Effectiveness and Safety of COVID-19 Vaccination in Patients with Malignant Disease
Abstract
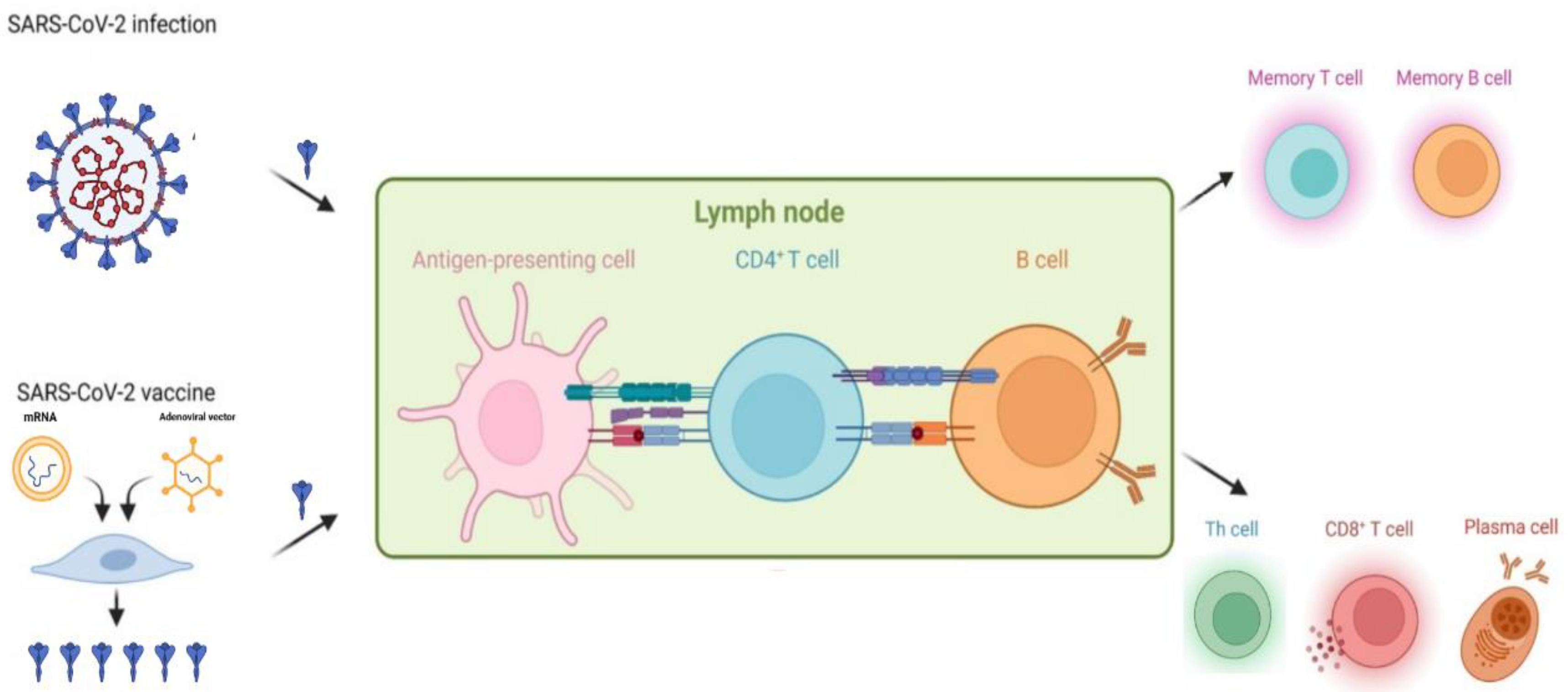
1. Introduction
2. Literature Search
3. Vaccine Effectiveness in Patients with Malignant Disease
3.1. Humoral Immunity: Serology and Neutralizing Antibodies
3.1.1. Effectiveness of Vaccination on Humoral Immunity
3.1.2. Risk Factors for Poor Humoral Immune Response to Vaccination
Solid Cancer
Hematological Malignancies
3.2. Cellular Immunity: T Cell Responses
3.2.1. Effectiveness of Vaccination on Cellular Immunity
3.2.2. Risk Factors for Poor Cellular Immune Response to Vaccination
Solid Cancer
Hematological Malignancies
3.3. Prime–Boost Vaccination: Solution for Waning Immunity and VOCs
4. Safety Concerns: Adverse Effects
5. Discussion and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Bakouny, Z.; Hawley, J.E.; Choueiri, T.K.; Peters, S.; Rini, B.I.; Warner, J.L.; Painter, C.A. COVID-19 and Cancer: Current Challenges and Perspectives. Cancer Cell 2020, 38, 629–646. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Ramaswamy, A.; Nayak, L.; Roy Moulik, N.; Sengar, M.; Chinnaswamy, G.; Jobanputra, K.; Shah, M.J.; Kapoor, A.; Joshi, A.; Kumar, A.; et al. COVID-19 in cancer patients on active systemic therapy—Outcomes from LMIC scenario with an emphasis on need for active treatment. Cancer Med. 2020, 9, 8747–8753. [Google Scholar] [CrossRef] [PubMed]
- Rüthrich, M.M.; Giessen-Jung, C.; Borgmann, S.; Classen, A.Y.; Dolff, S.; Grüner, B.; Hanses, F.; Isberner, N.; Köhler, P.; Lanznaster, J.; et al. COVID-19 in cancer patients: Clinical characteristics and outcome-an analysis of the LEOSS registry. Ann. Hematol. 2021, 100, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Salmanton-García, J.; Marchesi, F.; Busca, A.; Corradini, P.; Hoenigl, M.; Klimko, N.; Koehler, P.; Pagliuca, A.; Passamonti, F.; et al. COVID-19 infection in adult patients with hematological malignancies: A European Hematology Association Survey (EPICOVIDEHA). J. Hematol. Oncol. 2021, 14, 168. [Google Scholar] [CrossRef]
- Venkatesulu, B.P.; Chandrasekar, V.T.; Girdhar, P.; Advani, P.; Sharma, A.; Elumalai, T.; Hsieh, C.E.; Elghazawy, H.I.; Verma, V.; Krishnan, S. A Systematic Review and Meta-Analysis of Cancer Patients Affected by a Novel Coronavirus. JNCI Cancer Spectr. 2021, 5, pkaa102. [Google Scholar] [CrossRef]
- Mehta, V.; Goel, S.; Kabarriti, R.; Cole, D.; Goldfinger, M.; Acuna-Villaorduna, A.; Pradhan, K.; Thota, R.; Reissman, S.; Sparano, J.A.; et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020, 10, 935–941. [Google Scholar] [CrossRef]
- Hartman, H.E.; Sun, Y.; Devasia, T.P.; Chase, E.C.; Jairath, N.K.; Dess, R.T.; Jackson, W.C.; Morris, E.; Li, P.; Hochstedler, K.A.; et al. Integrated Survival Estimates for Cancer Treatment Delay Among Adults With Cancer During the COVID-19 Pandemic. JAMA Oncol. 2020, 6, 1881–1889. [Google Scholar] [CrossRef]
- Pinato, D.J.; Tabernero, J.; Bower, M.; Scotti, L.; Patel, M.; Colomba, E.; Dolly, S.; Loizidou, A.; Chester, J.; Mukherjee, U.; et al. Prevalence and impact of COVID-19 sequelae on treatment and survival of patients with cancer who recovered from SARS-CoV-2 infection: Evidence from the OnCovid retrospective, multicentre registry study. Lancet Oncol. 2021, 22, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, A.; de la Serna, J.; El Idrissi, M.; Oostvogels, L.; Quittet, P.; López-Jiménez, J.; Vural, F.; Pohlreich, D.; Zuckerman, T.; Issa, N.C.; et al. Effect of Recombinant Zoster Vaccine on Incidence of Herpes Zoster After Autologous Stem Cell Transplantation: A Randomized Clinical Trial. Jama 2019, 322, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Winston, D.J.; Mullane, K.M.; Cornely, O.A.; Boeckh, M.J.; Brown, J.W.; Pergam, S.A.; Trociukas, I.; Žák, P.; Craig, M.D.; Papanicolaou, G.A.; et al. Inactivated varicella zoster vaccine in autologous haemopoietic stem-cell transplant recipients: An international, multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2018, 391, 2116–2127. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Olivo, M.A.; Valerio, V.; Karpes Matusevich, A.R.; Brizio, M.; Kwok, M.; Geng, Y.; Suarez-Almazor, M.E.; Colmegna, I. Safety and Efficacy of Influenza Vaccination in Patients Receiving Immune Checkpoint Inhibitors. Systematic Review with Meta-Analysis. Vaccines 2022, 10, 1195. [Google Scholar] [CrossRef]
