Cellular and Molecular Mechanisms of Pathogenic and Protective Immune Responses to SARS-CoV-2 and Implications of COVID-19 Vaccines
Abstract
1. Introduction—Summary of the COVID-19 Pandemic
2. The Role of Host Immunity during SARS-CoV-2 Infection and Genesis of COVID-19
3. Diverse Clinical Presentations of COVID-19 and the Role of Host Immunity
4. The Role of Local and Systemic Host Immune Responses to SARS-CoV-2
5. The Pathogenic Role of Immune Response to SARS-CoV-2—Cytokine Storms and Severe COVID, Pneumonia, and Multiorgan Failure
6. Possible Mechanisms Underlying Failure of Containing SARS-CoV-2 in Nasal Mucosa and Development of Serious Illnesses
6.1. Mechanisms Underlying Failure of Containing SARS-CoV-2 in Nasal Mucosa
6.2. Mechanisms Underlying Development of Severe COVID-19 and Role of Immunity
7. Protective Immunity during SARS-CoV-2 Infection
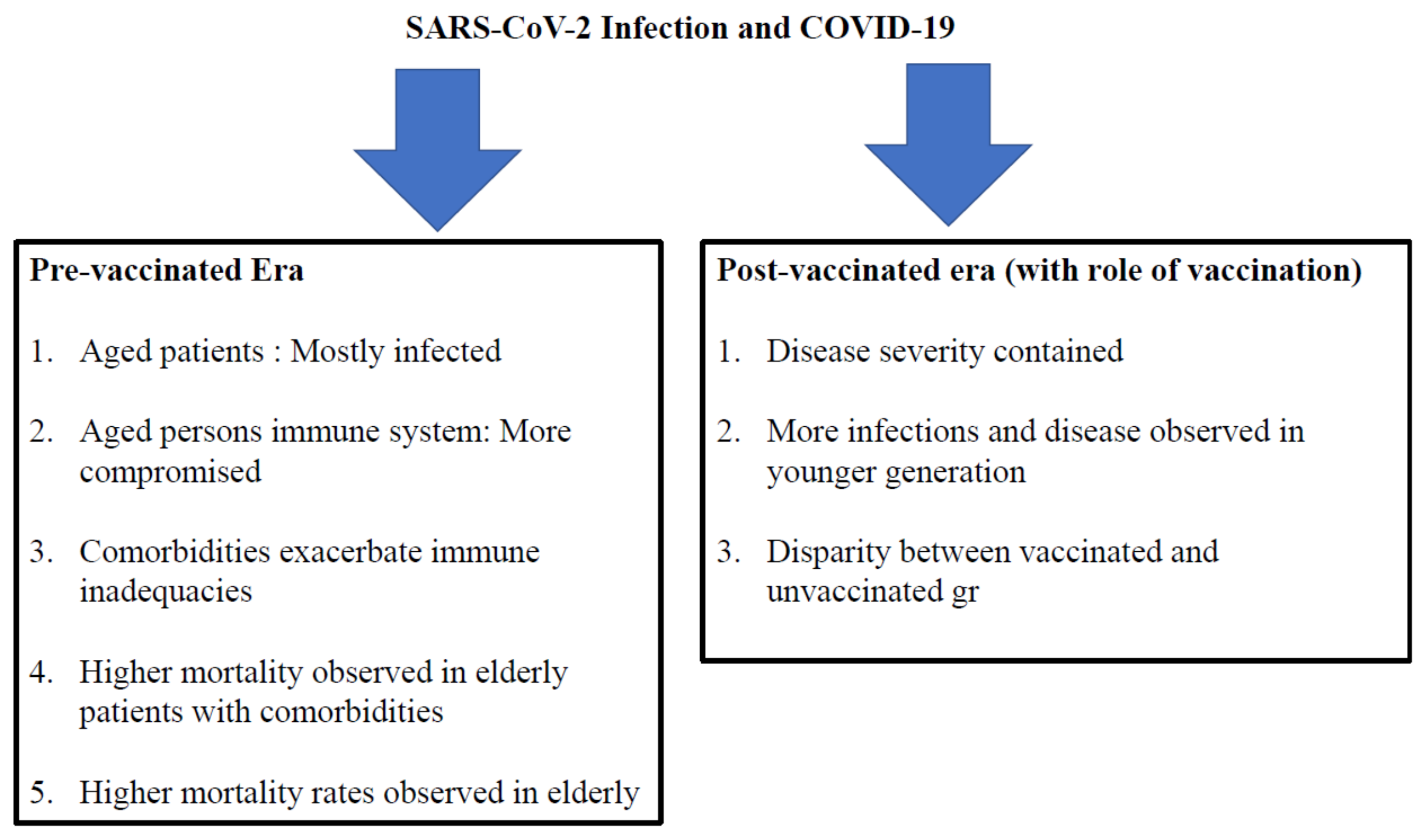
7.1. During the Pre-Vaccinated Era
7.2. Insights into COVID-19 during the Post-Vaccination Era
8. Vaccine Breakthrough Infection
9. Handling a Pandemic with Properties of a Double-Edged Sword
10. Queries from Scientists, Physicians, General Population, and Policy Makers Regarding SARS-CoV-2 and COVID-19
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chang, J.; Chen, S.; Wang, L.; Yau, T.O.; Zhao, Q.; Hong, Z.; Ruan, J.; Duan, G.; Gao, S. Genomic feature analysis of Betacoronavirus provides insights into SARS and COVID-19 pandemics. Front. Microbiol. 2021, 12, 614494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization. Naming the Coronavirus Disease (COVID-19) and the Virus That Causes It. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (accessed on 21 October 2022).
