Acceptance and Risk Perception of COVID-19 Vaccination among Pregnant and Non Pregnant Women in Sub-Saharan Africa: A Cross-Sectional Matched-Sample Study
Abstract
1. Introduction
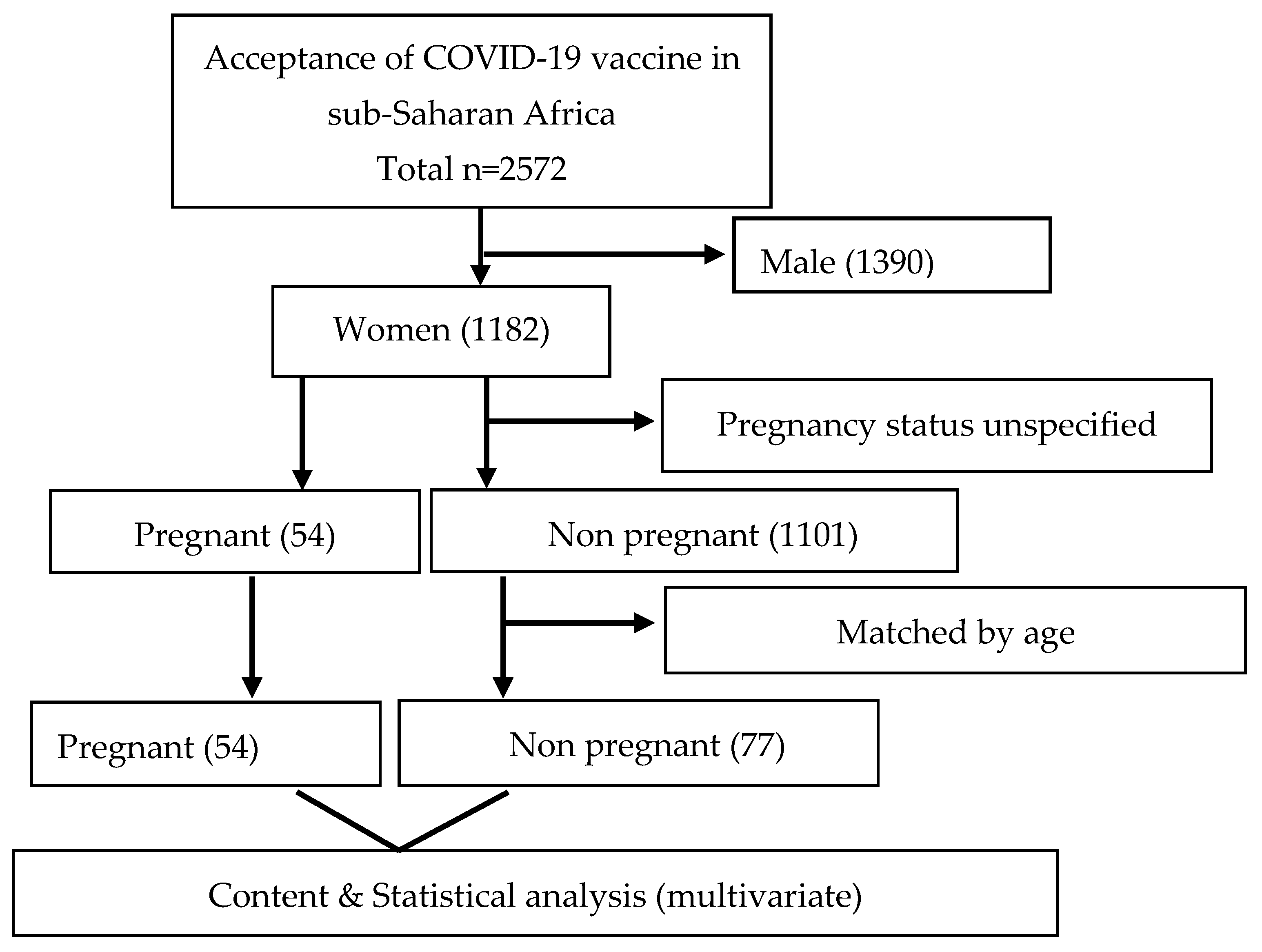
2. Materials and Methods
2.1. Study Design
2.2. Ethics
2.3. Data Collection
2.4. Content Analysis
2.5. Statistical Analysis
3. Results
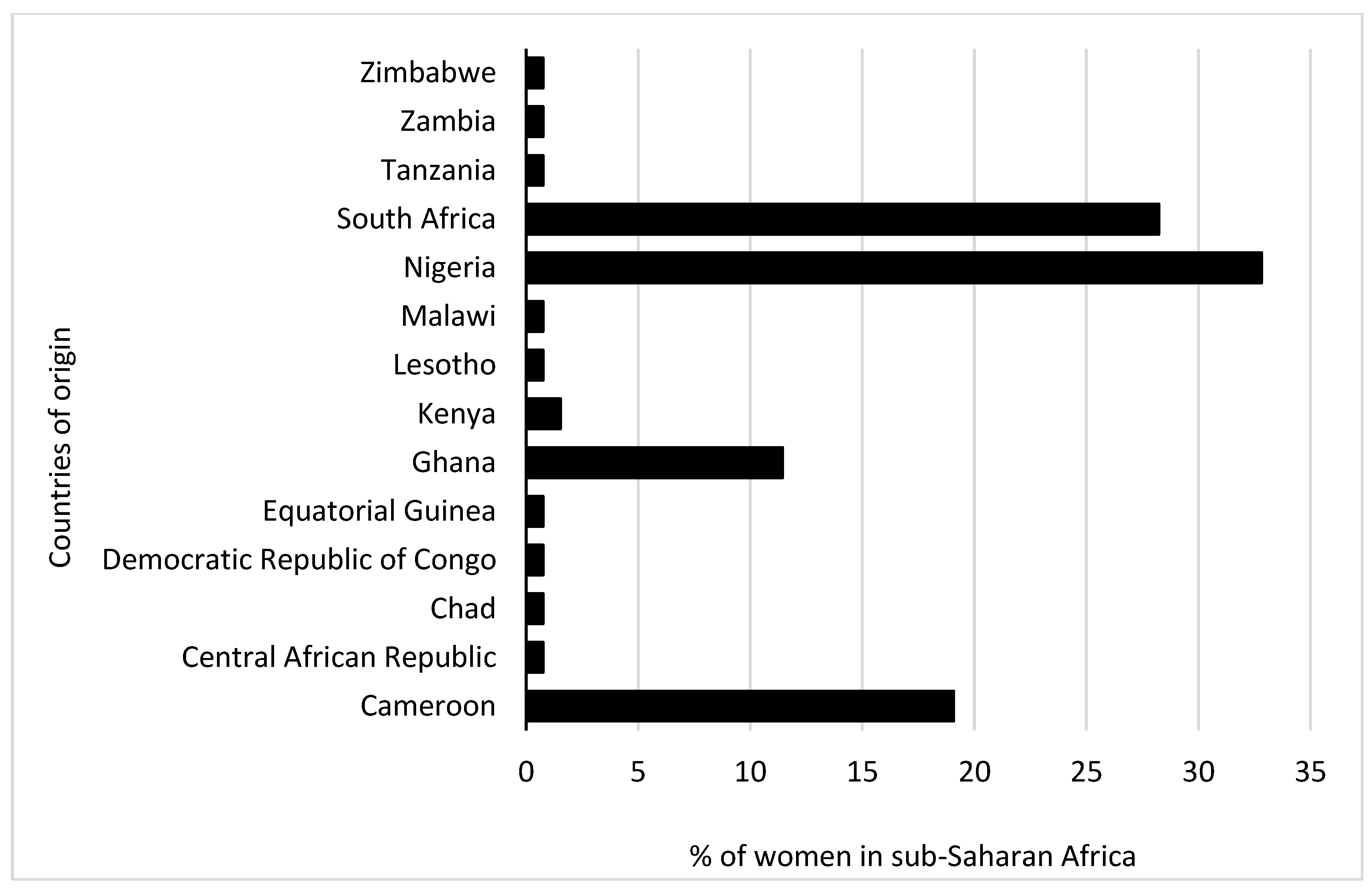
3.1. Comparison of Sociodemographic and COVID-19 Test Factors between Pregnant and Non Pregnant Women
3.2. Factors Associated with Non-Vaccination against COVID-19
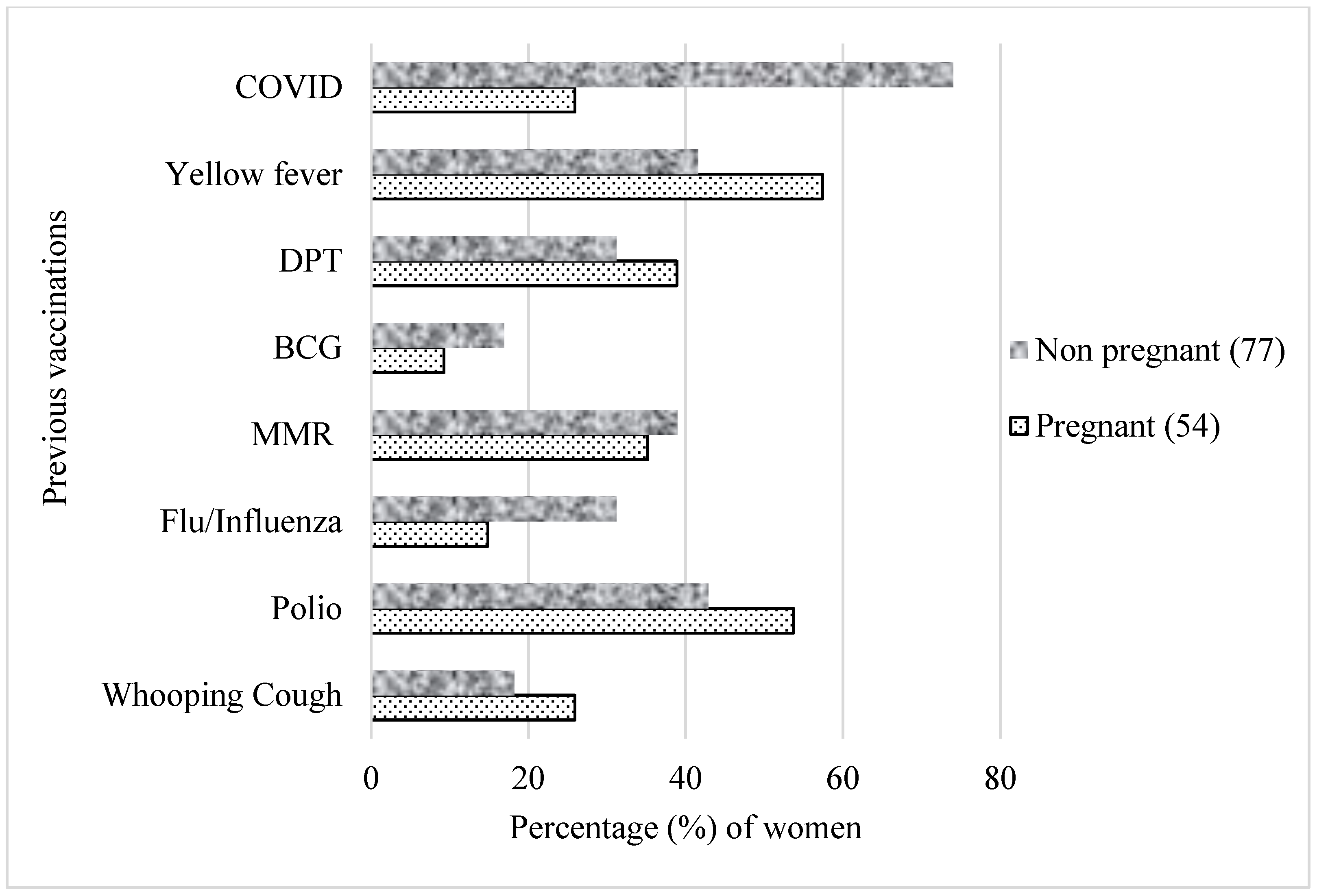
3.3. Common Misconceptions about the COVID-19 Vaccine
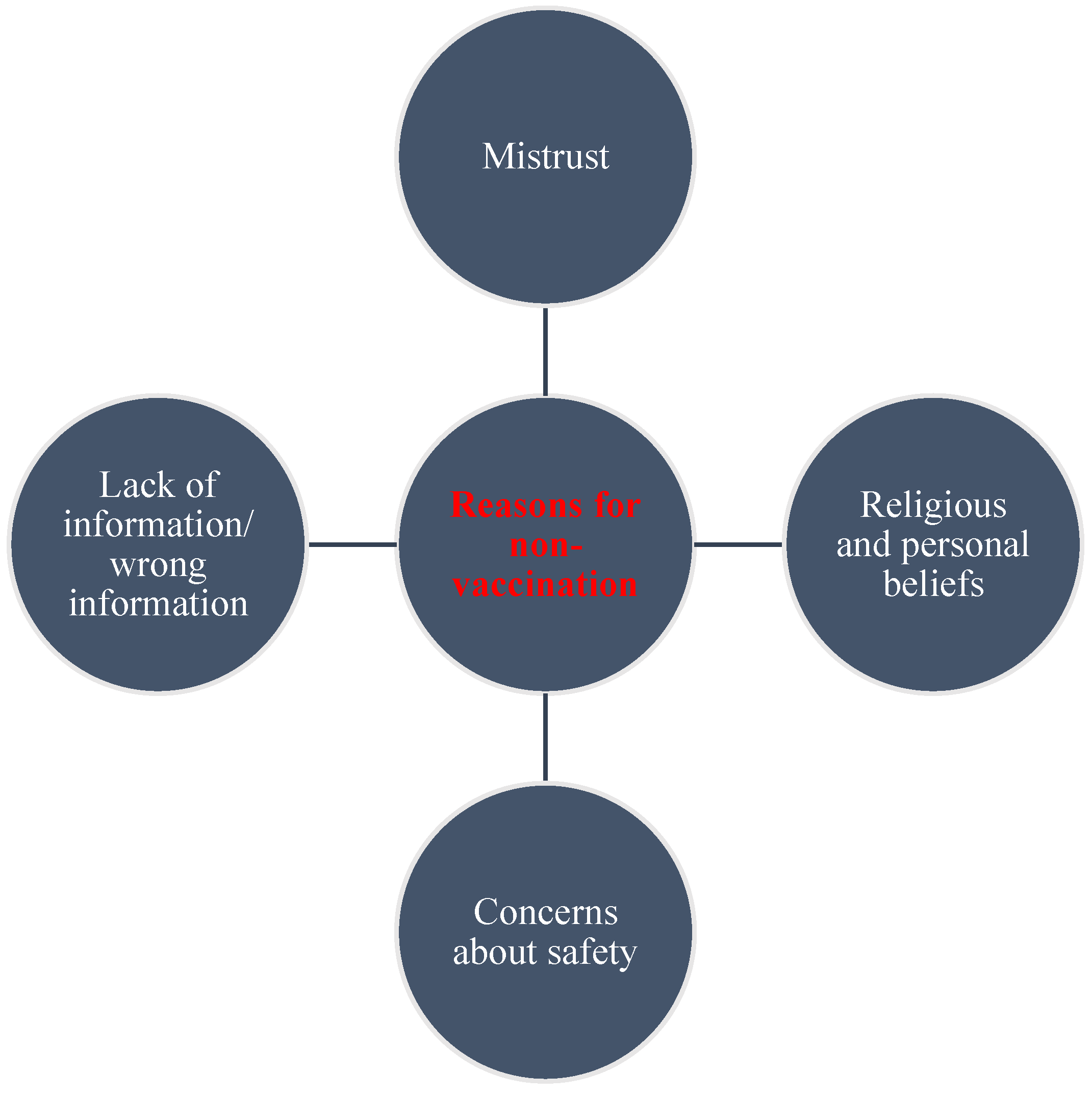
3.4. Reasons for Not Getting Vaccinated against COVID-19