- de Lavallade, H.; Garland, P.; Sekine, T.; Hoschler, K.; Marin, D.; Stringaris, K.; Loucaides, E.; Howe, K.; Szydlo, R.; Kanfer, E.; et al. Repeated vaccination is required to optimize seroprotection against H1N1 in the immunocompromised host. Haematologica 2011, 96, 307–314. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Byrne, F.; Cerrone, M.; Schmitt, A.M.; Joharatnam-Hogan, N.; Shum, B.; et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: The CAPTURE study. Nat. Cancer 2021, 2, 1305–1320. [Google Scholar] [CrossRef]
- Lim, S.H.; Campbell, N.; Johnson, M.; Joseph-Pietras, D.; Collins, G.P.; O’Callaghan, A.; Fox, C.P.; Ahearne, M.; Johnson, P.W.M.; Goldblatt, D.; et al. Antibody responses after SARS-CoV-2 vaccination in patients with lymphoma. Lancet Haematol. 2021, 8, e542–e544. [Google Scholar] [CrossRef]
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; Del Molino Del Barrio, I.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet Oncol. 2021, 22, 765–778. [Google Scholar] [CrossRef]
- Linardou, H.; Spanakis, N.; Koliou, G.A.; Christopoulou, A.; Karageorgopoulou, S.; Alevra, N.; Vagionas, A.; Tsoukalas, N.; Sgourou, S.; Fountzilas, E.; et al. Responses to SARS-CoV-2 Vaccination in Patients with Cancer (ReCOVer Study): A Prospective Cohort Study of the Hellenic Cooperative Oncology Group. Cancers 2021, 13, 4621. [Google Scholar] [CrossRef] [PubMed]
- Shmueli, E.S.; Itay, A.; Margalit, O.; Berger, R.; Halperin, S.; Jurkowicz, M.; Levin, E.G.; Levy, I.; Olmer, L.; Regev-Yochay, G.; et al. Efficacy and safety of BNT162b2 vaccination in patients with solid cancer receiving anticancer therapy—A single centre prospective study. Eur. J. Cancer 2021, 157, 124–131. [Google Scholar] [CrossRef]
- Agbarya, A.; Sarel, I.; Ziv-Baran, T.; Agranat, S.; Schwartz, O.; Shai, A.; Nordheimer, S.; Fenig, S.; Shechtman, Y.; Kozlener, E.; et al. Efficacy of the mRNA-Based BNT162b2 COVID-19 Vaccine in Patients with Solid Malignancies Treated with Anti-Neoplastic Drugs. Cancers 2021, 13, 4191. [Google Scholar] [CrossRef] [PubMed]
- Ligumsky, H.; Safadi, E.; Etan, T.; Vaknin, N.; Waller, M.; Croll, A.; Nikolaevski-Berlin, A.; Greenberg, I.; Halperin, T.; Wasserman, A.; et al. Immunogenicity and Safety of the BNT162b2 mRNA COVID-19 Vaccine Among Actively Treated Cancer Patients. JNCI J. Natl. Cancer Inst. 2022, 114, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Barrière, J.; Chamorey, E.; Adjtoutah, Z.; Castelnau, O.; Mahamat, A.; Marco, S.; Petit, E.; Leysalle, A.; Raimondi, V.; Carles, M. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann. Oncol. 2021, 32, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Becerril-Gaitan, A.; Vaca-Cartagena, B.F.; Ferrigno, A.S.; Mesa-Chavez, F.; Barrientos-Gutiérrez, T.; Tagliamento, M.; Lambertini, M.; Villarreal-Garza, C. Immunogenicity and risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection after Coronavirus Disease 2019 (COVID-19) vaccination in patients with cancer: A systematic review and meta-analysis. Eur. J. Cancer 2022, 160, 243–260. [Google Scholar] [CrossRef]
- Mair, M.J.; Berger, J.M.; Berghoff, A.S.; Starzer, A.M.; Ortmayr, G.; Puhr, H.C.; Steindl, A.; Perkmann, T.; Haslacher, H.; Strassl, R.; et al. Humoral Immune Response in Hematooncological Patients and Health Care Workers Who Received SARS-CoV-2 Vaccinations. JAMA Oncol. 2022, 8, 106–113. [Google Scholar] [CrossRef]
