Abstract
An important issue that is often neglected is the difference between male and female genders in response to medical treatments. In the context of COVID-19 vaccine administration, despite identical protocol strategies, it has been observed that females often suffer more adverse consequences than males. Here, we analyzed the adverse events (AEs) of the Comirnaty vaccine in a population of 2385 healthcare workers as a function of age, sex, COVID-19 history and BMI. Using logistic regression analysis, we showed that these variables may contribute to the development of AEs, particularly in young subjects, females and individuals with a BMI below 25 kg/m2. Moreover, partial dependence plots indicate a 50% probability of developing a mild AE for a long period of time (≥7 days) or a severe AE of any duration in women below 40 years old and with a BMI < 20 kg/m2. As this effect is more evident after the second dose of the vaccine, we propose to reduce the amount of vaccine for any additional booster dose in relation to age, sex and BMI. This strategy might reduce adverse events without affecting vaccine efficacy.
1. Introduction
The impact of vaccines is influenced by both biological differences (sex) and social or cultural factors (gender) [1]. A growing number of studies have identified numerous immunological, genetic, hormonal and environmental factors that contribute to differences in vaccine responses and outcomes (such as efficacy and side-effects) based on sex and gender [2]. Cisgender females often show a more robust antibody response and, as a result, have higher efficacy and an increased likelihood of experiencing side-effects than males, highlighting the need for different dosing regimens based on sex [2,3]. For example, research on the influenza vaccine shows that women can achieve the same immunological response with a half-dose as men with a full dose [4]. Although sex clearly plays a role in COVID-19 disease and the vaccine response (including the immune response and likelihood of adverse events), few COVID-19 vaccine studies consider biological sex and perform sex-disaggregated analysis [5,6,7]. The SARS-CoV-2 mRNA vaccination (Comirnaty) has proven to be effective in protecting against hospitalization and death across the world [8,9]. The Comirnaty vaccine was administered in two doses (30 μg/dose) separated by at least 21 days in people 12 years of age and older. Although mRNA vaccines are particularly safe, they are not devoid of adverse events (AEs) [10,11,12,13]. In this study, we analyzed the incidence of AEs according to biological sex, age, COVID-19 history and body mass index (BMI) in response to the Comirnaty vaccination.
2. Materials and Methods
This clinical trial is a longitudinal study on 4227 healthcare workers (HCW) from 7 different healthcare facilities in Lombardy, Italy. The subjects were vaccinated with 2 doses of the Comirnaty vaccine between January and March 2021 and were asked to self-report any AEs on a questionnaire (see Supplementary Data). The reporting of adverse events followed the Italian and European reference legislation, in compliance with the Italian Strategic Plan for the anti-SARS-CoV-2/COVID-19 Vaccination and the Italian Medicines Agency (AIFA), which envisages the anonymous sharing of spontaneous reports through the IT platform of the National Pharmacovigilance Network provided by AIFA.
We considered all the variables of interest (sex, age, BMI and COVID-19 history) and the correct completion of the questionnaire. After excluding those patients with missing height and weight information, we analyzed 2385 subjects, of whom 13.8% had a COVID-19 history. We verified the validity of the analyzed population by evaluating the distribution of sex and COVID-19 history before and after removing patients with missing information. In particular, we observed that the incidence of female subjects was similar before (61.8%) and after the filtering procedure (60.8% for the first dose population and 62.2% for the second dose population). Similarly, the incidence of COVID-19 history was unchanged, equaling to 13.8% before and 12.4% (first dose population) and 13.9% (second dose population) after filtering, thus suggesting that we did not introduce any bias by the requirement of sex and BMI-related information.