- Coronavirus Update. Worldometer. Available online: https://www.worldometers.info/coronavirus/ (accessed on 21 October 2022).
- Gopalan, A.; Tyagi, H. How reliable are test numbers for revealing the COVID-19 ground truth and applying Interventions? J. Indian Inst. Sci. 2020, 100, 863–884. [Google Scholar] [CrossRef] [PubMed]
- Jewell, N.P.; Lewnard, J.A. On the use of the reproduction number for SARS-CoV-2: Estimation, misinterpretations and relationships with other ecological measures. J. R. Stat. Soc. Ser. A Stat. Soc. 2022, 1–12. [Google Scholar] [CrossRef]
- COVID-19 Cases in Tokyo Likely 4 Times the Official Count. Available online: https://www.asahi.com/ajw/articles/14481175 (accessed on 21 October 2022).
- COVID-19: India’s Holiest River Is Swollen with Bodies. Available online: https://www.bbc.com/news/world-asia-india-57154564 (accessed on 21 October 2022).
- COVID-19 (PubMed). Available online: https://pubmed.ncbi.nlm.nih.gov/?term=COVID-19&sort=date&size=200 (accessed on 21 October 2022).
- Gu, H.; Chu, D.K.W.; Peiris, M.; Poon, L.L.M. Multivariate analyses of codon usage of SARS-CoV-2 and other betacoronaviruses. Virus Evol. 2020, 6, veaa032. [Google Scholar] [CrossRef]
- Murgolo, N.; Therien, A.G.; Howell, B.; Klein, D.; Koeplinger, K.; Lieberman, L.A.; Adam, G.C.; Flynn, J.; McKenna, P.; Swaminathan, G.; et al. SARS-CoV-2 tropism, entry, replication, and propagation: Considerations for drug discovery and development. PLoS. Pathog. 2021, 17, e1009225. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2002, 426, 450–454. [Google Scholar] [CrossRef]
- Chen, L.; Li, X.; Chen, M.; Feng, Y.; Xiong, C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020, 116, 1097–1100. [Google Scholar] [CrossRef]
- Harmer, D.; Gilbert, M.; Borman, R.; Clark, K.L. Quantitative mRNA expression profiling of ACE2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2020, 532, 107–110. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nao, N.; Shirato, K.; Kawase, M.; Saito, S.; Takayama, I.; Nagata, N.; Sekizuka, T.; Katoh, H.; Kato, F.; et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA 2020, 117, 7001–7003. [Google Scholar] [CrossRef]
- Glowacka, I.; Bertram, S.; Muller, M.A.; Allen, P.; Soilleux, E.; Pfefferle, S.; Steffen, I.; Tsegaye, T.S.; He, Y.; Gnirss, K.; et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011, 85, 4122–4134. [Google Scholar] [CrossRef]
- Shulla, A.; Heald-Sargent, T.; Subramanya, G.; Zhao, J.; Perlman, S.; Gallagher, T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011, 85, 582–873. [Google Scholar] [CrossRef]
- Kanneganti, T.D. Intracellular innate immune receptors: Life inside the cell. Immunol. Rev. 2020, 297, 5–12. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kanneganti, T.D. Innate immunity: The first line of defense against SARS-CoV-2. Nat Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef]
- E Sousa, C.R. Activation of dendritic cells: Translating innate into adaptive immunity. Curr. Opin. Immunol. 2004, 16, 21–25. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Eisenbarth, S.C. Dendritic cell subsets in T cell programming: Location dictates function. Nat. Rev. Immunol. 2019, 19, 89–103. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Hemmi, H. Dendritic cells: Translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 2006, 311, 17–58. [Google Scholar] [PubMed]
- Konno, Y.; Kimura, I.; Urio, K.; Fukushi, M.; Irie, T.; Koyanagi, Y.; Sauter, D.; Gifford, R.; USFQ-COVID19 Consortium; Nakagawa, S.; et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020, 32, 108185. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020, 181, 1036–1045. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef]
- Bayati, A.; Kumar, R.; Francis, V.; McPherson, P.S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2021, 296, 100306. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Chlamydas, S.; Papavassiliou, A.G.; Piperi, C. Epigenetic mechanisms regulating COVID-19 infection. Epigenetics 2021, 16, 263–270. [Google Scholar] [CrossRef]