- Embi, P.J.; Levy, M.E.; Naleway, A.L.; Patel, P.; Gaglani, M.; Natarajan, K.; Dascomb, K.; Ong, T.C.; Klein, N.P.; Liao, I.C.; et al. Effectiveness of 2-Dose Vaccination with mRNA COVID-19 Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults—Nine States, January–September 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1553–1559. [Google Scholar] [CrossRef]
- Wu, J.T.; La, J.; Branch-Elliman, W.; Huhmann, L.B.; Han, S.S.; Parmigiani, G.; Tuck, D.P.; Brophy, M.T.; Do, N.V.; Lin, A.Y.; et al. Association of COVID-19 Vaccination With SARS-CoV-2 Infection in Patients With Cancer: A US Nationwide Veterans Affairs Study. JAMA Oncol. 2022, 8, 281–286. [Google Scholar] [CrossRef]
- Cavanna, L.; Citterio, C.; Biasini, C.; Madaro, S.; Bacchetta, N.; Lis, A.; Cremona, G.; Muroni, M.; Bernuzzi, P.; Lo Cascio, G.; et al. COVID-19 vaccines in adult cancer patients with solid tumours undergoing active treatment: Seropositivity and safety. A prospective observational study in Italy. Eur. J. Cancer 2021, 157, 441–449. [Google Scholar] [CrossRef]
- Peeters, M.; Verbruggen, L.; Teuwen, L.; Vanhoutte, G.; Vande Kerckhove, S.; Peeters, B.; Raats, S.; Van der Massen, I.; De Keersmaecker, S.; Debie, Y.; et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open 2021, 6, 100274. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Chalasani, P.; Wei, R.; Pennington, D.; Quirk, G.; Schoenle, M.V.; Peyton, K.L.; Uhrlaub, J.L.; Ripperger, T.J.; Jergović, M.; et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat. Med. 2021, 27, 2002–2011. [Google Scholar] [CrossRef]
- McKenzie, D.R.; Muñoz-Ruiz, M.; Monin, L.; Alaguthurai, T.; Lechmere, T.; Abdul-Jawad, S.; Graham, C.; Pollock, E.; Graham, R.; Sychowska, K.; et al. Humoral and cellular immunity to delayed second dose of SARS-CoV-2 BNT162b2 mRNA vaccination in patients with cancer. Cancer Cell 2021, 39, 1445–1447. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Gonzalez-Lugo, J.D.; Goradia, N.; Gali, R.; Shapiro, L.C.; Pradhan, K.; Rahman, S.; Kim, S.Y.; Ko, B.; Sica, R.A.; et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell 2021, 39, 1081–1090. [Google Scholar] [CrossRef]
- Massarweh, A.; Eliakim-Raz, N.; Stemmer, A.; Levy-Barda, A.; Yust-Katz, S.; Zer, A.; Benouaich-Amiel, A.; Ben-Zvi, H.; Moskovits, N.; Brenner, B.; et al. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021, 7, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C.A.; Mehta, N.; Keogh, R.H.; Diaz-Ordaz, K.; Khunti, K.; Lyons, R.A.; Kee, F.; Sheikh, A.; Rahman, S.; et al. Risk prediction of COVID-19 related death and hospital admission in adults after covid-19 vaccination: National prospective cohort study. BMJ 2021, 374, n2244. [Google Scholar] [CrossRef]
- Matkowska-Kocjan, A.; Owoc-Lempach, J.; Chruszcz, J.; Kuźnik, E.; Szenborn, F.; Jurczenko, L.; Wójcik, M.; Banyś, D.; Szenborn, L.; Ussowicz, M. The COVID-19 mRNA BNT163b2 Vaccine Was Well Tolerated and Highly Immunogenic in Young Adults in Long Follow-Up after Haematopoietic Stem Cell Transplantation. Vaccines 2021, 9, 1209. [Google Scholar] [CrossRef]
- Wall, E.C.; Wu, M.; Harvey, R.; Kelly, G.; Warchal, S.; Sawyer, C.; Daniels, R.; Adams, L.; Hobson, P.; Hatipoglu, E.; et al. AZD1222-induced neutralising antibody activity against SARS-CoV-2 Delta VOC. Lancet 2021, 398, 207–209. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Oosting, S.F.; van der Veldt, A.A.M.; GeurtsvanKessel, C.H.; Fehrmann, R.S.N.; van Binnendijk, R.S.; Dingemans, A.C.; Smit, E.F.; Hiltermann, T.J.N.; den Hartog, G.; Jalving, M.; et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: A prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021, 22, 1681–1691. [Google Scholar] [CrossRef]
- Herzog Tzarfati, K.; Gutwein, O.; Apel, A.; Rahimi-Levene, N.; Sadovnik, M.; Harel, L.; Benveniste-Levkovitz, P.; Bar Chaim, A.; Koren-Michowitz, M. BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies. Am. J. Hematol. 2021, 96, 1195–1203. [Google Scholar] [CrossRef]