The AEs after the first and second dose are reported in Table 1, p-values were calculated using the , and the significance threshold was set to 0.001 according to Bonferroni’s correction to account for multiple AEs testing. We used the to evaluate the association between categorical variables, the Shapiro–Wilk test to check for normality, the ANOVA or Kruskal–Wallis test to evaluate differences in BMI when stratifying for sex and AEs, and a logistic regression analysis to evaluate the probability of AEs in relation to BMI while correcting for age, sex and COVID-19 history. Statistical significance was set for p < 0.05. Model improvement after the inclusion of BMI was assessed through the likelihood ratio test, and AEs probability varying age and BMI was analyzed by partial dependence plots for any symptoms equal or above the score of 4 for either males or females. We attributed a score of either 1 or 4 for a duration of 1 or 2 days, 2 or 8 for a duration of 3 to 6 days, and 4 or 16 for a duration ≥ 7 days for either mild or severe AEs, respectively. The analyses were conducted with Python 3.8.3.

Table 1.
AEs to Comirnaty vaccine reported after the first and second dose. Significant AEs are highlighted in bold. * means Severe AE, according to pharmacovigilance guidelines.
3. Results
Table 1 reports the occurrence of mild or severe AEs in relation to sex, age, BMI and COVID-19 history after both doses of vaccine. Univariate testing of AEs after the first dose showed a significant association with local reactions at the injection site (p < 0.001). Local reactions at the injection site, asthenia, headache, muscle pain and joint pain AEs were significantly associated with the second dose (p < 0.001).
COVID-19 history was associated with an increased incidence of AEs only after the first dose (p < 0.001) and not after the second dose (p > 0.05). Importantly, the presence of AEs after the second dose was associated with AEs after the first dose (p < 0.001) in a matched subgroup receiving both administrations. When considering the whole population, BMI was associated with AEs incidence in both the first (p = 0.002) and second (p < 0.001) doses. In order to exclude sex effects in BMI, we separately tested male and female populations, showing that a lower BMI was linked to a higher incidence of AEs in males after the first dose (p = 0.0431), and in both males (p = 0.0482) and females (p = 0.0074) after the second dose (Figure 1A).
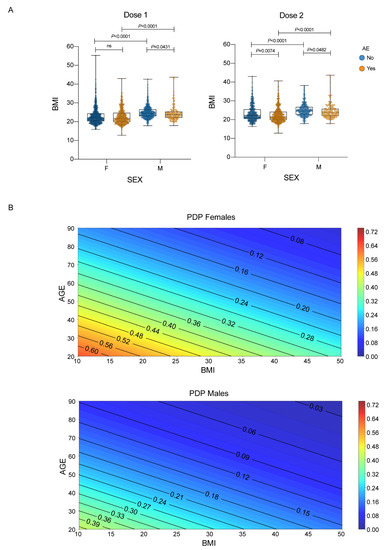
Figure 1.
Sex, age, and BMI and their association with AE. (A) BMI distributions stratified by sex and any adverse events. Each dot corresponds to an individual subject. The box plots show the interquartile range, the horizontal lines show the median values, and the whiskers indicate the minimum-to-maximum range. p-values were calculated using Kruskal–Wallis test, as data were not normally distributed according to Shapiro–Wilk test, followed by Dunn’s test after the first and second dose; (B) Partial dependence plots of the logistic regression model (with age, sex and BMI as covariates) showing the probability of having either a mild AE for 7 or more days or a severe AE of any duration after the second dose of vaccine stratified by sex. Female subjects show higher risk at the same age and BMI.
Table 2 shows the results of a logistic regression analysis, indicating that female and younger subjects are strongly associated with increased AEs after both vaccine doses (p < 0.001). For the first dose, there was no correlation between AEs and BMI. In contrast, BMI was inversely correlated with AEs after the second dose (p = 0.025), indicating that the second dose might be too high for young women with a low BMI. Additionally, having a history of COVID-19 is associated with increased AEs only for the first dose (p < 0.001). This implies that for individuals previously infected with SARS-CoV-2, the first dose may have a similar effect as the second dose for non-infected individuals.

Table 2.
Results of logistic regression after first and second dose. Features showing significant association with AEs after the first or second dose are highlighted in bold.