“Concerned about the effects after taking the vaccine. There are many myths concerning it, like, it can make a woman not fertile to depopulate us. Most importantly, our COVID strain in Africa is not that dangerous. They should make available the vaccine to be given to the developed countries like us and not another product”.
“I rather prefer self-protection for prevention purposes than trust the vaccine”.
“Personal conviction that the vaccine is not necessary for Africa, especially for young people who are not at risk. It could be a birth control procedure to reduce world population”.
“Vaccines have been used against black people for far too long-Kenya infertility, Tuskegee, etc. This vaccine is as questionable and its benefits for politicians far outweigh its care to manage this self-limiting bug”.
“Risk to my health as I have SLE with a severely compromised immune system”.
“I have a diagnosed allergy, which is the main cause of asthma and skin reactions, conjunctivitis. I am scared I might react to the vaccine”.
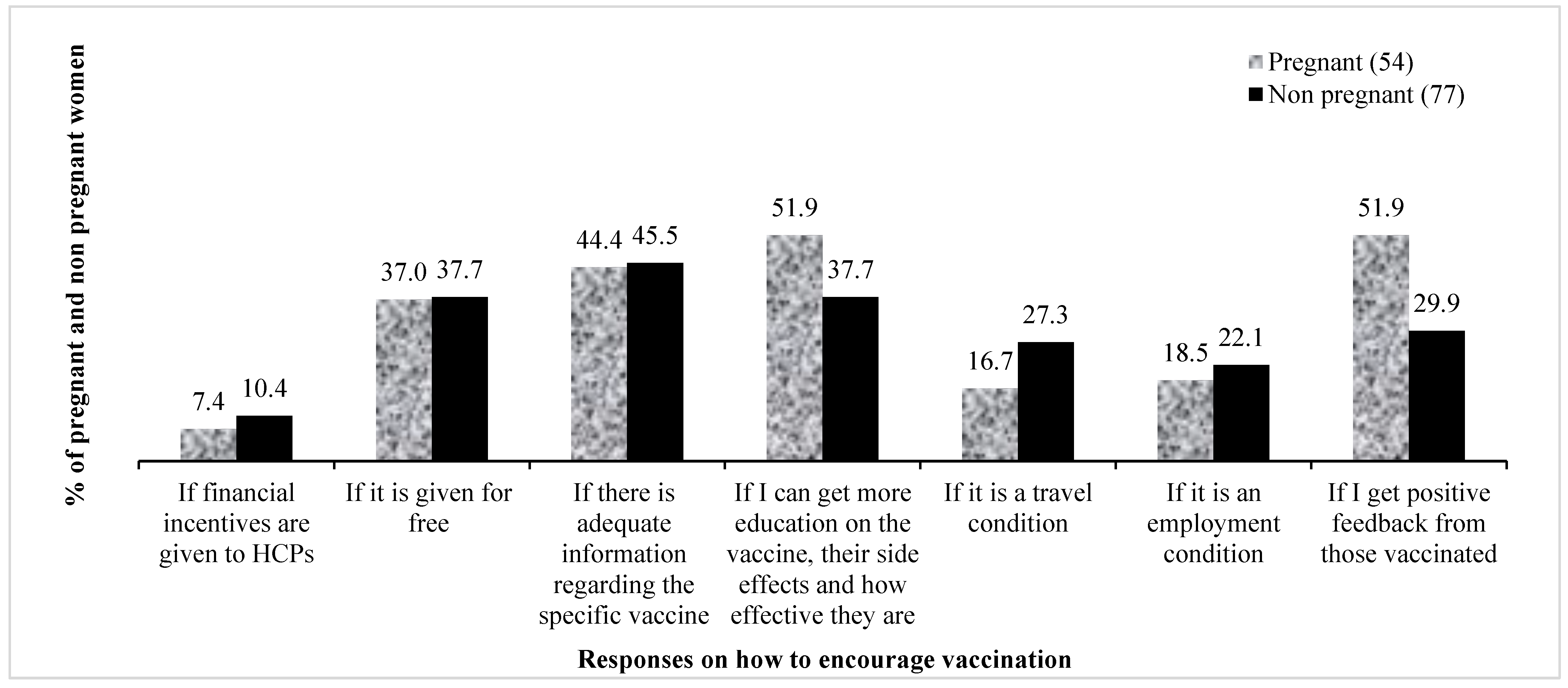
3.5. Motivations to Get COVID-19 Vaccines
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phoswa, W.N.; Khaliq, O.P. Is pregnancy a risk factor of COVID-19? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 605. [Google Scholar] [CrossRef] [PubMed]
- Celewicz, A.; Celewicz, M.; Michalczyk, M.; Woźniakowska-Gondek, P.; Krejczy, K.; Misiek, M.; Rzepka, R. Pregnancy as a Risk Factor of Severe COVID-19. J. Clin. Med. 2021, 10, 5458. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.; Fernandez, S.; Bonet, M.; Stallings, E.; Yap, M.; Kew, T.; Zhou, D.; Coomar, D.; Sheikh, J.; Lawson, H.