- Buttiron Webber, T.; Provinciali, N.; Musso, M.; Ugolini, M.; Boitano, M.; Clavarezza, M.; D’Amico, M.; Defferrari, C.; Gozza, A.; Briata, I.M.; et al. Predictors of poor seroconversion and adverse events to SARS-CoV-2 mRNA BNT162b2 vaccine in cancer patients on active treatment. Eur. J. Cancer 2021, 159, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Grinshpun, A.; Rottenberg, Y.; Ben-Dov, I.Z.; Djian, E.; Wolf, D.G.; Kadouri, L. Serologic response to COVID-19 infection and/or vaccine in cancer patients on active treatment. ESMO Open 2021, 6, 100283. [Google Scholar] [CrossRef] [PubMed]
- Goshen-Lago, T.; Waldhorn, I.; Holland, R.; Szwarcwort-Cohen, M.; Reiner-Benaim, A.; Shachor-Meyouhas, Y.; Hussein, K.; Fahoum, L.; Baruch, M.; Peer, A.; et al. Serologic Status and Toxic Effects of the SARS-CoV-2 BNT162b2 Vaccine in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021, 7, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Liontos, M.; Terpos, E.; Markellos, C.; Zagouri, F.; Briasoulis, A.; Katsiana, I.; Skafida, E.; Fiste, O.; Kunadis, E.; Andrikopoulou, A.; et al. Immunological Response to COVID-19 Vaccination in Ovarian Cancer Patients Receiving PARP Inhibitors. Vaccines 2021, 9, 1148. [Google Scholar] [CrossRef]
- Di Noia, V.; Pimpinelli, F.; Renna, D.; Barberi, V.; Maccallini, M.T.; Gariazzo, L.; Pontone, M.; Monti, A.; Campo, F.; Taraborelli, E.; et al. Immunogenicity and Safety of COVID-19 Vaccine BNT162b2 for Patients with Solid Cancer: A Large Cohort Prospective Study from a Single Institution. Clin. Cancer Res. 2021, 27, 6815–6823. [Google Scholar] [CrossRef]
- Malard, F.; Gaugler, B.; Gozlan, J.; Bouquet, L.; Fofana, D.; Siblany, L.; Eshagh, D.; Adotevi, O.; Laheurte, C.; Ricard, L.; et al. Weak immunogenicity of SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Cancer J. 2021, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv, T.; Shorer Arbel, Y.; Scarfò, L.; et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021, 137, 3165–3173. [Google Scholar] [CrossRef]
- Chan, W.Y.; Howells, L.; Wilson, W.; Sanchez, E.; Ainley, L.; Chavda, S.J.; Dowling, E.; Correia, N.; Lecat, C.S.Y.; McMillan, A.; et al. Serological response to the BNT162b2 mRNA or ChAdOx1 nCoV-19 COVID-19 vaccine after first and second doses in patients with plasma cell disorders: Influence of host and disease factors. Br. J. Haematol. 2022, 196, e21–e26. [Google Scholar] [CrossRef]
- Stampfer, S.D.; Goldwater, M.S.; Jew, S.; Bujarski, S.; Regidor, B.; Daniely, D.; Chen, H.; Xu, N.; Li, M.; Green, T.; et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia 2021, 35, 3534–3541. [Google Scholar] [CrossRef]
- Roeker, L.E.; Knorr, D.A.; Thompson, M.C.; Nivar, M.; Lebowitz, S.; Peters, N.; Deonarine, I., Jr.; Momotaj, S.; Sharan, S.; Chanlatte, V.; et al. COVID-19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia 2021, 35, 2703–2705. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Pernat, C.A.; Gavralidis, A.; St Denis, K.J.; Lam, E.C.; Spring, L.M.; Isakoff, S.J.; Farmer, J.R.; Zubiri, L.; Hobbs, G.S.; et al. Immunogenicity and Reactogenicity of SARS-CoV-2 Vaccines in Patients With Cancer: The CANVAX Cohort Study. J. Clin. Oncol. 2022, 40, 12–23. [Google Scholar] [CrossRef]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wu, M.; Harvey, R.; Schmitt, A.M.; Tippu, Z.; Shum, B.; Farag, S.; Rogiers, A.; et al. Omicron neutralising antibodies after third COVID-19 vaccine dose in patients with cancer. Lancet 2022, 399, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Ehmsen, S.; Asmussen, A.; Jeppesen, S.S.; Nilsson, A.C.; Østerlev, S.; Vestergaard, H.; Justesen, U.S.; Johansen, I.S.; Frederiksen, H.; Ditzel, H.J. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell 2021, 39, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.; Panopoulou, A.; Shea, R.L.; Tsui, M.; Saso, R.; Sud, A.; West, S.; Smith, K.; Barwood, J.; Kaczmarek, E.; et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021, 8, e389–e392. [Google Scholar] [CrossRef]