BMI inclusion significantly improved model performance after the second dose (p = 0.023, likelihood ratio test). Interestingly, the AEs that mostly correlated with a lower BMI (<25 kg/m2) were local reactions (after both doses) and headache, muscle and joint pain (only after the second dose). We then evaluated, through partial dependence plots, the model’s output, i.e., the probability of developing an AE equal or above the score of 4 (meaning either a mild symptom lasting 7 or more days or a severe symptom for any duration of time), taking into account age, sex and BMI after the second dose. The partial dependence plots in Figure 1B show that one out of two females (below 40 years old and BMI below 20 kg/m2) are at risk of having long-lasting mild or any duration severe adverse events. Males are less susceptible to score 4 AEs.
4. Discussion
A recent study analyzed data from four cross-sectional studies on the Comirnaty vaccine’s AEs and found that females experience more AEs compared to males at all ages [13]. In our study, we explored the development of AEs while taking into account not only biological sex and age but also COVID-19 history and BMI variables in a logistic regression analysis. Our findings show that the Comirnaty vaccine induces AEs, particularly in young subjects, females, and individuals with a BMI below 25 kg/m2. Women under 40 years old and with a BMI < 20 kg/m2 had a 50% probability of developing a mild AE lasting for at least 7 days or a severe AE of any duration, especially after the second dose of the vaccine. These results suggest that biological sex, age and BMI differences should be considered when determining vaccine doses for booster shots, with a possible reduction in the dose for women under 40 years old and with a BMI < 20 kg/m2.
Fractional dosing has been successfully used for various diseases. For example, between 2016 and 2018, some countries utilized 1/5 doses of yellow fever vaccine to control epidemics following guidance from the World Health Organization (WHO) [14]. Immunogenicity data combined with model-based analysis for COVID-19 vaccines, developed by W. Wiecek and collaborators, suggest that half or quarter doses of some vaccines could be nearly as effective as current doses and more effective than other vaccines in use [15]. Moreover, fractional doses may induce fewer side effects, as suggested by clinical data on the ChAdOx1 nCoV-19 (AZD1222) vaccine [16]. For the BNT162b2 vaccine, 10 μg and 20 μg doses are predicted to have an efficacy of 70–85%, compared to roughly 95% for the standard dose [15]. These predictions need confirmation, and a randomized trial of immunogenicity comparing low dose (20 μg) with a full dose of BNT162b2 was recently completed (https://clinicaltrials.gov/ct2/show/NCT04852861, estimated study completion date: 30 September 2022). Lower doses of BNT162b2 vaccine have been tested and used for children between 5 and 11 years old, which should not affect vaccine efficacy but significantly reduce adverse events [17]. Even if fractional doses are less effective than standard doses, epidemiological analysis indicates that increasing vaccination in countries still facing supply constraints would decrease overall infections and deaths [15]. Reducing the dosage would save vaccine doses and make them available to many low- and middle-income countries where the vaccine is not yet accessible. In these settings, reduced doses of mRNA vaccines could potentially be more efficacious than the standard of care. From an ethical point of view, it is important to evaluate the best dose of the Comirnaty vaccine in relation to age, biological sex and BMI to obtain maximal protection while avoiding adverse events, without jeopardizing vaccine efficacy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11030505/s1, Supplementary Data: Questionnaire.
Author Contributions
Conceptualization, M.R.; methodology, R.B., M.M. and L.U.; formal analysis, M.M. and L.U.; investigation, E.A., C.P. and M.M.; resources, E.A. and C.S.; data curation, C.P.; writing—original draft preparation, M.R.; supervision, M.R., R.B. and A.M.; project administration, E.A.; funding acquisition, M.R. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MINISTERO DELLA SALUTE, Ricerca Corrente.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board IRCCS Humanitas Research Hospital (ClinicalTrials.gov NCT04387929).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
According to the policy of the hosting Institution, data will be made available through Zenodo.