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccination—Pregnancy, Breastfeeding, and COVID-19 Vaccines|Australian Government Department of Health and Aged Care. Available online: https://www.health.gov.au/resources/publications/covid-19-vaccination-pregnancy-breastfeeding-and-covid-19-vaccines?language=en (accessed on 4 January 2023).
- Luppi, P. How immune mechanisms are affected by pregnancy. Vaccine 2003, 21, 3352–3357. [Google Scholar] [CrossRef]
- Buckley, S.J. Executive Summary of Hormonal Physiology of Childbearing: Evidence and Implications for Women, Babies, and Maternity Care. J. Perinat. Educ. 2015, 24, 145. [Google Scholar] [CrossRef]
- Blakeway, H.; Prasad, S.; Kalafat, E.; Heath, P.T.; Ladhani, S.N.; Le Doare, K.; Magee, L.A.; O’Brien, P.; Rezvani, A.; von Dadelszen, P.; et al. COVID-19 vaccination during pregnancy: Coverage and safety. Am. J. Obstet. Gynecol. 2021, 226, 236.e1–236.e14. [Google Scholar] [CrossRef]
- Adhikari, E.H.; Spong, C.Y. COVID-19 Vaccination in Pregnant and Lactating Women. JAMA 2021, 325, 1039. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Vaccines Advice. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice (accessed on 1 January 2023).
- Government, U. COVID-19 Vaccination: A Guide on Pregnancy and Breastfeeding—GOV.UK. Available online: https://www.gov.uk/government/publications/covid-19-vaccination-women-of-childbearing-age-currently-pregnant-planning-a-pregnancy-or-breastfeeding/covid-19-vaccination-a-guide-on-pregnancy-and-breastfeeding (accessed on 1 January 2023).
- Vaccination Considerations for People Who Are Pregnant or Breastfeeding. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html (accessed on 14 December 2022).