- Parry, H.; McIlroy, G.; Bruton, R.; Ali, M.; Stephens, C.; Damery, S.; Otter, A.; McSkeane, T.; Rolfe, H.; Faustini, S.; et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Briasoulis, A.; Gumeni, S.; Malandrakis, P.; Fotiou, D.; Papanagnou, E.D.; Migkou, M.; Theodorakakou, F.; et al. The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J. 2021, 11, 138. [Google Scholar] [CrossRef]
- Marasco, V.; Carniti, C.; Guidetti, A.; Farina, L.; Magni, M.; Miceli, R.; Calabretta, L.; Verderio, P.; Ljevar, S.; Serpenti, F.; et al. T-cell immune response after mRNA SARS-CoV-2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br. J. Haematol. 2022, 196, 548–558. [Google Scholar] [CrossRef]
- Maneikis, K.; Šablauskas, K.; Ringelevičiūtė, U.; Vaitekėnaitė, V.; Čekauskienė, R.; Kryžauskaitė, L.; Naumovas, D.; Banys, V.; Pečeliūnas, V.; Beinortas, T.; et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: A national prospective cohort study. Lancet Haematol. 2021, 8, e583–e592. [Google Scholar] [CrossRef]
- Addeo, A.; Shah, P.K.; Bordry, N.; Hudson, R.D.; Albracht, B.; Di Marco, M.; Kaklamani, V.; Dietrich, P.Y.; Taylor, B.S.; Simand, P.F.; et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell 2021, 39, 1091–1098. [Google Scholar] [CrossRef]
- Greenberger, L.M.; Saltzman, L.A.; Senefeld, J.W.; Johnson, P.W.; DeGennaro, L.J.; Nichols, G.L. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell 2021, 39, 1031–1033. [Google Scholar] [CrossRef]
- Chung, D.J.; Shah, G.L.; Devlin, S.M.; Ramanathan, L.V.; Doddi, S.; Pessin, M.S.; Hoover, E.; Marcello, L.T.; Young, J.C.; Boutemine, S.R.; et al. Disease- and Therapy-Specific Impact on Humoral Immune Responses to COVID-19 Vaccination in Hematologic Malignancies. Blood Cancer Discov. 2021, 2, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Crombie, J.L.; Sherman, A.C.; Cheng, C.A.; Ryan, C.E.; Zon, R.; Desjardins, M.; Baker, P.; McDonough, M.; Izaguirre, N.; Bausk, B.; et al. Activity of mRNA COVID-19 vaccines in patients with lymphoid malignancies. Blood Adv. 2021, 5, 3062–3065. [Google Scholar] [CrossRef] [PubMed]
- Ghione, P.; Gu, J.J.; Attwood, K.; Torka, P.; Goel, S.; Sundaram, S.; Mavis, C.; Johnson, M.; Thomas, R.; McWhite, K.; et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell-directed therapies. Blood 2021, 138, 811–814. [Google Scholar] [CrossRef]
- Tadmor, T.; Benjamini, O.; Braester, A.; Rahav, G.; Rokach, L. Antibody persistence 100 days following the second dose of BNT162b mRNA Covid19 vaccine in patients with chronic lymphocytic leukemia. Leukemia 2021, 35, 2727–2730. [Google Scholar] [CrossRef]
- Mellinghoff, S.C.; Robrecht, S.; Mayer, L.; Weskamm, L.M.; Dahlke, C.; Gruell, H.; Vanshylla, K.; Schlösser, H.A.; Thelen, M.; Fink, A.M.; et al. SARS-CoV-2 specific cellular response following COVID-19 vaccination in patients with chronic lymphocytic leukemia. Leukemia 2022, 36, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, F.; Pimpinelli, F.; Sperandio, E.; Papa, E.; Falcucci, P.; Pontone, M.; di Martino, S.; de Latouliere, L.; Orlandi, G.; Morrone, A.; et al. The 12-week kinetics of anti-SARS-CoV-2 antibodies in different haematological cancers after vaccination with BNT162b2. Br. J. Haematol. 2022, 196, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Upadhyaya, B.; Tuballes, K.; Kappes, K.; Gleason, C.R.; Beach, K.; Agte, S.; Srivastava, K.; Van Oekelen, O.; Barcessat, V.; et al. Variable cellular responses to SARS-CoV-2 in fully vaccinated patients with multiple myeloma. Cancer Cell 2021, 39, 1442–1444. [Google Scholar] [CrossRef]