- Anifandis, G.; Tempest, H.G.; Oliva, R.; Swanson, G.M.; Simopoulou, M.; Easley, C.A.; Primig, M.; Messini, C.I.; Turek, P.J.; Sutovsky, P.; et al. COVID-19 and human reproduction: A pandemic that packs a serious punch. Syst. Biol. Reprod. Med. 2021, 67, 3–23. [Google Scholar] [CrossRef]
- Evans, J.P.; Liu, S.L. Role of host factors in SARS-CoV-2 entry. J. Biol. Chem. 2021, 297, 100847. [Google Scholar] [CrossRef]
- Parthasarathy, U.; Martinelli, R.; Vollmann, E.H.; Best, K.; Therien, A.G. The impact of DAMP-mediated inflammation in severe COVID-19 and related disorders. Biochem. Pharmacol. 2022, 195, 114847. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; COVID Human Genetic Effort; Cobat, A.; Casanova, J.L. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature 2022, 603, 587–598. [Google Scholar] [CrossRef]
- Stravalaci, M.; Pagani, I.; Paraboschi, E.M.; Pedotti, M.; Doni, A.; Scavello, F.; Mapelli, S.N.; Sironi, M.; Perucchini, C.; Varani, L. Recognition and inhibition of SARS-CoV-2 by humoral innate immunity pattern recognition molecules. Nat. Immunol. 2022, 23, 275–286. [Google Scholar] [CrossRef]
- Silva-Lagos, L.A.; Pillay, J.; van Meurs, M.; Smink, A.; van der Voort, P.H.J.; de Vos, P. DAMPening COVID-19 Severity by Attenuating Danger Signals. Front. Immunol. 2021, 12, 720192. [Google Scholar] [CrossRef]
- Park, A.; Iwasaki, A. Type I and type III interferons—Induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe 2020, 27, 870–878. [Google Scholar] [CrossRef]
- Nie, X.; Qian, L.; Sun, R.; Huang, B.; Dong, X.; Xiao, Q.; Zhang, Q.; Lu, T.; Yue, L.; Chen, S.; et al. Multi-organ proteomic landscape of COVID-19 autopsies. Cell 2021, 184, 775–791.e14. [Google Scholar] [CrossRef]
- Khanam, A.; Chua, J.V.; Kottilil, S. Immunopathology of Chronic Hepatitis B Infection: Role of Innate and Adaptive Immune Response in Disease Progression. Int. J. Mol. Sci. 2021, 22, 5497. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Tian, L.; Chen, Y. Cytokine-Mediated Immunopathogenesis of Hepatitis B Virus Infections. Clin. Rev. Allergy Immunol. 2016, 50, 41–54. [Google Scholar] [CrossRef]
- Chisari, F.V.; Ferrari, C. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 1995, 13, 29–60. [Google Scholar] [CrossRef]
- Akbar, S.M.; Horiike, N.; Onji, M.; Hino, O. Dendritic cells and chronic hepatitis virus carriers. Intervirology 2001, 44, 199–208. [Google Scholar] [CrossRef]
- Akbar, S.M.; Abe, M.; Masumoto, T.; Horiike, N.; Onji, M. Mechanism of action of vaccine therapy in murine hepatitis B virus carriers: Vaccine-induced activation of antigen presenting dendritic cells. J. Hepatol. 1999, 30, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.K.; Onji, M. Hepatitis B virus (HBV)-transgenic mice as an investigative tool to study immunopathology during HBV infection. Int. J. Exp. Pathol. 1998, 79, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Hanan, N.; Donald, R.L., Jr.; Park, I.-W.; Jones, H.P.; Mathew, S.O. The many faces of innate immunity in SARS-CoV-2 infection. Vaccines 2021, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Garcia-Telles, N.; Aggarwal, G.; Lavie, C.; Lippi, G.; Henry, B.M. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): Early report from the United States. Diagnosis 2020, 7, 91–96. [Google Scholar] [CrossRef]
- Gupta, R.; Ghosh, A.; Singh, A.K.; Misra, A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab. Syndr. 2020, 14, 211–212. [Google Scholar] [CrossRef]
- Ackermann, M.; Mentzer, S.J.; Jonigk, D. Visualization of SARS-CoV-2 in the Lung. Reply. N. Engl. J. Med. 2020, 383, 2689–2690. [Google Scholar]
- Bharat, A.; Querrey, M.; Markov, N.S.; Kim, S.; Kurihara, C.; Garza-Castillon, R.; Manerikar, A.; Shilatifard, A.; Tomic, R.; Politanska, Y.; et al. Lung transplantation for patients with severe COVID-19. Sci. Transl. Med. 2020, 12, eabe4282. [Google Scholar] [CrossRef]
- Delory, T.M.; Ziegler, C.G.; Heimberg, G.; Normand, R.; Yang, Y.; Segerstole, A.; Abbondaza, D.; Fleming, S.J.; Subramanian, A.; Montoro, D.; et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature 2021, 595, 107–113. [Google Scholar] [CrossRef]