- Magnus, M.C.; Gjessing, H.K.; Eide, H.N.; Wilcox, A.J.; Fell, D.B.; Håberg, S.E. COVID-19 Vaccination during Pregnancy and First-Trimester Miscarriage. N. Engl. J. Med. 2021, 385, 2008–2010. [Google Scholar] [CrossRef]
- Zauche, L.H.; Wallace, B.; Smoots, A.N.; Olson, C.K.; Oduyebo, T.; Kim, S.Y.; Petersen, E.E.; Ju, J.; Beauregard, J.; Wilcox, A.J.; et al. Receipt of mRNA COVID-19 Vaccines and Risk of Spontaneous Abortion. N. Engl. J. Med. 2021, 385, 1533–1535. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef]
- Levy, A.T.; Singh, S.; Riley, L.E.; Prabhu, M. Acceptance of COVID-19 vaccination in pregnancy: A survey study. Am. J. Obstet. Gynecol. MFM 2021, 3, 100399. [Google Scholar] [CrossRef] [PubMed]
- Goncu Ayhan, S.; Oluklu, D.; Atalay, A.; Menekse Beser, D.; Tanacan, A.; Moraloglu Tekin, O.; Sahin, D. COVID-19 vaccine acceptance in pregnant women. Int. J. Gynaecol. Obstet. 2021, 154, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Hailemariam, S.; Mekonnen, B.; Shifera, N.; Endalkachew, B.; Asnake, M.; Assefa, A.; Qanche, Q. Predictors of pregnant women’s intention to vaccinate against coronavirus disease 2019: A facility-based cross-sectional study in southwest Ethiopia. SAGE Open Med. 2021, 9, 20503121211038454. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.; Brigden, A.; Davies, A.; Shepherd, E.; Ingram, J. Maternal vaccines during the COVID-19 pandemic: A qualitative interview study with UK pregnant women. Midwifery 2021, 100, 103062. [Google Scholar] [CrossRef] [PubMed]
- Galanis, P.; Vraka, I.; Siskou, O.; Konstantakopoulou, O.; Katsiroumpa, A.; Kaitelidou, D. Uptake of COVID-19 Vaccines among Pregnant Women: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 766. [Google Scholar] [CrossRef]
- Iliyasu, Z.; Perkins, J.M.; Tsiga-Ahmed, F.I.; Galadanci, H.S.; Jibo, A.M.; Amole, T.G.; Umar, A.A.; Abdullahi, H.M.; Kwaku, A.A.; Salihu, H.M.; et al. COVID-19 Vaccine Acceptability Among Pregnant Women in Northern Nigeria. J. Obstet. Gynaecol. Can. 2022, 44, 349–350.e1. [Google Scholar] [CrossRef] [PubMed]
- Januszek, S.M.; Faryniak-Zuzak, A.; Barnaś, E.; Łoziński, T.; Góra, T.; Siwiec, N.; Szczerba, P.; Januszek, R.; Kluz, T. The Approach of Pregnant Women to Vaccination Based on a COVID-19 Systematic Review. Medicina 2021, 57, 977. [Google Scholar] [CrossRef]
- Skjefte, M.; Ngirbabul, M.; Akeju, O.; Escudero, D.; Hernandez-Diaz, S.; Wyszynski, D.F.; Wu, J.W. COVID-19 vaccine acceptance among pregnant women and mothers of young children: Results of a survey in 16 countries. Eur. J. Epidemiol. 2021, 36, 197–211. [Google Scholar] [CrossRef]
- Sutton, D.; D'Alton, M.; Zhang, Y.; Kahe, K.; Cepin, A.; Goffman, D.; Staniczenko, A.; Yates, H.; Burgansky, A.; Coletta, J.; et al. COVID-19 vaccine acceptance among pregnant, breastfeeding, and nonpregnant reproductive-aged women. Am. J. Obstet. Gynecol. MFM 2021, 3, 100403. [Google Scholar] [CrossRef]
- Mose, A.; Yeshaneh, A. COVID-19 Vaccine Acceptance and Its Associated Factors Among Pregnant Women Attending Antenatal Care Clinic in Southwest Ethiopia: Institutional-Based Cross-Sectional Study. Int. J. Gen. Med. 2021, 14, 2385–2395. [Google Scholar] [CrossRef]
- Jayagobi, P.A.; Ong, C.; Thai, Y.K.; Lim, C.C.; Jiun, S.M.; Koon, K.L.; Wai, K.C.; Chan, J.K.Y.; Mathur, M.; Chien, C.M. Perceptions and acceptance of COVID-19 vaccine among pregnant and lactating women in Singapore: A cross-sectional study. medRxiv 2021. [Google Scholar] [CrossRef]
- Tostrud, L.; Thelen, J.; Palatnik, A. Models of determinants of COVID-19 vaccine hesitancy in non pregnant and pregnant population: Review of current literature. Hum. Vaccines Immunother. 2022, 18, 2138047. [Google Scholar] [CrossRef]