- Van Oekelen, O.; Gleason, C.R.; Agte, S.; Srivastava, K.; Beach, K.F.; Aleman, A.; Kappes, K.; Mouhieddine, T.H.; Wang, B.; Chari, A.; et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell 2021, 39, 1028–1030. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Trougakos, I.P.; Gavriatopoulou, M.; Papassotiriou, I.; Sklirou, A.D.; Ntanasis-Stathopoulos, I.; Papanagnou, E.D.; Fotiou, D.; Kastritis, E.; Dimopoulos, M.A. Low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood 2021, 137, 3674–3676. [Google Scholar] [CrossRef]
- Piñana, J.L.; López-Corral, L.; Martino, R.; Montoro, J.; Vazquez, L.; Pérez, A.; Martin-Martin, G.; Facal-Malvar, A.; Ferrer, E.; Pascual, M.J.; et al. SARS-CoV-2-reactive antibody detection after SARS-CoV-2 vaccination in hematopoietic stem cell transplant recipients: Prospective survey from the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group. Am. J. Hematol. 2022, 97, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Mairhofer, M.; Kausche, L.; Kaltenbrunner, S.; Ghanem, R.; Stegemann, M.; Klein, K.; Pammer, M.; Rauscher, I.; Salzer, H.J.F.; Doppler, S.; et al. Humoral and cellular immune responses in SARS-CoV-2 mRNA-vaccinated patients with cancer. Cancer Cell 2021, 39, 1171–1172. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Solís Arce, J.S.; Warren, S.S.; Meriggi, N.F.; Scacco, A.; McMurry, N.; Voors, M.; Syunyaev, G.; Malik, A.A.; Aboutajdine, S.; Adeojo, O.; et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 2021, 27, 1385–1394. [Google Scholar] [CrossRef]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef]
- Shapiro, L.C.; Thakkar, A.; Campbell, S.T.; Forest, S.K.; Pradhan, K.; Gonzalez-Lugo, J.D.; Quinn, R.; Bhagat, T.D.; Choudhary, G.S.; McCort, M.; et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell 2022, 40, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4(+) and CD8(+) T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Bange, E.M.; Han, N.A.; Wileyto, P.; Kim, J.Y.; Gouma, S.; Robinson, J.; Greenplate, A.R.; Hwee, M.A.; Porterfield, F.; Owoyemi, O.; et al. CD8(+) T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat. Med. 2021, 27, 1280–1289. [Google Scholar] [CrossRef]
- Fendler, A.; Au, L.; Shepherd, S.T.C.; Byrne, F.; Cerrone, M.; Boos, L.A.; Rzeniewicz, K.; Gordon, W.; Shum, B.; Gerard, C.L.; et al. Functional antibody and T cell immunity following SARS-CoV-2 infection, including by variants of concern, in patients with cancer: The CAPTURE study. Nat. Cancer 2021, 2, 1321–1337. [Google Scholar] [CrossRef]
- Lindemann, M.; Klisanin, V.; Thümmler, L.; Fisenkci, N.; Tsachakis-Mück, N.; Ditschkowski, M.; Schwarzkopf, S.; Klump, H.; Reinhardt, H.C.; Horn, P.A.; et al. Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients. Vaccines 2021, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Ram, R.; Hagin, D.; Kikozashvilli, N.; Freund, T.; Amit, O.; Bar-On, Y.; Beyar-Katz, O.; Shefer, G.; Moshiashvili, M.M.; Karni, C.; et al. Safety and Immunogenicity of the BNT162b2 mRNA COVID-19 Vaccine in Patients after Allogeneic HCT or CD19-based CART therapy-A Single-Center Prospective Cohort Study. Transplant. Cell. Ther. 2021, 27, 788–794. [Google Scholar] [CrossRef]
- Harrington, P.; Doores, K.J.; Radia, D.; O’Reilly, A.; Lam, H.P.J.; Seow, J.; Graham, C.; Lechmere, T.; McLornan, D.; Dillon, R.; et al. Single dose of BNT162b2 mRNA vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) induces neutralising antibody and polyfunctional T-cell responses in patients with chronic myeloid leukaemia. Br. J. Haematol. 2021, 194, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Harrington, P.; de Lavallade, H.; Doores, K.J.; O’Reilly, A.; Seow, J.; Graham, C.; Lechmere, T.; Radia, D.; Dillon, R.; Shanmugharaj, Y.; et al. Single dose of BNT162b2 mRNA vaccine against SARS-CoV-2 induces high frequency of neutralising antibody and polyfunctional T-cell responses in patients with myeloproliferative neoplasms. Leukemia 2021, 35, 3573–3577. [Google Scholar] [CrossRef] [PubMed]