- Jamal, S.J.E.; Pujadas, E.; Ramos, I.; Bryce, C.B.; Grimes, Z.M.; Amanat, F.; Tsankova, N.M.; Musa, Z.; Olson, S.; Salem, F.; et al. Tissue-based SARS-CoV-2 in fatal COVID-19 infections: Sustained direct viral-induced damage is not necessary to drive disease progression. Hum. Pathol. 2021, 114, 110–119. [Google Scholar] [CrossRef]
- Massoth, L.R.; Desai, N.; Szabolcs, A.; Harris, C.K.; Neyaz, A.; Crotty, R.; Chebib, I.; Rivera, M.N.; Sholl, L.M.; Stone, J.R.; et al. Comparison of RNA in situ hybridization and immunohistochemistry techniques for the detection and localization of SARS-CoV-2 in human tissues. Am. J. Surg. Pathol. 2021, 45, 14–24. [Google Scholar] [CrossRef]
- Best Rocha, A.; Stroberg, E.; Barton, L.M.; Duval, E.J.; Mukhopadhyay, S.; Yarid, N.; Caza, T.; Wilson, J.D.; Kenan, D.J.; Kuperman, M.; et al. Detection of SARS-CoV-2 in formalin-fixed paraffin-embedded tissue sections using commercially available reagents. Lab. Investig. J. Tech. Methods Pathol. 2020, 100, 1485–1489. [Google Scholar] [CrossRef]
- Dorward, D.A.; Russell, C.D.; Um, I.H.; Elshani, M.; Armstrong, S.D.; Penrice-Randal, R.; Millar, T.; Lerpiniere, C.E.; Tagliavini, G.; Hartley, C.S.; et al. Tissue-specific immunopathology in fatal SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2021, 203, 192–201. [Google Scholar] [CrossRef]
- Parasher, A. COVID-19: Current understanding of its pathophysiology, clinical presentation and treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef]
- Halpin, D.M.G.; Criner, G.J.; Papi, A.; Singh, D.; Anzueto, A.; Martinez, F.J.; Agusti, A.A.; Vogelmeier, C.F. Global Initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD Science Committee Report on COVID-19 and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2021, 203, 24–36. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. Post-COVID-19 syndrome: Epidemiology, diagnostic criteria and pathogenic mechanisms involved. Rev. Neurol. 2021, 72, 384–396. [Google Scholar]
- Mirbeyk, M.; Saghazadeh, A.; Rezaei, N. A systematic review of pregnant women with COVID-19 and their neonates. Arch. Gynecol. Obstet. 2021, 304, 5–38. [Google Scholar] [CrossRef]
- Carrillo-Larco, R.M.; Altez-Fernandez, C. Anosmia and dysgeusia in COVID-19: A systematic review. Wellcome Open Res. 2020, 5, 94. [Google Scholar] [CrossRef]
- Chang, T.H.; Chou, C.C.; Chang, L.Y. Effect of obesity and body mass index on coronavirus disease 2019 severity: A systematic review and meta-analysis. Obes. Rev. 2020, 2, e13089. [Google Scholar] [CrossRef]
- Fraser, J.; Mousley, J.; Testro, A.; Smibert, O.C.; Koshy, A.N. Clinical presentation, treatment, and mortality rate in liver transplant recipients with coronavirus disease 2019: A systematic review and quantitative analysis. Transplant. Proc. 2020, 52, 2676–2683. [Google Scholar] [CrossRef]
- Biagianti, B.; Di Liberto, A.; Nicolò Edoardo, A.; Lisi, I.; Nobilia, L.; de Ferrabonc, G.D.; Zanier, E.R.; Stocchetti, N.; Brambilla, P. Cognitive assessment in SARS-CoV-2 patients: A systematic review. Front. Aging Neurosci. 2022, 14, 909661. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Understanding the complexities of SARS-CoV2 infection and its immunology: A road to immune-based therapeutics. Int. Immunopharmacol. 2020, 88, 106980. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, J.; Gary, J.; Reagan-Steiner, S.; Estetter, L.B.; Tong, S.; Tao, Y.; Denison, A.M.; Lee, E.; DeLeon-Carnes, M.; Li, Y.; et al. Evidence of severe acute respiratory syndrome coronavirus 2 replication and tropism in the lungs, airways, and vascular endothelium of patients with fatal coronavirus disease 2019: An autopsy case series. J. Infect. Dis. 2021, 223, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Bussani, R.; Schneider, E.; Zentilin, L.; Collesi, C.; Ali, H.; Braga, L.; Volpe, M.C.; Colliva, A.; Zanconati, F.; Berlot, G.; et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine 2020, 61, 103104. [Google Scholar] [CrossRef]
- NIH COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 1 November 2022).