- Makoni, M. The quest for more COVID-19 vaccinations in Africa. Lancet Respir. Med. 2022, 10, e70–e71. [Google Scholar] [CrossRef]
- Woodcock, T.; Novov, V.; Skirrow, H.; Butler, J.; Lovett, D.; Adeleke, Y.; Blair, M.; Saxena, S.; Majeed, A.; Aylin, P. Characteristics associated with influenza vaccination uptake in pregnancy: A retrospective cohort study. Br. J. Gen. Pract. 2023, 73, e148–e155. [Google Scholar] [PubMed]
- Yazdanbakhsh, M.; Kremsner, P.G. Influenza in Africa. PLoS Med. 2009, 6, e1000182. [Google Scholar] [CrossRef] [PubMed]
- Troiano, G.; Nardi, A. Vaccine hesitancy in the era of COVID-19. Public Health 2021, 194, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Remmel, A. COVID vaccines and safety: What the research says. Nature 2021, 590, 538–540. [Google Scholar] [CrossRef]
- Larson, H.J.; Clarke, R.M.; Jarrett, C.; Eckersberger, E.; Levine, Z.; Schulz, W.S.; Paterson, P. Measuring trust in vaccination: A systematic review. Hum. Vaccines Immunother. 2018, 14, 1599–1609. [Google Scholar] [CrossRef]
- Larson, H.J.; De Figueiredo, A.; Xiahong, Z.; Schulz, W.S.; Verger, P.; Johnston, I.G.; Cook, A.R.; Jones, N.S. The State of Vaccine Confidence 2016: Global Insights Through a 67-Country Survey. EBioMedicine 2016, 12, 295–301. [Google Scholar] [CrossRef]
- Osuagwu, U.L.; Mashige, K.P.; Ovenseri-Ogbomo, G.; Envuladu, E.A.; Abu, E.K.; Miner, C.A.; Timothy, C.G.; Ekpenyong, B.N.; Langsi, R.; Amiebenomo, O.M.; et al. The impact of information sources on COVID-19 vaccine hesitancy and resistance in sub-Saharan Africa. BMC Public Health 2023, 23, 1–16. [Google Scholar] [CrossRef]
- Skirrow, H.; Barnett, S.; Bell, S.; Riaposova, L.; Mounier-Jack, S.; Kampmann, B.; Holder, B. Women’s views on accepting COVID-19 vaccination during and after pregnancy, and for their babies: A multi-methods study in the UK. BMC Pregnancy Childbirth 2022, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Murewanhema, G.; Musuka, G.; Denhere, K.; Chingombe, I.; Mapingure, M.P.; Dzinamarira, T. The Landscape of COVID-19 Vaccination in Zimbabwe: A Narrative Review and Analysis of the Strengths, Weaknesses, Opportunities and Threats of the Programme. Vaccines 2022, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Usigbe, L. Nigeria: COVID-19 Vaccine Rollout Kicks off in Africa’s Most Populous Country. Africa Renewal 2021. Available online: https://www.un.org/africarenewal/magazine/april-2021/nigeria-covid-19-vaccine-rollout-kicks-africas-most-populous-country (accessed on 13 February 2023).
- Kyobutungi, C. The Ins and Outs of Kenya’s COVID-19 Vaccine Rollout Plan. The Conversation. Available online: https://webcache.googleusercontent.com/search?q=cache:4ttNkmgD87cJ:https://theconversation.com/the-ins-and-outs-of-kenyas-covid-19-vaccine-rollout-plan-156310&cd=1&hl=zh-CN&ct=clnk&gl=sg&client=firefox-b-d (accessed on 13 February 2023).
- Osuagwu, U.L.; Timothy, C.G.; Langsi, R.; Abu, E.K.; Goson, P.C.; Mashige, K.P.; Ekpenyong, B.; Ovenseri-Ogbomo, G.O.; Miner, C.A.; Oloruntoba, R.; et al. Differences in Perceived Risk of Contracting SARS-CoV-2 during and after the Lockdown in Sub-Saharan African Countries. Int. J. Environ. Res. Public Health 2021, 18, 11091. [Google Scholar] [CrossRef] [PubMed]

| Variable | Pregnant Women (n = 54, 41.2%) | Non Pregnant Women (n = 77, 58.8%) | p-Value |
|---|---|---|---|
| Demography | |||
| Region of origin | |||
| West Africa | 30 (56.6) | 28 (36.36) | 0.037 |
| East Africa | 4 (7.55) | 2 (2.60) | |
| Central Africa | 8 (15.09) | 20 (25.97) | |
| Southern Africa | 11 (20.75) | 27 (35.06) | |
| Age | |||
| 18–34 years | 32 (60.38) | 35 (50) | 0.252 |
| 35 and older | 21 (39.62) | 35 (50) | |
| Marital status | |||
| Unmarried | 15 (27.78) | 64 (83.12) | <0.001 |