- Nelli, F.; Fabbri, A.; Onorato, A.; Giannarelli, D.; Silvestri, M.A.; Giron Berrios, J.R.; Virtuoso, A.; Marrucci, E.; Signorelli, C.; Chilelli, M.G.; et al. Effects of active cancer treatment on safety and immunogenicity of COVID-19 mRNA-BNT162b2 vaccine: Preliminary results from the prospective observational Vax-On study. Ann. Oncol. 2022, 33, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Gounant, V.; Ferré, V.M.; Soussi, G.; Charpentier, C.; Flament, H.; Fidouh, N.; Collin, G.; Namour, C.; Assoun, S.; Bizot, A.; et al. Efficacy of Severe Acute Respiratory Syndrome Coronavirus-2 Vaccine in Patients With Thoracic Cancer: A Prospective Study Supporting a Third Dose in Patients With Minimal Serologic Response After Two Vaccine Doses. J. Thorac. Oncol. 2022, 17, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Palich, R.; Veyri, M.; Vozy, A.; Marot, S.; Gligorov, J.; Benderra, M.A.; Maingon, P.; Morand-Joubert, L.; Adjoutah, Z.; Marcelin, A.G.; et al. High seroconversion rate but low antibody titers after two injections of BNT162b2 (Pfizer-BioNTech) vaccine in patients treated with chemotherapy for solid cancers. Ann. Oncol. 2021, 32, 1294–1295. [Google Scholar] [CrossRef]
- Figueiredo, J.C.; Merin, N.M.; Hamid, O.; Choi, S.Y.; Lemos, T.; Cozen, W.; Nguyen, N.; Finster, L.J.; Foley, J.; Darrah, J.; et al. Longitudinal SARS-CoV-2 mRNA Vaccine-Induced Humoral Immune Responses in Patients with Cancer. Cancer Res. 2021, 81, 6273–6280. [Google Scholar] [CrossRef]
- Redjoul, R.; Le Bouter, A.; Parinet, V.; Fourati, S.; Maury, S. Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT. Lancet Haematol. 2021, 8, e681–e683. [Google Scholar] [CrossRef]
- Debie, Y.; Vandamme, T.; Goossens, M.E.; van Dam, P.A.; Peeters, M. Antibody titres before and after a third dose of the SARS-CoV-2 BNT162b2 vaccine in patients with cancer. Eur. J. Cancer 2022, 163, 177–179. [Google Scholar] [CrossRef]
- Rottenberg, Y.; Grinshpun, A.; Ben-Dov, I.Z.; Oiknine Djian, E.; Wolf, D.G.; Kadouri, L. Assessment of Response to a Third Dose of the SARS-CoV-2 BNT162b2 mRNA Vaccine in Patients With Solid Tumors Undergoing Active Treatment. JAMA Oncol. 2022, 8, 300–301. [Google Scholar] [CrossRef]
- Re, D.; Seitz-Polski, B.; Brglez, V.; Carles, M.; Graça, D.; Benzaken, S.; Liguori, S.; Zahreddine, K.; Delforge, M.; Bailly-Maitre, B.; et al. Humoral and cellular responses after a third dose of SARS-CoV-2 BNT162b2 vaccine in patients with lymphoid malignancies. Nat. Commun. 2022, 13, 864. [Google Scholar] [CrossRef]
- Fenioux, C.; Teixeira, L.; Fourati, S.; Melica, G.; Lelievre, J.D.; Gallien, S.; Zalcman, G.; Pawlotsky, J.M.; Tournigand, C. SARS-CoV-2 Antibody Response to 2 or 3 Doses of the BNT162b2 Vaccine in Patients Treated With Anticancer Agents. JAMA Oncol. 2022, 8, 612–617. [Google Scholar] [CrossRef]
- Ligumsky, H.; Dor, H.; Etan, T.; Golomb, I.; Nikolaevski-Berlin, A.; Greenberg, I.; Halperin, T.; Angel, Y.; Henig, O.; Spitzer, A.; et al. Immunogenicity and safety of BNT162b2 mRNA vaccine booster in actively treated patients with cancer. Lancet Oncol. 2022, 23, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; St Denis, K.J.; Lam, E.C.; Ofoman, O.; Garcia-Beltran, W.F.; Mairena, C.B.; Bhan, A.K.; Gainor, J.F.; Balazs, A.B.; Iafrate, A.J. Neutralization breadth of SARS-CoV-2 viral variants following primary series and booster SARS-CoV-2 vaccines in patients with cancer. Cancer Cell 2022, 40, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Luangdilok, S.; Wanchaijiraboon, P.; Pakvisal, N.; Susiriwatananont, T.; Zungsontiporn, N.; Sriuranpong, V.; Sainamthip, P.; Suntronwong, N.; Vichaiwattana, P.; Wanlapakorn, N.; et al. Immunogenicity after a Third COVID-19 mRNA Booster in Solid Cancer Patients Who Previously Received the Primary Heterologous CoronaVac/ChAdOx1 Vaccine. Vaccines 2022, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Reimann, P.; Ulmer, H.; Mutschlechner, B.; Benda, M.; Severgnini, L.; Volgger, A.; Lang, T.; Atzl, M.; Huynh, M.; Gasser, K.; et al. Efficacy and safety of heterologous booster vaccination with Ad26.COV2.S after BNT162b2 mRNA COVID-19 vaccine in haemato-oncological patients with no antibody response. Br. J. Haematol. 