Acknowledgments
This work was supported by the Italian Ministry of Health (Ricerca Corrente). We would like to thank all the employees that volunteered to participate in this study, all the vaccinating doctors, the nurses, Humanitas Operations Management and Customer Care that coordinated vaccinations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- Flanagan, K.L.; Fink, A.L.; Plebanski, M.; Klein, S.L. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu. Rev. Cell Dev. Biol. 2017, 33, 577–599. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef]
- Engler, R.J.; Nelson, M.R.; Klote, M.M.; VanRaden, M.J.; Huang, C.Y.; Cox, N.J.; Klimov, A.; Keitel, W.A.; Nichol, K.L.; Carr, W.W.; et al. Half- vs. full-dose trivalent inactivated influenza vaccine (2004–2005): Age, dose, and sex effects on immune responses. Arch. Intern. Med. 2008, 168, 2405–2414. [Google Scholar] [CrossRef]
- Bignucolo, A.; Scarabel, L.; Mezzalira, S.; Polesel, J.; Cecchin, E.; Toffoli, G. Sex Disparities in Efficacy in COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2021, 9, 825. [Google Scholar] [CrossRef]
- Vassallo, A.; Shajahan, S.; Harris, K.; Hallam, L.; Hockham, C.; Womersley, K.; Woodward, M.; Sheel, M. Sex and Gender in COVID-19 Vaccine Research: Substantial Evidence Gaps Remain. Front. Glob. Womens Health 2021, 2, 761511. [Google Scholar] [CrossRef]
- Vijayasingham, L.; Bischof, E.; Wolfe, J. Sex-disaggregated data in COVID-19 vaccine trials. Lancet 2021, 397, 966–967. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Scobie, H.M.; Johnson, A.G.; Suthar, A.B.; Severson, R.; Alden, N.B.; Balter, S.; Bertolino, D.; Blythe, D.; Brady, S.; Cadwell, B.; et al. Monitoring Incidence of COVID-19 Cases, Hospitalizations, and Deaths, by Vaccination Status—13 U.S. Jurisdictions, April 4-July 17, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 1284–1290. [Google Scholar] [CrossRef]
- Ripabelli, G.; Tamburro, M.; Buccieri, N.; Adesso, C.; Caggiano, V.; Cannizzaro, F.; Di Palma, M.A.; Mantuano, G.; Montemitro, V.G.; Natale, A.; et al. Active Surveillance of Adverse Events in Healthcare Workers Recipients After Vaccination with COVID-19 BNT162b2 Vaccine (Pfizer-BioNTech, Comirnaty): A Cross-Sectional Study. J. Community Health 2021, 47, 211–225. [Google Scholar] [CrossRef]
- Amanzio, M.; Mitsikostas, D.D.; Giovannelli, F.; Bartoli, M.; Cipriani, G.E.; Brown, W.A. Adverse events of active and placebo groups in SARS-CoV-2 vaccine randomized trials: A systematic review. Lancet Reg. Health-Eur. 2021, 12, 100253. [Google Scholar] [CrossRef]
- Lim, S.M.; Chan, H.C.; Santosa, A.; Quek, S.C.; Liu, E.H.C.; Somani, J. Safety and side effect profile of Pfizer-BioNTech COVID-19 vaccination among healthcare workers: A tertiary hospital experience in Singapore. Ann. Acad. Med. Singap. 2021, 50, 703–711. [Google Scholar] [CrossRef]
- Green, M.S.; Peer, V.; Magid, A.; Hagani, N.; Anis, E.; Nitzan, D. Gender Differences in Adverse Events Following the Pfizer-BioNTech COVID-19 Vaccine. Vaccines 2022, 10, 233. [Google Scholar] [CrossRef]
- Vannice, K.; Wilder-Smith, A.; Hombach, J. Fractional-Dose Yellow Fever Vaccination—Advancing the Evidence Base. N. Engl. J. Med. 2018, 379, 603–605. [Google Scholar] [CrossRef]
- Więcek, W.; Ahuja, A.; Chaudhuri, E.; Kremer, M.; Simoes Gomes, A.; Snyder, C.M.; Tabarrok, A.; Tan, B.J. Testing fractional doses of COVID-19 vaccines. Proc. Natl. Acad. Sci. USA 2022, 119, e2116932119. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Munoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2021, 386, 35–46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).