveness of 2-Dose BNT162b2 (Pfizer BioNTech) MRNA Vaccine in Preventing SARS-CoV-2 Infection Among Children Aged 5–11 Years and Adolescents Aged 12–15 Years—PROTECT Cohort, July 2021–February 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2021, 386, 35–46. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. MRNA Vaccines Induce Durable Immune Memory to SARS-CoV-2 and Variants of Concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef] [PubMed]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody Persistence through 6 Months after the Second Dose of MRNA-1273 Vaccine for COVID-19. N. Engl. J. Med. 2021, 384, 2259–2261. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Scott, M.K.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.; Lai, L.; et al. Systems Vaccinology of the BNT162b2 MRNA Vaccine in Humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef]
- Li, C.; Lee, A.; Grigoryan, L.; Arunachalam, P.S.; Scott, M.K.; Trisal, M.; Wimmers, F.; Sanyal, M.; Weidenbacher, P.A.; Feng, Y.; et al. Mechanisms of Innate and Adaptive Immunity to the Pfizer-BioNTech BNT162b2 Vaccine. Nat. Immunol. 2022, 23, 543–555. [Google Scholar] [CrossRef]
- Pettengill, M.A.; van Haren, S.D.; Li, N.; Dowling, D.J.; Bergelson, I.; Jans, J.; Ferwerda, G.; Levy, O. Distinct TLR-Mediated Cytokine Production and Immunoglobulin Secretion in Human Newborn Naïve B Cells. Innate Immun. 2016, 22, 433–443. [Google Scholar] [CrossRef]
- Weerkamp, F.; de Haas, E.; Naber, B.; Comans-Bitter, M.W.; Bogers, A.; van Dongen, J.; Staal, F. Age-Related Changes in the Cellular Composition of the Thymus in Children. J. Allergy Clin. Immunol. 2005, 115, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Harpur, C.M.; Piers, A.T.; Auladell, M.; Perriman, L.; Li, S.; An, K.; Anderson, J.; Berzins, S.P.; Licciardi, P.V.; et al. A High-dimensional Cytometry Atlas of Peripheral Blood over the Human Life Span. Immunol. Cell Biol. 2022, 100, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, T.R.; Levy, O.; Montgomery, R.R.; Goriely, S. Innate Immune Function by Toll-like Receptors: Distinct Responses in Newborns and the Elderly. Immunity 2012, 37, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Reikie, B.A.; Adams, R.C.; Ruck, C.E.; Ho, K.; Leligdowicz, A.; Pillay, S.; Naidoo, S.; Fortuno, E.S.; de Beer, C.; Preiser, W.; et al. Ontogeny of Toll-Like Receptor Mediated Cytokine Responses of South African Infants throughout the First Year of Life. PLoS ONE 2012, 7, e44763. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beijnen, E.M.S.; Odumade, O.A.; Haren, S.D.v. Molecular Determinants of the Early Life Immune Response to COVID-19 Infection and Immunization. Vaccines 2023, 11, 509. https://doi.org/10.3390/vaccines11030509
Beijnen EMS, Odumade OA, Haren SDv. Molecular Determinants of the Early Life Immune Response to COVID-19 Infection and Immunization. Vaccines. 2023; 11(3):509. https://doi.org/10.3390/vaccines11030509
Chicago/Turabian StyleBeijnen, Elisabeth M. S., Oludare A. Odumade, and Simon D. van Haren. 2023. "Molecular Determinants of the Early Life Immune Response to COVID-19 Infection and Immunization" Vaccines 11, no. 3: 509. https://doi.org/10.3390/vaccines11030509
APA StyleBeijnen, E. M. S., Odumade, O. A., & Haren, S. D. v. (2023). Molecular Determinants of the Early Life Immune Response to COVID-19 Infection and Immunization. Vaccines, 11(3), 509. https://doi.org/10.3390/vaccines11030509