- Hou, Y.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H., 3rd; Kato, T.; Kato, T.; Lee, R.E.; Yount, B.L.; et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 2020, 182, 429–446.e14. [Google Scholar] [CrossRef]
- Hou, Y.J.; Chiba, S.; Halfmann, P.; Ehre, C.; Kuroda, M.; Dinnon, K.H., 3rd; Leist, S.R.; Schäfer, A.; Nakajima, N.; Takahashi, K.Y.J.; et al. SARS-CoV-2 D614G variant exhibits efficient replication ex vivo and transmission in vivo. Science 2020, 370, 1464–1468. [Google Scholar] [CrossRef]
- Ehre, C. SARS-CoV-2 infection of airway cells. N. Engl. J. Med. 2020, 383, 969. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, W.; Liu, Z.; Liang, C.; Wang, W.; Ye, F.; Huang, B.; Zhao, L.; Wang, H.; Zhou, W.; et al. Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat. Commun. 2020, 11, 3910. [Google Scholar] [CrossRef]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef]
- Shah, V.K.; Firmal, P.; Alam, A.; Ganguly, D.; Chattopadhyay, S. Overview of immune response during SARS-CoV-2 infection. Lessons from the past. Front. Immunol. 2020, 11, 1949. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, T.; Hassani, F.; Ghaffari, N.; Ebrahimi, B.; Yarahmadi, A.; Hassanzadeh, G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J. Mol. Histol. 2020, 51, 613–628. [Google Scholar] [CrossRef]
- Wang, Y.; Perlman, S. COVID-19: Inflammatory profile. Annu. Rev. Med. 2022, 73, 65–80. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Khunti, K.; Nafilyan, V.; Maddox, T.; Humberstone, B.; Diamond, I.; Banerjee, A. Post-covid syndrome in individuals admitted to hospital with COVID-19: Retrospective cohort study. BMJ 2021, 372, n693. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Tirelli, U.; Taibi, R.; Chirumbolo, S. Post COVID syndrome: A new challenge for medicine. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4422–4425. [Google Scholar]
- Li, X.; Ma, X. Acute respiratory failure in COVID-19: Is it “typical” ARDS? Crit. Care 2020, 24, 198. [Google Scholar] [CrossRef] [PubMed]
- SeyedAlinaghi, S.; Karimi, A.; Barzegary, A.; Mojdeganlou, H.; Vahedi, F.; Mirghaderi, S.P.; Shobeiri, P.; Ramezani, M.; Yousefi Konjdar, P.; Mirzapour, P.; et al. COVID-19 mortality in patients with immunodeficiency and its predictors: A systematic review. Eur. J. Med. Res. 2022, 27, 195. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.S.; Chen, B.; Ze, B.; Zhou, W.H. Human genetic basis of severe or critical illness in COVID-19. Front. Cell. Infect. Microbiol. 2022, 12, 963239. [Google Scholar] [CrossRef]
- Twitchell, D.K.; Christensen, M.B.; Hackett, G.; Morgentaler, A.; Saad, F.; Pastuszak, A.W. Examining Male Predominance of Severe COVID-19 Outcomes: A Systematic Review. Androg. Clin. Res. Ther. 2022, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Murata, D.; Azuma, K.; Tokisawa, S.; Tokito, T.; Hoshino, T. A case of cytokine release syndrome accompanied with COVID-19 infection during treatment with immune checkpoint inhibitors for non-small cell lung cancer. Thorac. Cancer 2022, 13, 2911–2914. [Google Scholar] [CrossRef]
- Tahsini Tekantapeh, S.; Ghojazadeh, M.; Ghamari, A.A.; Mohammadi, A.; Soleimanpour, H. Therapeutic and anti-inflammatory effects of baricitinib on mortality, ICU transfer, clinical improvement, and CRS-related laboratory parameters of hospitalized patients with moderate to severe COVID-19 pneumonia: A systematic review and meta-analysis. Expert Rev. Respir. Med. 2022, 16, 1109–1132. [Google Scholar] [CrossRef]
- Gonzalez-Rubio, J.; Navarro-Lopez, C.; Lopez-Najera, E.; Lopez-Najera, A.; Jimenez-Diaz, L.; Navarro-Lopez, J.D.; Najera, A. Cytokine Release Syndrome (CRS) and Nicotine in COVID-19 Patients: Trying to Calm the Storm. Front. Immunol. 2020, 11, 1359. [Google Scholar] [CrossRef]