| Married | 39 (72.22) | 13 (16.88) | |
| Education | |||
| Tertiary | 50 (92.59) | 37 (48.05) | <0.001 |
| Secondary | 4 (7.41) | 40 (51.95) | |
| Employment status | |||
| Unemployed | 14 (25.93) | 28 (36.36) | 0.208 |
| Employed | 40 (74.07) | 49 (63.64) | |
| Occupation | |||
| Non-healthcare worker | 36 (66.67) | 59 (76.62) | 0.209 |
| Healthcare worker | 18 (33.33) | 18 (23.38) | |
| Place of residence | n = 53 | ||
| Africa | 52 (98.11) | 73 (94.81) | 0.335 |
| Diaspora | 1 (1.89) | 4 (5.19) | |
| COVID-19 test factors | |||
| COVID-19 vaccine can prevent COVID-19 infection and its complications | |||
| Disagree | 11 (20.37) | 26 (33.77) | 0.492 |
| Agree | 43 (79.63) | 51 (66.23) | |
| Have you ever been tested for coronavirus disease (COVID-19)? | |||
| No | 35 (64.81) | 59 (76.62) | 0.139 |
| Yes | 19 (35.19) | 18 (23.38) | |
| Have you ever tested positive for coronavirus disease (COVID-19)? | |||
| No | 48 (88.89) | 72 (93.51) | 0.348 |
| Yes | 6 (11.11) | 5 (6.49) | |
| Common misconceptions about the COVID-19 vaccine | |||
| COVID-19 vaccines cause infertility in women | |||
| Disagree | 29 (53.70) | 46 (59.74) | 0.492 |
| Agree | 25 (46.30) | 31 (40.26) | |
| COVID-19 vaccine is a means to digitally implant a microchip | |||
| Disagree | 31 (57.41) | 53 (68.83) | 0.094 |
| Agree | 23 (42.59) | 24 (31.17) | |
| COVID-19 vaccines alter DNA | |||
| Disagree | 11 (20.37) | 26 (33.77) | 0.180 |
| Agree | 43 (79.63) | 59 (66.23) | |
| Perception of risk of COVID-19 infection | |||
| Mean (SD) | 3.74 (2.26) | 5.78 (2.89) | <0.001 |
| Variable | AOR [95%CI] | p-Value |
|---|---|---|
| Education | ||
| Tertiary | 1.00 | |
| Secondary | 0.04 [0.01, 0.18] | <0.001 |
| Marital status | ||
| Unmarried | 1.00 | |
| Married | 37.54 [9.30, 151.56] | <0.001 |
| COVID-19 vaccine is a means to implant a digital microchip | ||
| No | 1.00 | |
| Yes | 3.63 [1.12, 11.79] | 0.032 |
| Perception of risk of COVID-19 infection | 1.58 [1.24, 2.01] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amiebenomo, O.M.; Osuagwu, U.L.; Envuladu, E.A.; Miner, C.A.; Mashige, K.P.; Ovenseri-Ogbomo, G.; Abu, E.K.; Timothy, C.G.; Ekpenyong, B.N.; Langsi, R.; et al. Acceptance and Risk Perception of COVID-19 Vaccination among Pregnant and Non Pregnant Women in Sub-Saharan Africa: A Cross-Sectional Matched-Sample Study. Vaccines 2023, 11, 484. https://doi.org/10.3390/vaccines11020484
Amiebenomo OM, Osuagwu UL, Envuladu EA, Miner CA, Mashige KP, Ovenseri-Ogbomo G, Abu EK, Timothy CG, Ekpenyong BN, Langsi R, et al. Acceptance and Risk Perception of COVID-19 Vaccination among Pregnant and Non Pregnant Women in Sub-Saharan Africa: A Cross-Sectional Matched-Sample Study. Vaccines. 2023; 11(2):484. https://doi.org/10.3390/vaccines11020484
Chicago/Turabian StyleAmiebenomo, Onyekachukwu M., Uchechukwu L. Osuagwu, Esther Awazzi Envuladu, Chundung Asabe Miner, Khathutshelo P. Mashige, Godwin Ovenseri-Ogbomo, Emmanuel Kwasi Abu, Chikasirimobi Goodhope Timothy, Bernadine N. Ekpenyong, Raymond Langsi, and et al. 2023. "Acceptance and Risk Perception of COVID-19 Vaccination among Pregnant and Non Pregnant Women in Sub-Saharan Africa: A Cross-Sectional Matched-Sample Study" Vaccines 11, no. 2: 484. https://doi.org/10.3390/vaccines11020484
APA StyleAmiebenomo, O. M., Osuagwu, U. L., Envuladu, E. A., Miner, C. A., Mashige, K. P., Ovenseri-Ogbomo, G., Abu, E. K., Timothy, C. G., Ekpenyong, B. N., Langsi, R., Oloruntoba, R., Goson, P. C., Charwe, D. D., Ishaya, T., & Agho, K. E. (2023). Acceptance and Risk Perception of COVID-19 Vaccination among Pregnant and Non Pregnant Women in Sub-Saharan Africa: A Cross-Sectional Matched-Sample Study. Vaccines, 11(2), 484. https://doi.org/10.3390/vaccines11020484