2022, 196, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Sablerolles, R.S.G.; Rietdijk, W.J.R.; Goorhuis, A.; Postma, D.F.; Visser, L.G.; Geers, D.; Schmitz, K.S.; Garcia Garrido, H.M.; Koopmans, M.P.G.; Dalm, V.; et al. Immunogenicity and Reactogenicity of Vaccine Boosters after Ad26.COV2.S Priming. N. Engl. J. Med. 2022, 386, 951–963. [Google Scholar] [CrossRef]
- Waissengrin, B.; Agbarya, A.; Safadi, E.; Padova, H.; Wolf, I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021, 22, 581–583. [Google Scholar] [CrossRef]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef]
- Chen, Y.W.; Tucker, M.D.; Beckermann, K.E.; Iams, W.T.; Rini, B.I.; Johnson, D.B. COVID-19 mRNA vaccines and immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Eur. J. Cancer 2021, 155, 291–293. [Google Scholar] [CrossRef]
- Hussain, K.; Kawsar, A.; Weir, J.; Au, L.; Turajlic, S.; Larkin, J.; Fearfield, L. Severe cutaneous adverse reaction following COVID-19 vaccination and immunotherapy: A second hit? Clin. Exp. Dermatol. 2022, 47, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Au, L.; Fendler, A.; Shepherd, S.T.C.; Rzeniewicz, K.; Cerrone, M.; Byrne, F.; Carlyle, E.; Edmonds, K.; Del Rosario, L.; Shon, J.; et al. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat. Med. 2021, 27, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Ngo, D.; Aribi, A.; Arslan, S.; Dadwal, S.; Marcucci, G.; Nakamura, R.; Forman, S.J.; Chen, J.; Al Malki, M.M. Safety and Tolerability of SARS-CoV2 Emergency-Use Authorized Vaccines for Allogeneic Hematopoietic Stem Cell Transplant Recipients. Transplant. Cell. Ther. 2021, 27, e931–e938. [Google Scholar] [CrossRef] [PubMed]
- Garreffa, E.; Hamad, A.; O’Sullivan, C.C.; Hazim, A.Z.; York, J.; Puri, S.; Turnbull, A.; Robertson, J.F.; Goetz, M.P. Regional lymphadenopathy following COVID-19 vaccination: Literature review and considerations for patient management in breast cancer care. Eur. J. Cancer 2021, 159, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Waldhorn, I.; Holland, R.; Goshen-Lago, T.; Shirman, Y.; Szwarcwort-Cohen, M.; Reiner-Benaim, A.; Shachor-Meyouhas, Y.; Hussein, K.; Fahoum, L.; Peer, A.; et al. Six-Month Efficacy and Toxicity Profile of BNT162b2 Vaccine in Cancer Patients with Solid Tumors. Cancer Discov. 2021, 11, 2430–2435. [Google Scholar] [CrossRef]
- Özütemiz, C.; Krystosek, L.A.; Church, A.L.; Chauhan, A.; Ellermann, J.M.; Domingo-Musibay, E.; Steinberger, D. Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncologic Patients. Radiology 2021, 300, E296–E300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Fu, L.; He, Y.; Li, H.; Song, Y.; Liu, S. Effectiveness and Safety of COVID-19 Vaccination in Patients with Malignant Disease. Vaccines 2023, 11, 486. https://doi.org/10.3390/vaccines11020486
Zhao L, Fu L, He Y, Li H, Song Y, Liu S. Effectiveness and Safety of COVID-19 Vaccination in Patients with Malignant Disease. Vaccines. 2023; 11(2):486. https://doi.org/10.3390/vaccines11020486
Chicago/Turabian StyleZhao, Li, Lin Fu, Yuqin He, Han Li, Yixuan Song, and Shaoyan Liu. 2023. "Effectiveness and Safety of COVID-19 Vaccination in Patients with Malignant Disease" Vaccines 11, no. 2: 486. https://doi.org/10.3390/vaccines11020486
APA StyleZhao, L., Fu, L., He, Y., Li, H., Song, Y., & Liu, S. (2023). Effectiveness and Safety of COVID-19 Vaccination in Patients with Malignant Disease. Vaccines, 11(2), 486. https://doi.org/10.3390/vaccines11020486