- Iannaccone, G.; Scacciavillani, R.; Del Buono, M.G.; Camilli, M.; Ronco, C.; Lavie, C.J.; Abbate, A.; Crea, F.; Massetti, M.; Aspromonte, N. Weathering the cytokine storm in COVID-19: Therapeutic implications. Cardiorenal Med. 2020, 10, 277–287. [Google Scholar] [CrossRef]
- Zust, R.; Cervantes-Barragan, L.; Habjan, M.; Maier, R.; Neuman, B.W.; Ziebuhr, J.; Szretter, K.J.; Baker, S.C.; Barchet, W.; Diamond, M.S.; et al. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat. Immunol. 2011, 12, 137–143. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Drabek, D.; Okba, N.M.A.; van Haperen, R.; Osterhaus, A.D.M.E.; van Kuppeveld, F.J.M.; Haagmans, B.L.; Grosveld, F.; Bosch, B.J. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020, 11, 2251. [Google Scholar] [CrossRef]
- Taefehshokr, N.; Taefehshokr, S.; Hemmat, N.; Heit, B. COVID-19: Perspectives on innate immune evasion. Front. Immunol. 2020, 11, 580641. [Google Scholar] [CrossRef]
- Eskandarian Boroujeni, M.; Sekrecka, A.; Antonczyk, A.; Hassani, S.; Sekrecki, M.; Nowicka, H.; Lopacinska, N.; Olya, A.; Kluzek, K.; Wesoly, J.; et al. Dysregulated interferon response and immune hyperactivation in severe COVID-19: Targeting STATs as a novel therapeutic strategy. Front. Immunol. 2022, 13, 888897. [Google Scholar] [CrossRef]
- Shin, J.; Toyoda, S.; Nishitani, S.; Onodera, T.; Fukuda, S.; Kita, S.; Fukuhara, A.; Shimomura, I. SARS-CoV-2 infection impairs the insulin/IGF signaling pathway in the lung, liver, adipose tissue, and pancreatic cells via IRF1. Metabolism 2022, 133, 155236. [Google Scholar] [CrossRef]
- Mazzoni, A.; Salvati, L.; Maggi, L.; Annunziato, F.; Cosmi, L. Hallmarks of immune response in COVID-19: Exploring dysregulation and exhaustion. Semin. Immunol. 2021, 55, 101508. [Google Scholar] [CrossRef]
- Kim, Y.M.; Shin, Y.C. Type I and III interferon responses in SARS-CoV-2 infection. Exp. Mol. Med. 2021, 53, 750–760. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Latifi-Pupovci, H. Molecular mechanisms involved in pathogenicity of SARS-CoV-2: Immune evasion and implications for therapeutic strategies. Biomed. Biopharm. 2022, 133, 113368. [Google Scholar] [CrossRef]
- Haagmans, B.L.; Lamers, M. SARS-CoV-2 pathogenesis. Nat. Rev. Muicobiol. 2022, 20, 270–284. [Google Scholar]
- Mo, W.; Li, Q.; Zhou, H.; Shi, X.; Yang, H.; Xiao, Z.; Wei, J.; Lv, X. Bibliometric analysis of global research trends on pyroptosis in lung disease. Front. Immunol. 2022, 13, 978552. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Wu, P.; Wu, M.; Gan, L.; Jin, J.; Wu, R.; Liu, W.; Zhang, K.; Li, D.; et al. Complanatuside alleviates inflammatory cell damage induced by pro-inflammatory cytokines in skin keratinocytes. Front. Chem. 2022, 10, 909651. [Google Scholar] [CrossRef]
- Majchrzak, M.; Poręba, M. The roles of cellular protease interactions in viral infections and programmed cell death: A lesson learned from the SARS-CoV-2 outbreak and COVID-19 pandemic. Pharmacol. Rep. 2022, 74, 1149–1165. [Google Scholar] [CrossRef]
- Wang, M.; Chang, W.; Zhang, L.; Zhang, Y. Pyroptotic cell death in SARS-CoV-2 infection: Revealing its roles during the immunopathogenesis of COVID-19. Int. J. Biol. Sci. 2022, 18, 5827–5848. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, P.; Liu, Q.; Cao, L.; Xu, J. Pyroptotic Patterns in Blood Leukocytes Predict Disease Severity and Outcome in COVID-19 Patients. Front. Immunol. 2022, 13, 888661. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Soares, V.C.; de Azevedo-Quintanilha, I.G.; Dias, S.D.S.G.; Fintelman-Rodrigues, N.; Sacramento, C.Q.; Mattos, M.; de Freitas, C.S.; Temerozo, J.R.; Teixeira, L.; et al. SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes. Cell Death Discov. 2021, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Sefik, E.; Qu, R.; Junqueira, C.; Kaffe, E.; Mirza, H.; Zhao, J.; Brewer, J.R.; Han, A.; Steach, H.R.; Israelow, B.; et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature 2022, 606, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.S.; de Sa, K.S.G.; Ishimoto, A.; Becerra, A.; Oliveria, S.; Almeida, L.; Goncalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, L. Inflammasomes are activated in response to SARS-CoV-2 infection and associated with COVID-19 severity in patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef]
- Eisfeld, H.S.; Simonis, A.; Winter, S.; Chhen, J.; Ströh, L.J.; Krey, T.; Koch, M.; Theobald, S.J.; Rybniker, J. Viral glycoproteins induce NLRP3 inflammasome activation and pyroptosis in macrophages. Viruses 2021, 13, 2076. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, J.; Li, L.; Wang, J.; Zhao, Y.; Ma, H. Therapy targets SARS-CoV-2 infection-induced cell death. Front. Immunol. 2022, 13, 870216. [Google Scholar] [CrossRef]
- Bittner, Z.A.; Schrader, M.; George, S.E.; Amann, R. Pyroptosis and its role in SARS-CoV-2 infection. Cells 2022, 11, 1717. [Google Scholar] [CrossRef]
- Yin, M.; Marrne, L.; Peace, C.G.; O’Neill, L.A.J. NLPR3, the inflamasome and COVID-19 infection. QJM 2023. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef]
- Ulhaq, Z.S.; Soraya, G.V. Interleukin-6 as a potential biomarker of COVID-19 progression. Med. Mal. Infect. 2020, 50, 382–383. [Google Scholar] [CrossRef]
- Ing, A.J.; Cocks, C.; Green, P.J. COVID-19: In the footsteps of Ernest Shackleton. Thorax 2020, 75, 693–694. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, J.; Liu, K.; Kang, L.; Liu, R.; Jing, W.; Wu, Y.; Liu, M. Global Percentage of Asymptomatic SARS-CoV-2 Infections Among the Tested Population and Individuals With Confirmed COVID-19 Diagnosis. JAMA Netw. Open 2021, 4, e2137257. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fett, C.; Zhao, J.; Meyerholz, M.D.; Perlman, S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol. 2014, 88, 11034–11044. [Google Scholar] [CrossRef]
- Kirkcaldy, R.D.; King, B.A.; Books, J.T. COVID-19 and Postinfection Immunity: Limited Evidence, Many Remaining Questions. JAMA 2020, 323, 2245–2246. [Google Scholar] [CrossRef]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strlin, K.; Gorin, J.-B.; Olosson, A.; Llewllyn, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158–168.e14. [Google Scholar] [CrossRef]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, eabc8511. [Google Scholar] [CrossRef]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef]
- Salimi, S.; Hamlyn, J.M. COVID-19 and Crosstalk with the hallmarks of aging. J. Gerontol. A. Biol. Sci. Med. Sci. 2020, 75, e34–e41. [Google Scholar] [CrossRef]
- Lima-Martínez, M.M.; Carrera Boada, C.; Madera-Silva, M.D.; Marín, W.; Contreras, M. COVID-19 and diabetes: A bidirectional relationship. Clin. Investig. Arterioscler. 2021, 33, 151–157. [Google Scholar] [CrossRef]
- Pathania, A.S.; Prathipati, P.; Abdul, B.A.; Chava, S.; Katta, S.S.; Gupta, S.C.; Gangula, P.R.; Pandey, M.K.; Durden, D.L.; Byrareddy, S.N.; et al. COVID-19 and Cancer Comorbidity: Therapeutic Opportunities and Challenges. Theranostics 2021, 11, 731–753. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Bhadada, S.K. COVID-19 and diabetes mellitus: An unholy interaction of two pandemics. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Rubio Herrera, M.A.; Bretón Lesmes, I. Obesity in the COVID era: A global health challenge. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2021, 68, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar]
- Forchette, L.; Sebastian, W.; Liu, T. A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics. Curr. Med. Sci. 2021, 41, 1037–1051. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef]
- Hadj Hassine, I. COVID-19 Vaccines And Variants Of Concern: A Review. Rev. Med. Virol. 2022, 32, e2313. [Google Scholar] [CrossRef]
- Soiza, R.L.; Scicluna, C.; Thomson, E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing 2021, 50, 279–283. [Google Scholar] [CrossRef]
- Park, J.W.; Lagniton, P.N.P.; Liu, Y.; Xu, R.H. mRNA vaccines for COVID-19: What, why and how. Int. J. Biol. Sci. 2021, 17, 1446–1460. [Google Scholar] [CrossRef]
- Chung, J.Y.; Thone, M.N.; Kwon, Y.J. COVID-19 vaccines: The status and perspectives in delivery points of view. Adv. Drug Deliv. Rev. 2021, 170, 1–25. [Google Scholar] [CrossRef]
- Harder, T.; Külper-Schiek, W.; Reda, S.; Treskova-Schwarzbach, M.; Koch, J.; Vygen-Bonnet, S.; Wichmann, O. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection with the Delta (B.1.617.2) variant: Second interim results of a living systematic review and meta-analysis, 1 January to 25 August 2021. Eurosurveillance 2021, 26, 2100920. [Google Scholar] [CrossRef]
- Jiang, L.; Tang, K.; Levin, M.; Irfan, O.; Morris, S.K.; Wilson, K.; Klein, J.D.; Bhutta, Z.A. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020, 20, e276–e288. [Google Scholar] [CrossRef]
- Gupta, R.K.; Topol, E.J. COVID-19 vaccine breakthrough infections. Science 2021, 374, 1561–1562. [Google Scholar] [CrossRef]
- Amanatidou, E.; Gkiouliava, A.; Pella, E.; Serafidi, M.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Dalamaga, M. Breakthrough infections after COVID-19 vaccination: Insights, perspectives and challenges. Metab. Open 2022, 14, 100180. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus Disease (COVID-19): Herd Immunity, Lockdowns and COVID-19. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/herd-immunity-lockdowns-and-covid-19?gclid=CjwKCAjwh4ObBhAzEiwAHzZYU4tbL37LIPSV46T4vtGw69suA8cuH32eCkAYk8c5lk2EeDmQKuA5WhoCzLkQAvD_BwE (accessed on 2 November 2022).
- Levine-Tiefenbrun, M.; Yelin, I.; Katz, R.; Herzel, E.; Golan, Z.; Schreiber, L.; Wolf, T.; Nadler, V.; Ben-Tov, A.; Kuint, J.; et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat. Med. 2021, 27, 790–792. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, Y.; Sun, C.; Wang, X.; Guo, Y.; Qiu, S.; Ma, K. A systematic review of asymptomatic infections with COVID-19. J. Microbiol. Immunol. Infect. 2021, 54, 12–16. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Brodin, P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021, 27, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.J.; Tiderman, S.; Diaz, G.A.; French, T.; Parsons, G.T.; Robicsek, A. Comparative vaccine effectiveness against severe COVID-19 over time in US hospital administration data: A case-control study. Lancet Respir. Med. Lancet. 2022, 10, 557–565. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbar, S.M.F.; Al Mahtab, M.; Khan, S. Cellular and Molecular Mechanisms of Pathogenic and Protective Immune Responses to SARS-CoV-2 and Implications of COVID-19 Vaccines. Vaccines 2023, 11, 615. https://doi.org/10.3390/vaccines11030615
Akbar SMF, Al Mahtab M, Khan S. Cellular and Molecular Mechanisms of Pathogenic and Protective Immune Responses to SARS-CoV-2 and Implications of COVID-19 Vaccines. Vaccines. 2023; 11(3):615. https://doi.org/10.3390/vaccines11030615
Chicago/Turabian StyleAkbar, Sheikh Mohammad Fazle, Mamun Al Mahtab, and Sakirul Khan. 2023. "Cellular and Molecular Mechanisms of Pathogenic and Protective Immune Responses to SARS-CoV-2 and Implications of COVID-19 Vaccines" Vaccines 11, no. 3: 615. https://doi.org/10.3390/vaccines11030615
APA StyleAkbar, S. M. F., Al Mahtab, M., & Khan, S. (2023). Cellular and Molecular Mechanisms of Pathogenic and Protective Immune Responses to SARS-CoV-2 and Implications of COVID-19 Vaccines. Vaccines, 11(3), 615. https://doi.org/10.3390/vaccines11030615





